In Situ Evaluation of Epoxy Self-Healing Coating by Encapsulated Linseed Oil in Poly(Urea–Formaldehyde–Melamine) Microcapsules
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Microcapsule Synthesis
2.3. Microcapsule Characterization
2.4. Preparation of Epoxy-Coated Mild Steel Samples
2.5. Characterization of Epoxy-Coated Mild Steel Samples
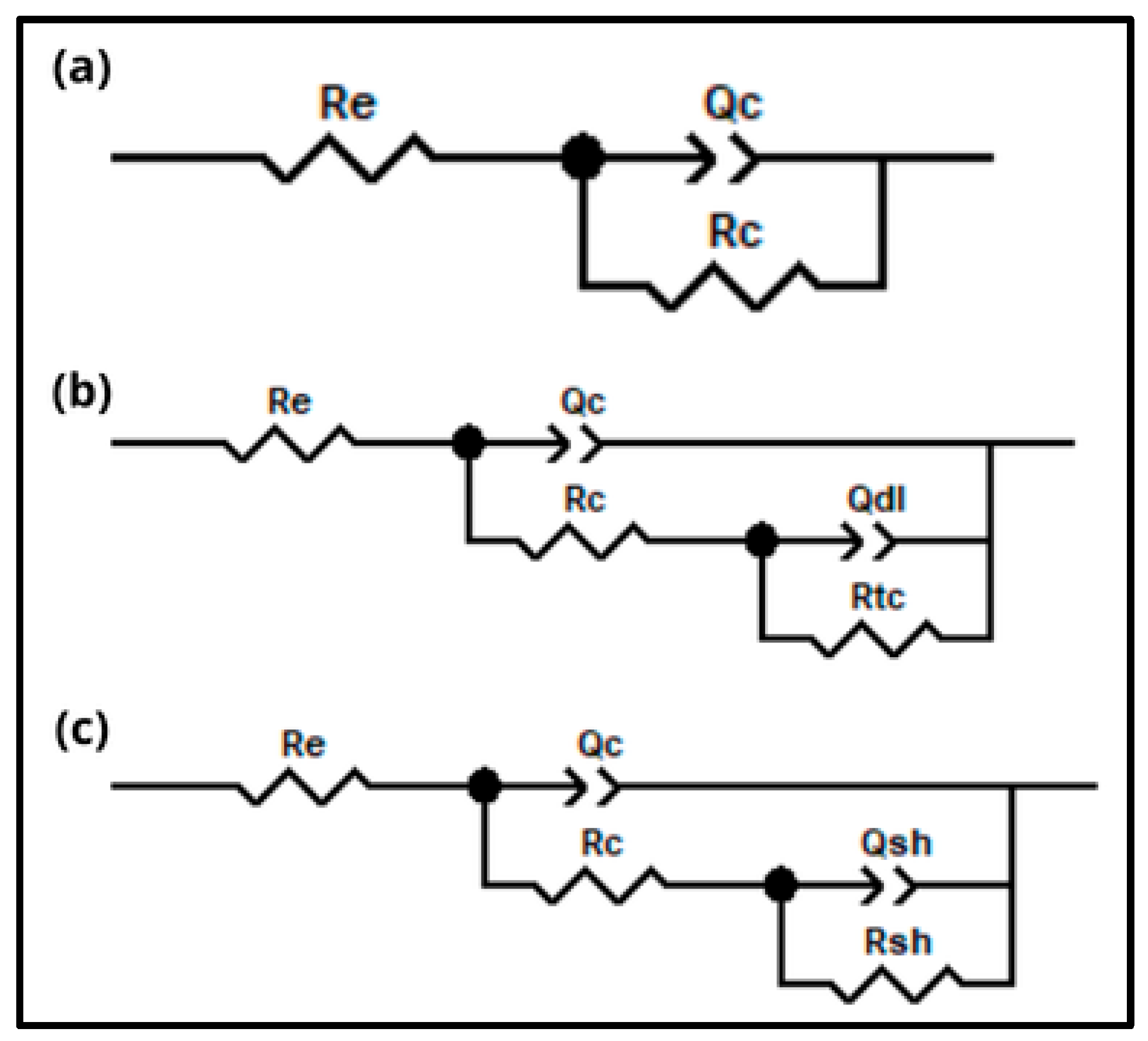
2.6. Electrochemical Impedance Spectroscopy
2.7. Raman Spectroscopy in Situ Characterization
3. Results and Discussion
3.1. Physicochemical Characterization of the Pigments
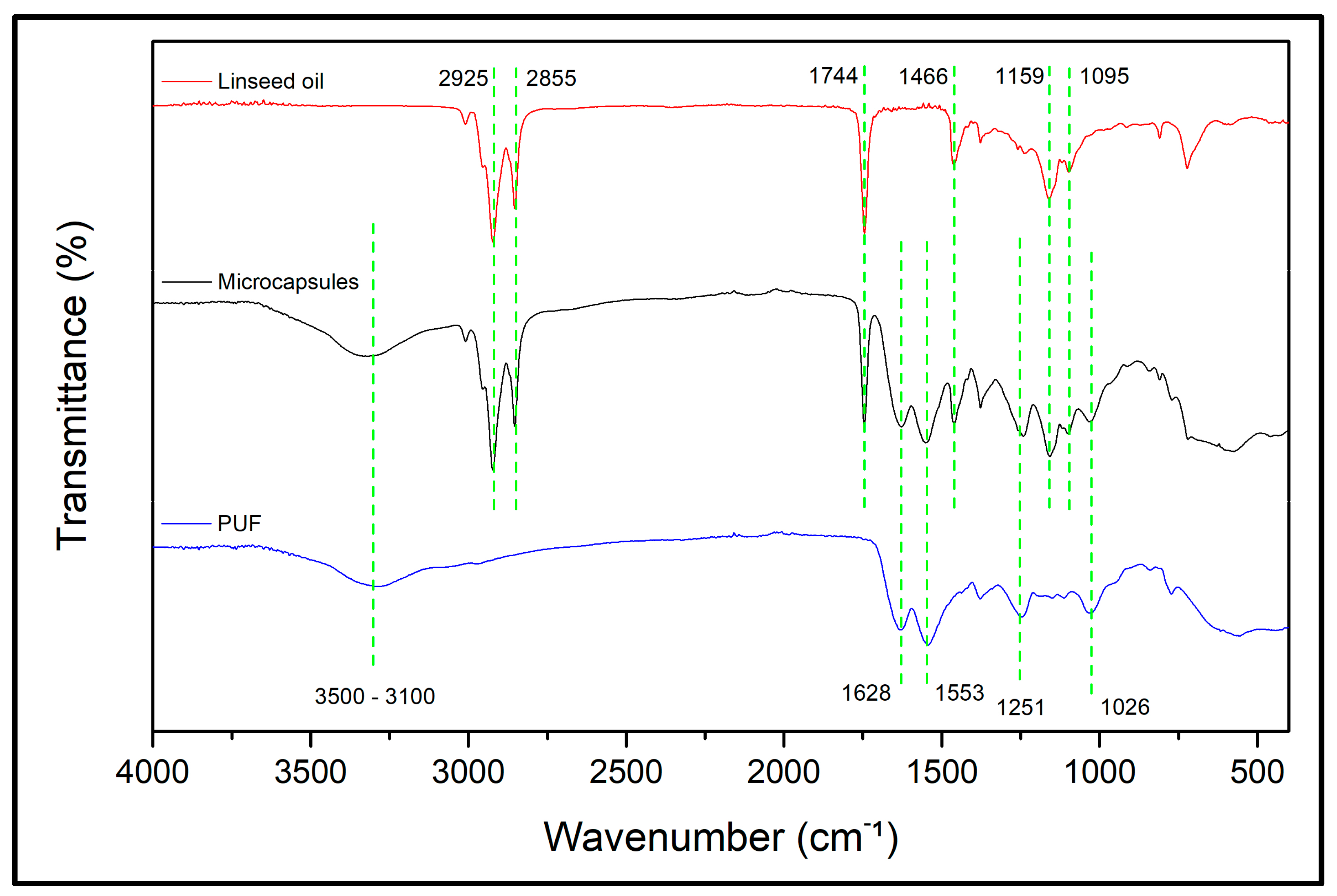
3.1.1. Fourier Transform Infrared Spectroscopy
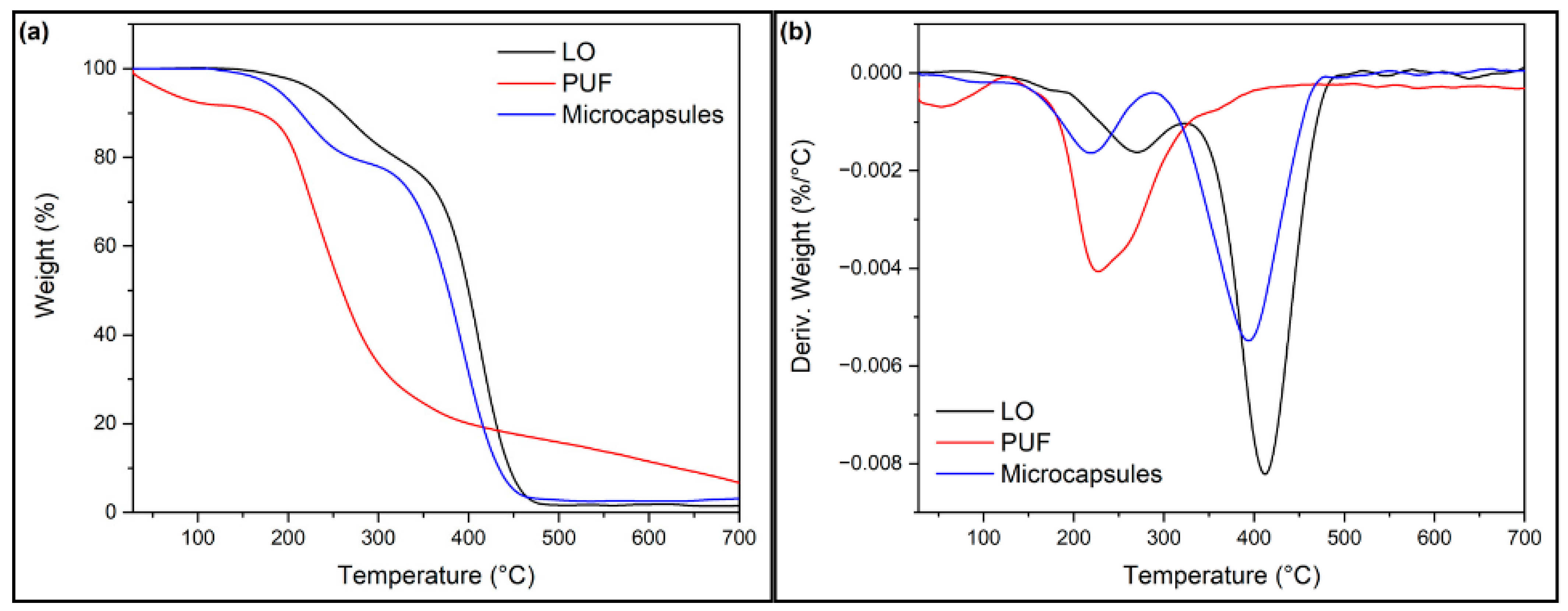
3.1.2. Thermogravimetric Analysis
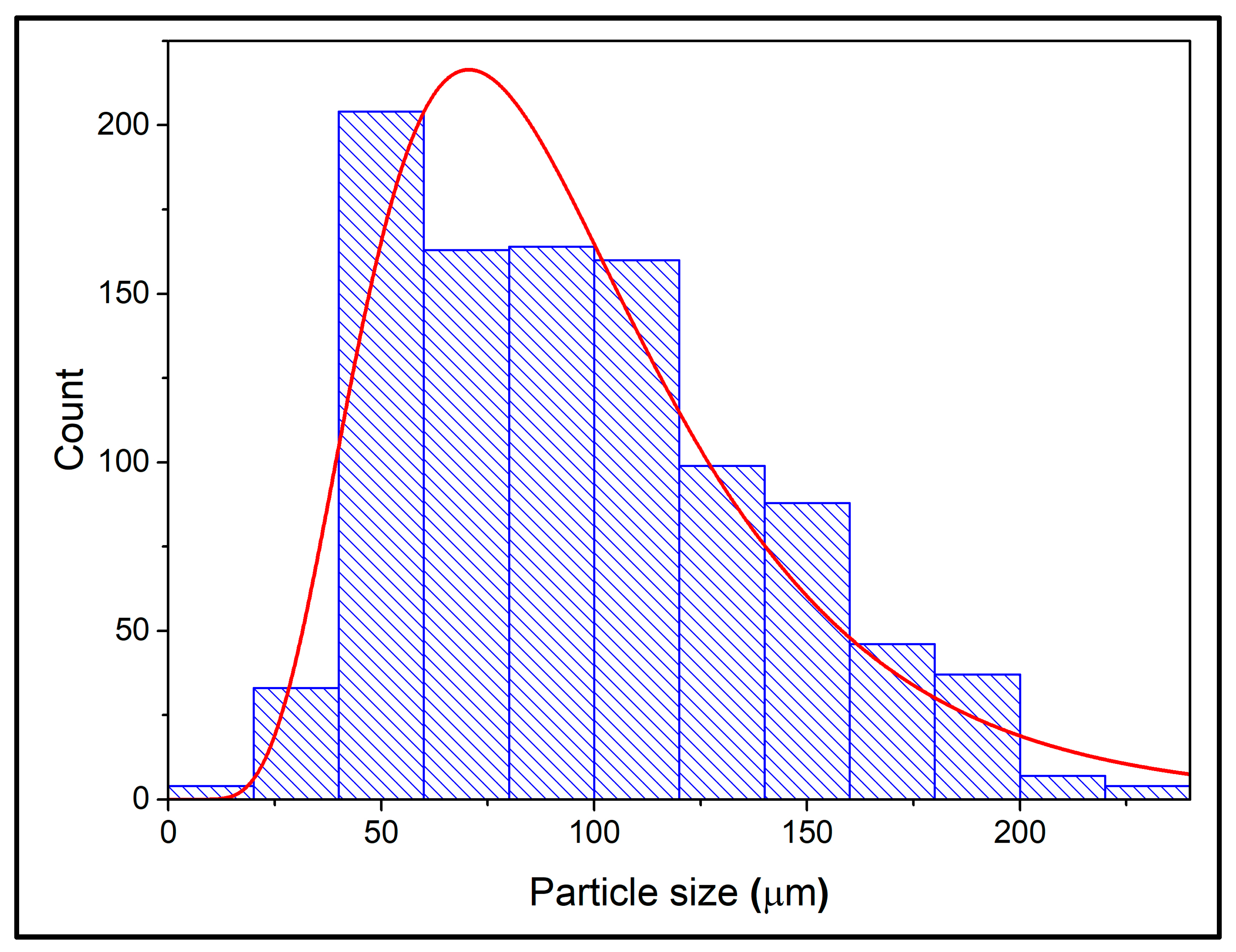
3.1.3. Particle Size and Linseed Oil Content of the Microcapsules
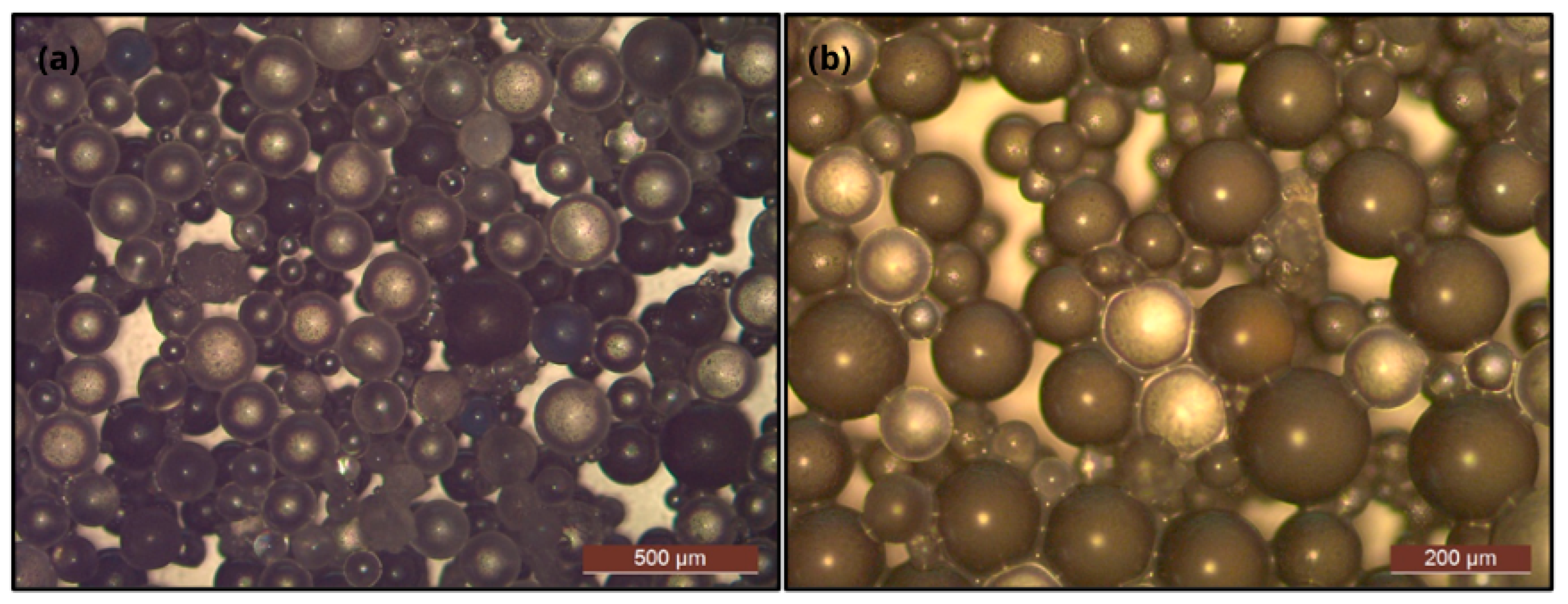
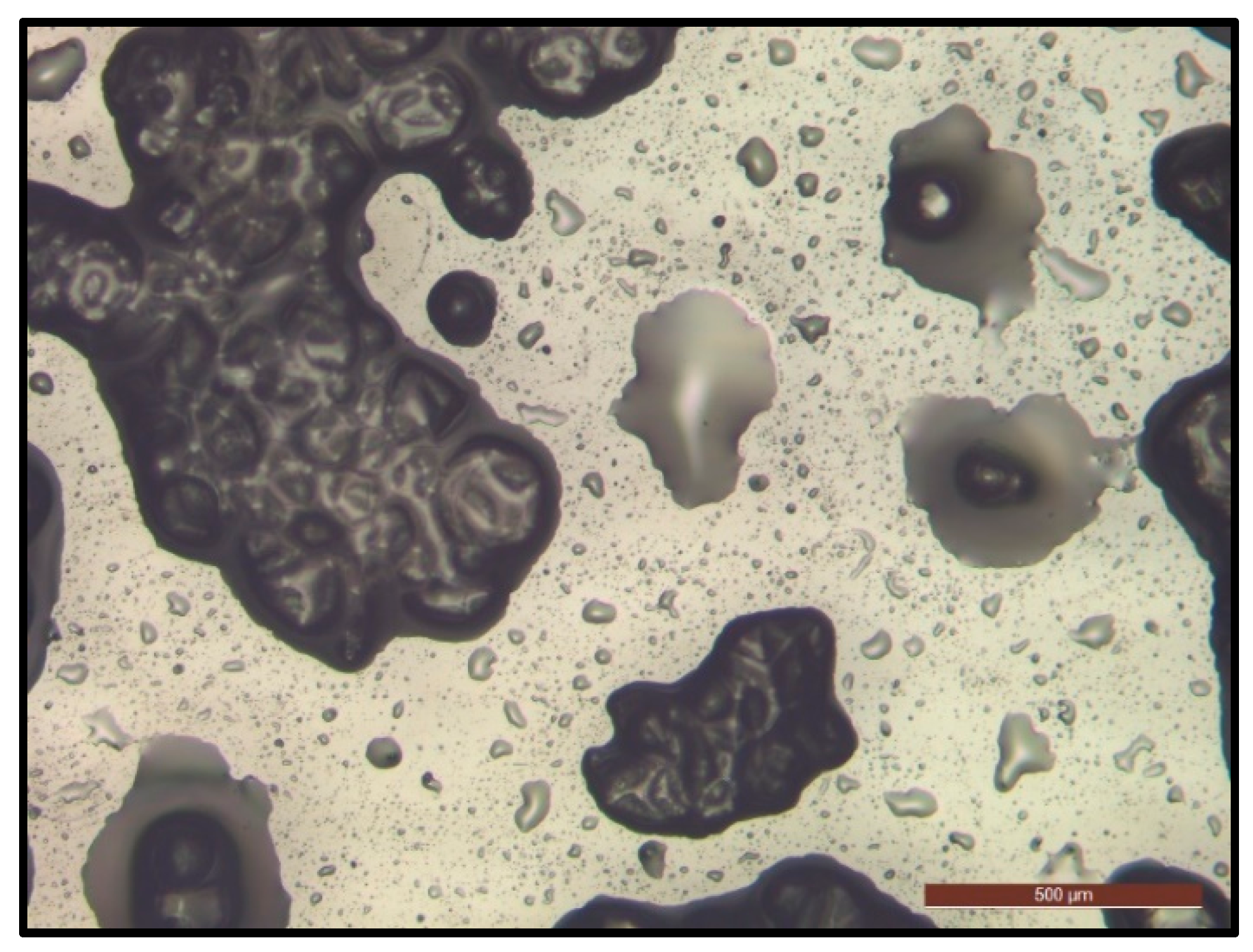
3.1.4. Microcapsule Morphology
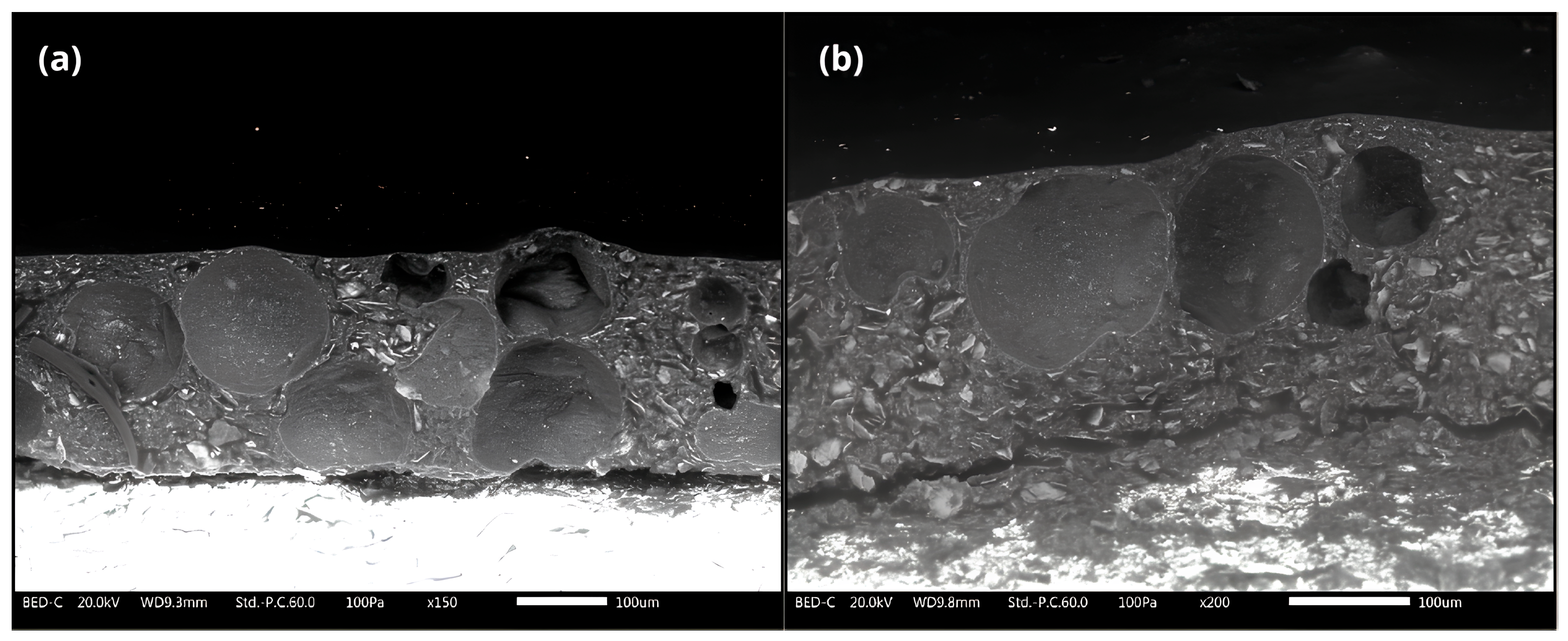
3.1.5. Scanning Electron Microscopy of Cross-Section of Epoxy Coating
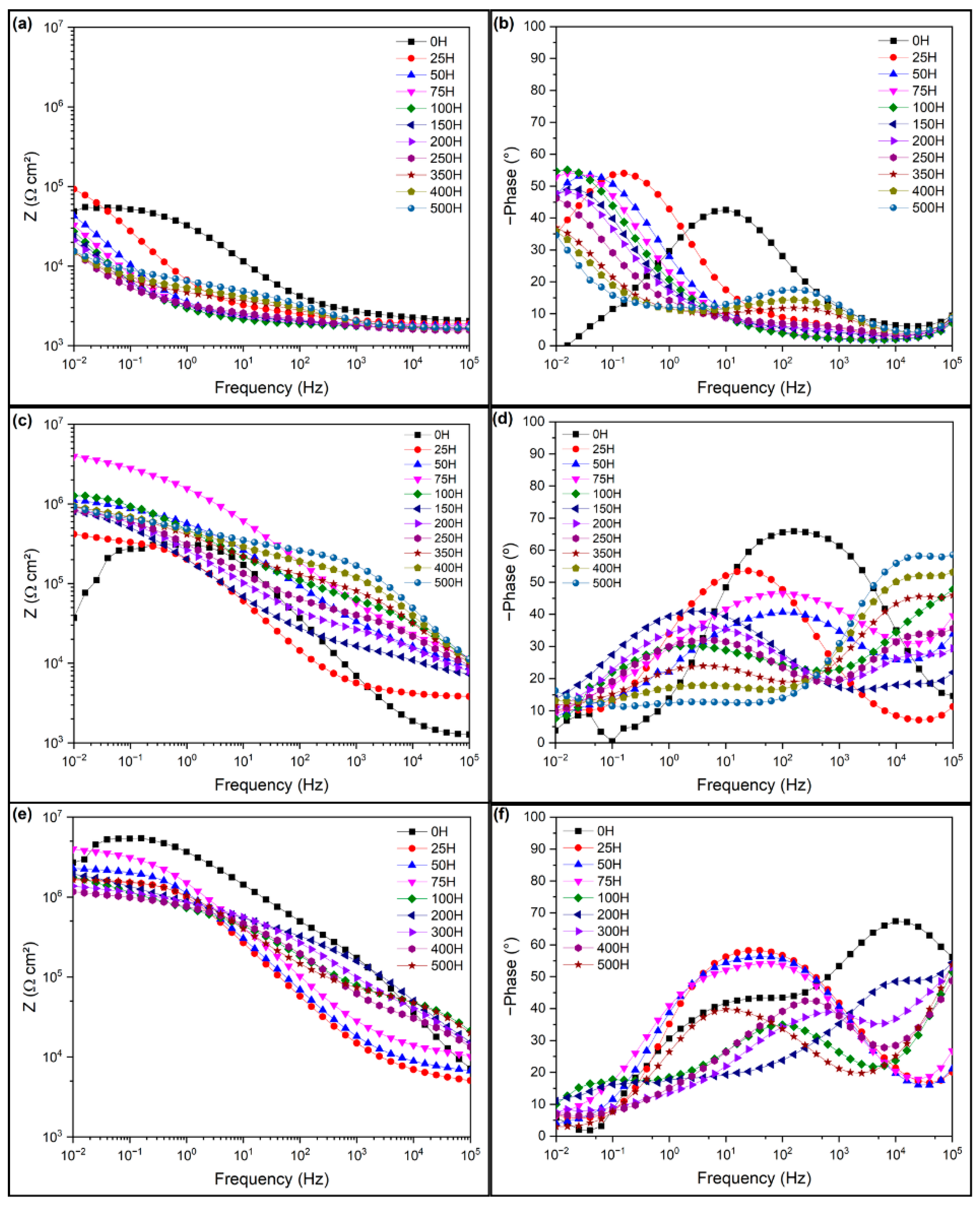
3.2. Electrochemical Impedance Spectroscopy of the Intact Coatings
3.3. Electrochemical Impedance Spectroscopy of the Damaged Coatings
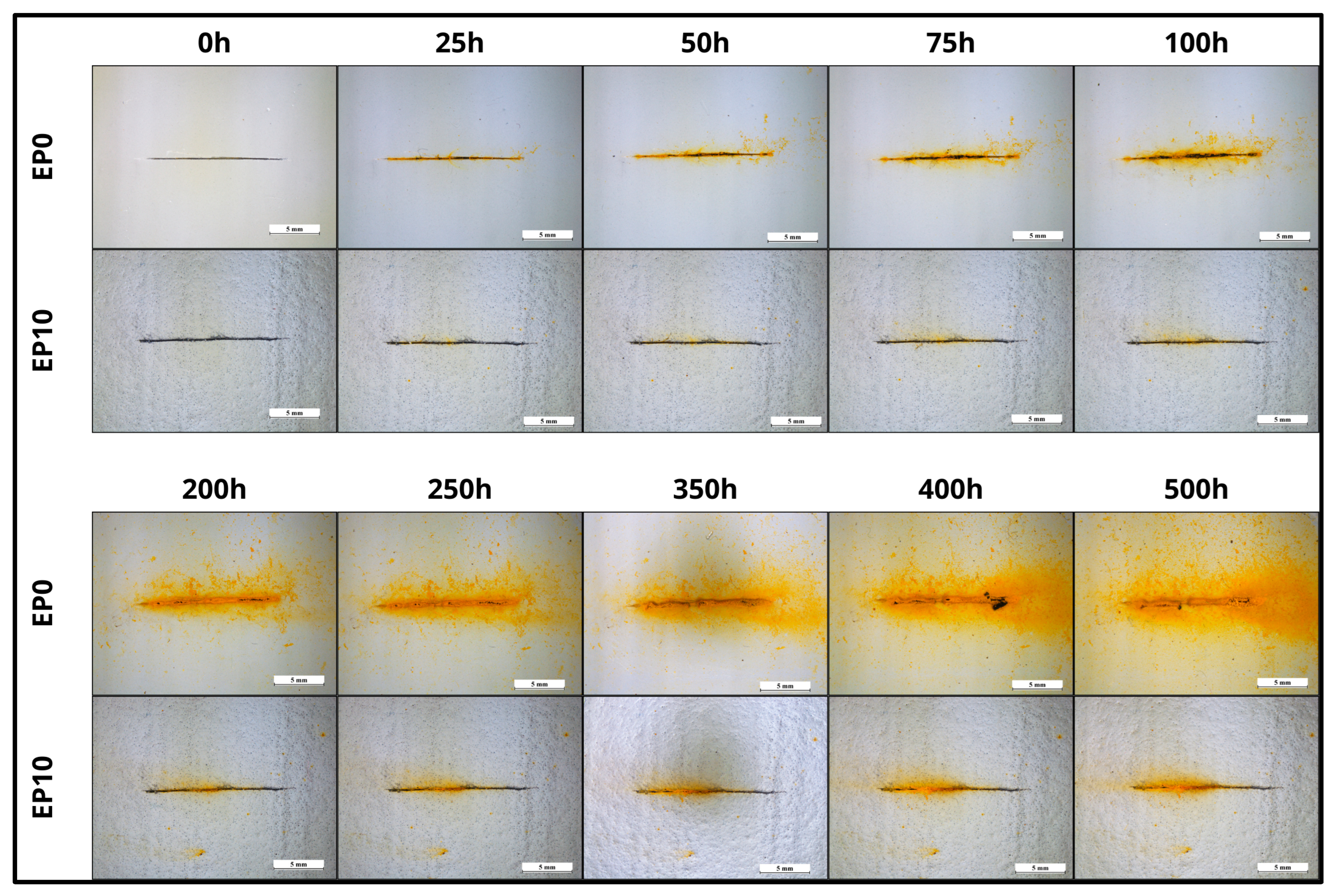
3.4. Corrosion Resistance by Immersion Study
3.5. In Situ Characterization by Raman Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SEM | Scanning Electron Microscopy |
| OM | Optical Microscopy |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| TGA | Thermogravimetric Analysis |
| EIS | Electrochemical Impedance Spectroscopy |
| OCP | Open-Circuit Potential |
| PUF | Poly(Urea–Formaldehyde–Melamine) |
| DTG | Derivative Thermogravimetric |
| LO | Linseed Oil |
| EP0 | Pure Epoxy Resin Coating |
| EP10 | Composite Coating With 10 wt% Microcapsules |
| CPE | Constant Phase Element |
References
- Lang, S.; Zhou, Q. Synthesis and Characterization of Poly(Urea-Formaldehyde) Microcapsules Containing Linseed Oil for Self-Healing Coating Development. Prog. Org. Coat. 2017, 105, 99–110. [Google Scholar] [CrossRef]
- Yang, H.; Mo, Q.; Li, W.; Gu, F. Preparation and Properties of Self-Healing and Self-Lubricating Epoxy Coatings with Polyurethane Microcapsules Containing Bifunctional Linseed Oil. Polymers 2019, 11, 1578. [Google Scholar] [CrossRef] [PubMed]
- Thanawala, K.; Mutneja, N.; Khanna, A.S.; Singh Raman, R.K. Development of Self-Healing Coatings Based on Linseed Oil as Autonomous Repairing Agent for Corrosion Resistance. Materials 2014, 7, 7324–7338. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ma, L.; Wang, X.; Wang, J.; Qian, H.; Zhang, D.; Li, X. Self-Healing Corrosion Protective Coatings Based on Micro/Nanocarriers: A Review. Corros. Commun. 2021, 1, 18–25. [Google Scholar] [CrossRef]
- Mahajan, M.S.; Gite, V.V. Self-Healing Polyurethane Coatings of Eugenol-Based Polyol Incorporated with Linseed Oil Encapsulated Cardanol-Formaldehyde Microcapsules: A Sustainable Approach. Prog. Org. Coat. 2022, 162, 106534. [Google Scholar] [CrossRef]
- Poornima Vijayan, P.; Al-Maadeed, M. Self-Repairing Composites for Corrosion Protection: A Review on Recent Strategies and Evaluation Methods. Materials 2019, 12, 2754. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, K.; Chen, Y.; Liu, R.; Luo, J. The Preparation of Linseed Oil Loaded Graphene/Polyaniline Microcapsule via Emulsion Template Method for Self-Healing Anticorrosion Coatings. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129771. [Google Scholar] [CrossRef]
- Zhang, F.; Ju, P.; Pan, M.; Zhang, D.; Huang, Y.; Li, G.; Li, X. Self-Healing Mechanisms in Smart Protective Coatings: A Review. Corros. Sci. 2018, 144, 74–88. [Google Scholar] [CrossRef]
- Frauches-Santos, C.; Albuquerque, M.A.; Oliveira, M.C.C.; Echevarria, A. A Corrosão e Os Agentes Anticorrosivos. Rev. Virtual De Química 2014, 6, 293–309. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Q. Evaluation and Failure Analysis of Linseed Oil Encapsulated Self-Healing Anticorrosive Coating. Prog. Org. Coat. 2018, 118, 108–115. [Google Scholar] [CrossRef]
- Cotting, F.; Koebsch, A.; Aoki, I.V. Epoxy Self-Healing Coating by Encapsulated Epoxy Ester Resin in Poly(Urea-Formaldehyde-Melamine) Microcapsules. Front. Mater. 2019, 6, 314. [Google Scholar] [CrossRef]
- Ong, G.; Kasi, R.; Subramaniam, R. A Review on Plant Extracts as Natural Additives in Coating Applications. Prog. Org. Coat. 2021, 151, 106091. [Google Scholar] [CrossRef]
- Ecco, L.G.; Li, J.; Fedel, M.; Deflorian, F.; Pan, J. EIS and in Situ AFM Study of Barrier Property and Stability of Waterborne and Solventborne Clear Coats. Prog. Org. Coat. 2014, 77, 600–608. [Google Scholar] [CrossRef]
- Pilbáth, A.; Szabó, T.; Telegdi, J.; Nyikos, L. SECM Study of Steel Corrosion under Scratched Microencapsulated Epoxy Resin. Prog. Org. Coat. 2012, 75, 480–485. [Google Scholar] [CrossRef]
- Mahmoudian, M.; Nozad, E.; Kochameshki, M.G.; Enayati, M. Preparation and Investigation of Hybrid Self-Healing Coatings Containing Linseed Oil Loaded Nanocapsules, Potassium Ethyl Xanthate and Benzotriazole on Copper Surface. Prog. Org. Coat. 2018, 120, 167–178. [Google Scholar] [CrossRef]
- Li, J.; Ecco, L.; Fedel, M.; Ermini, V.; Delmas, G.; Pan, J. In-Situ AFM and EIS Study of a Solventborne Alkyd Coating with Nanoclay for Corrosion Protection of Carbon Steel. Prog. Org. Coat. 2015, 87, 179–188. [Google Scholar] [CrossRef]
- Li, K.; Liu, Z.; Wang, C.; Fan, W.; Liu, F.; Li, H.; Zhu, Y.; Wang, H. Preparation of Smart Coatings with Self-Healing and Anti-Wear Properties by Embedding PU-Fly Ash Absorbing Linseed Oil Microcapsules. Prog. Org. Coat. 2020, 145, 105668. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Poznyak, S.K.; Rodrigues, L.M.; Raps, D.; Hack, T.; Dick, L.F.; Nunes, T.; Ferreira, M.G.S. Active Protection Coatings with Layered Double Hydroxide Nanocontainers of Corrosion Inhibitor. Corros. Sci. 2010, 52, 602–611. [Google Scholar] [CrossRef]
- Behzadnasab, M.; Mirabedini, S.M.; Esfandeh, M.; Farnood, R.R. Evaluation of Corrosion Performance of a Self-Healing Epoxy-Based Coating Containing Linseed Oil-Filled Microcapsules via Electrochemical Impedance Spectroscopy. Prog. Org. Coat. 2017, 105, 212–224. [Google Scholar] [CrossRef]
- Montemor, M.F. Functional and Smart Coatings for Corrosion Protection: A Review of Recent Advances. Surf. Coatings Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Kanellopoulos, A.; Giannaros, P.; Al-Tabbaa, A. The Effect of Varying Volume Fraction of Microcapsules on Fresh, Mechanical and Self-Healing Properties of Mortars. Constr. Build. Mater. 2016, 122, 577–593. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Rong, M.Z.; Zhang, M.Q. Self-Healing Polymeric Materials Based on Microencapsulated Healing Agents: From Design to Preparation. Prog. Polym. Sci. 2015, 49–50, 175–220. [Google Scholar] [CrossRef]
- Jadhav, R.S.; Hundiwale, D.G.; Mahulikar, P.P. Synthesis and Characterization of Phenol–Formaldehyde Microcapsules Containing Linseed Oil and Its Use in Epoxy for Self-Healing and Anticorrosive Coating. J. Appl. Polym. Sci. 2011, 119, 2911–2916. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Feng, Q.; Qiu, H.; Yang, G.; Zheng, S.; Yang, J. Encapsulation of Linseed Oil in Graphene Oxide Shells for Preparation of Self-Healing Composite Coatings. Prog. Org. Coat. 2019, 129, 285–291. [Google Scholar] [CrossRef]
- Navarchian, A.H.; Najafipoor, N.; Ahangaran, F. Surface-Modified Poly(Methyl Methacrylate) Microcapsules Containing Linseed Oil for Application in Self-Healing Epoxy-Based Coatings. Prog. Org. Coat. 2019, 132, 288–297. [Google Scholar] [CrossRef]
- Stankiewicz, A.; Szczygieł, I.; Szczygieł, B. Self-Healing Coatings in Anti-Corrosion Applications. J. Mater. Sci. 2013, 48, 8041–8051. [Google Scholar] [CrossRef]
- Udoh, I.I.; Shi, H.; Daniel, E.F.; Li, J.; Gu, S.; Liu, F.; Han, E.H. Active Anticorrosion and Self-Healing Coatings: A Review with Focus on Multi-Action Smart Coating Strategies. J. Mater. Sci. Technol. 2022, 116, 224–237. [Google Scholar] [CrossRef]
- Blaiszik, B.J.; Caruso, M.M.; McIlroy, D.A.; Moore, J.S.; White, S.R.; Sottos, N.R. Microcapsules Filled with Reactive Solutions for Self-Healing Materials. Polymer 2009, 50, 990–997. [Google Scholar] [CrossRef]
- Nesterova, T.; Dam-Johansen, K.; Pedersen, L.T.; Kiil, S. Microcapsule-Based Self-Healing Anticorrosive Coatings: Capsule Size, Coating Formulation, and Exposure Testing. Prog. Org. Coat. 2012, 75, 309–318. [Google Scholar] [CrossRef]
- Kouhi, M.; Mohebbi, A.; Mirzaei, M.; Peikari, M. Optimization of Smart Self-Healing Coatings Based on Micro/Nanocapsules in Heavy Metals Emission Inhibition. Prog. Org. Coat. 2013, 76, 1006–1015. [Google Scholar] [CrossRef]
- Cotting, F.; Aoki, I.V. Smart Protection Provided by Epoxy Clear Coating Doped with Polystyrene Microcapsules Containing Silanol and Ce (III) Ions as Corrosion Inhibitors. Surf. Coatings Technol. 2015, 303, 310–318. [Google Scholar] [CrossRef]
- Kurt Çömlekçi, G.; Ulutan, S. Encapsulation of Linseed Oil and Linseed Oil Based Alkyd Resin by Urea Formaldehyde Shell for Self-Healing Systems. Prog. Org. Coat. 2018, 121, 190–200. [Google Scholar] [CrossRef]
- de la Paz Miguel, M.; Vallo, C.I. Influence of the Emulsifying System to Obtain Linseed Oil-Filled Microcapsules with a Robust Poly(Melamine-Formaldehyde)-Based Shell. Prog. Org. Coat. 2019, 129, 236–246. [Google Scholar] [CrossRef]
- Hegde, M.B.; Mohana, K.N.S.; Rajitha, K.; Madhusudhana, A.M. Reduced Graphene Oxide-Epoxidized Linseed Oil Nanocomposite: A Highly Efficient Bio-Based Anti-Corrosion Coating Material for Mild Steel. Prog. Org. Coat. 2021, 159, 106399. [Google Scholar] [CrossRef]
- Leal, D.A.; Riegel-Vidotti, I.C.; Ferreira, M.G.S.; Marino, C.E.B. Smart Coating Based on Double Stimuli-Responsive Microcapsules Containing Linseed Oil and Benzotriazole for Active Corrosion Protection. Corros. Sci. 2018, 130, 56–63. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Rao, K.C.; Kumar, D. Preparation and Characterization of Microcapsules Containing Linseed Oil and Its Use in Self-Healing Coatings. Prog. Org. Coat. 2008, 63, 72–78. [Google Scholar] [CrossRef]
- Grinapel Lachtermacher, M.; Coelho, J.F.; Altoe Ferreira, P.; Solymossy, V.; Vieira Aoki, I. Revestimento Autorregenerante Contendo Agentes de Autorreparação. WO2014032130A1, 28 August 2012. [Google Scholar]
- Behzadnasab, M.; Esfandeh, M.; Mirabedini, S.M.; Zohuriaan-Mehr, M.J.; Farnood, R.R. Preparation and Characterization of Linseed Oil-Filled Urea-Formaldehyde Microcapsules and Their Effect on Mechanical Properties of an Epoxy-Based Coating. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 16–26. [Google Scholar] [CrossRef]
- PETROBRAS N-2680; Tinta Epóxi, Sem Solventes, Tolerante a Superfícies Molhadas. PETROBRAS: Buenos Aires, Argentina, 2007.
- De La Paz Miguel, M.; Ollier, R.; Alvarez, V.; Vallo, C. Effect of the Preparation Method on the Structure of Linseed Oil-Filled Poly(Urea-Formaldehyde) Microcapsules. Prog. Org. Coat. 2016, 97, 194–202. [Google Scholar] [CrossRef]
- Siva, T.; Sathiyanarayanan, S. Self Healing Coatings Containing Dual Active Agent Loaded Urea Formaldehyde (UF) Microcapsules. Prog. Org. Coat. 2015, 82, 57–67. [Google Scholar] [CrossRef]
- Abdipour, H.; Rezaei, M.; Abbasi, F. Synthesis and Characterization of High Durable Linseed Oil-Urea Formaldehyde Micro/Nanocapsules and Their Self-Healing Behaviour in Epoxy Coating. Prog. Org. Coat. 2018, 124, 200–212. [Google Scholar] [CrossRef]
- Tong, X.M.; Zhang, T.; Yang, M.Z.; Zhang, Q. Preparation and Characterization of Novel Melamine Modified Poly(Urea-Formaldehyde) Self-Repairing Microcapsules. Colloids Surf. A Physicochem. Eng. Asp. 2010, 371, 91–97. [Google Scholar] [CrossRef]
- Alias, J.; Johari, N.A.; Zanurin, A.; Alang, N.A.; Zain, M.Z.M. Self-Healing Epoxy Coating with Microencapsulation of Linseed Oil for the Corrosion Protection of Magnesium (Mg). J. Phys. Conf. Ser. 2021, 2129, 012008. [Google Scholar] [CrossRef]
- Paolini, N.A.; Cordeiro Neto, A.G.; Pellanda, A.C.; Carvalho Jorge, A.R.D.; Barros Soares, B.D.; Floriano, J.B.; Berton, M.A.C.; Vijayan P, P.; Thomas, S. Evaluation of Corrosion Protection of Self-Healing Coatings Containing Tung and Copaiba Oil Microcapsules. Int. J. Polym. Sci. 2021, 2021, 6650499. [Google Scholar] [CrossRef]
- Ebrahiminiya, A.; Khorram, M.; Hassanajili, S.; Javidi, M. Modeling and Optimization of the Parameters Affecting the In-Situ Microencapsulation Process for Producing Epoxy-Based Self-Healing Anti-Corrosion Coatings. Particuology 2018, 36, 59–69. [Google Scholar] [CrossRef]
- Bouvet, G.; Nguyen, D.D.; Mallarino, S.; Touzain, S. Analysis of the Non-Ideal Capacitive Behaviour for High Impedance Organic Coatings. Prog. Org. Coat. 2014, 77, 2045–2053. [Google Scholar] [CrossRef]
- Margarit-Mattos, I.C.P. EIS and Organic Coatings Performance: Revisiting Some Key Points. Electrochim. Acta 2020, 354, 136725. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, J.; Li, X.; Guo, M. Study of Degradation of Organic Coatings in Seawater by Using EIS and AFM Methods. J. Appl. Polym. Sci. 2008, 109, 1890–1899. [Google Scholar] [CrossRef]
- Yuan, X.; Yue, Z.F.; Chen, X.; Wen, S.F.; Li, L.; Feng, T. EIS Study of Effective Capacitance and Water Uptake Behaviors of Silicone-Epoxy Hybrid Coatings on Mild Steel. Prog. Org. Coat. 2015, 86, 41–48. [Google Scholar] [CrossRef]
- Tatiya, P.D.; Hedaoo, R.K.; Mahulikar, P.P.; Gite, V.V. Novel Polyurea Microcapsules Using Dendritic Functional Monomer: Synthesis, Characterization, and Its Use in Self-Healing and Anticorrosive Polyurethane Coatings. Ind. Eng. Chem. Res. 2013, 52, 1562–1570. [Google Scholar] [CrossRef]
- Covaciu, F.D.; Berghian-Grosan, C.; Feher, I.; Magdas, D.A. Edible Oils Differentiation Based on the Determination of Fatty Acids Profile and Raman Spectroscopy—A Case Study. Appl. Sci. 2020, 10, 8347. [Google Scholar] [CrossRef]
- Berghian-Grosan, C.; Magdas, D.A. Raman Spectroscopy and Machine-Learning for Edible Oils Evaluation. Talanta 2020, 218, 121176. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, P.; Wehling, B.; Moens, L.; Edwards, H.; De Reu, M.; Van Hooydonk, G. Analysis with Micro-Raman Spectroscopy of Natural Organic Binding Media and Varnishes Used in Art. Anal. Chim. Acta 2000, 407, 261–274. [Google Scholar] [CrossRef]
- Schönemann, A.; Edwards, H.G.M. Raman and FTIR Microspectroscopic Study of the Alteration of Chinese Tung Oil and Related Drying Oils during Ageing. Anal. Bioanal. Chem. 2011, 400, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.; Reggio, D.; Lazzari, M. Linseed Oil as a Model System for Surface Enhanced Raman Spectroscopy Detection of Degradation Products in Artworks. J. Raman Spectrosc. 2019, 50, 242–249. [Google Scholar] [CrossRef]



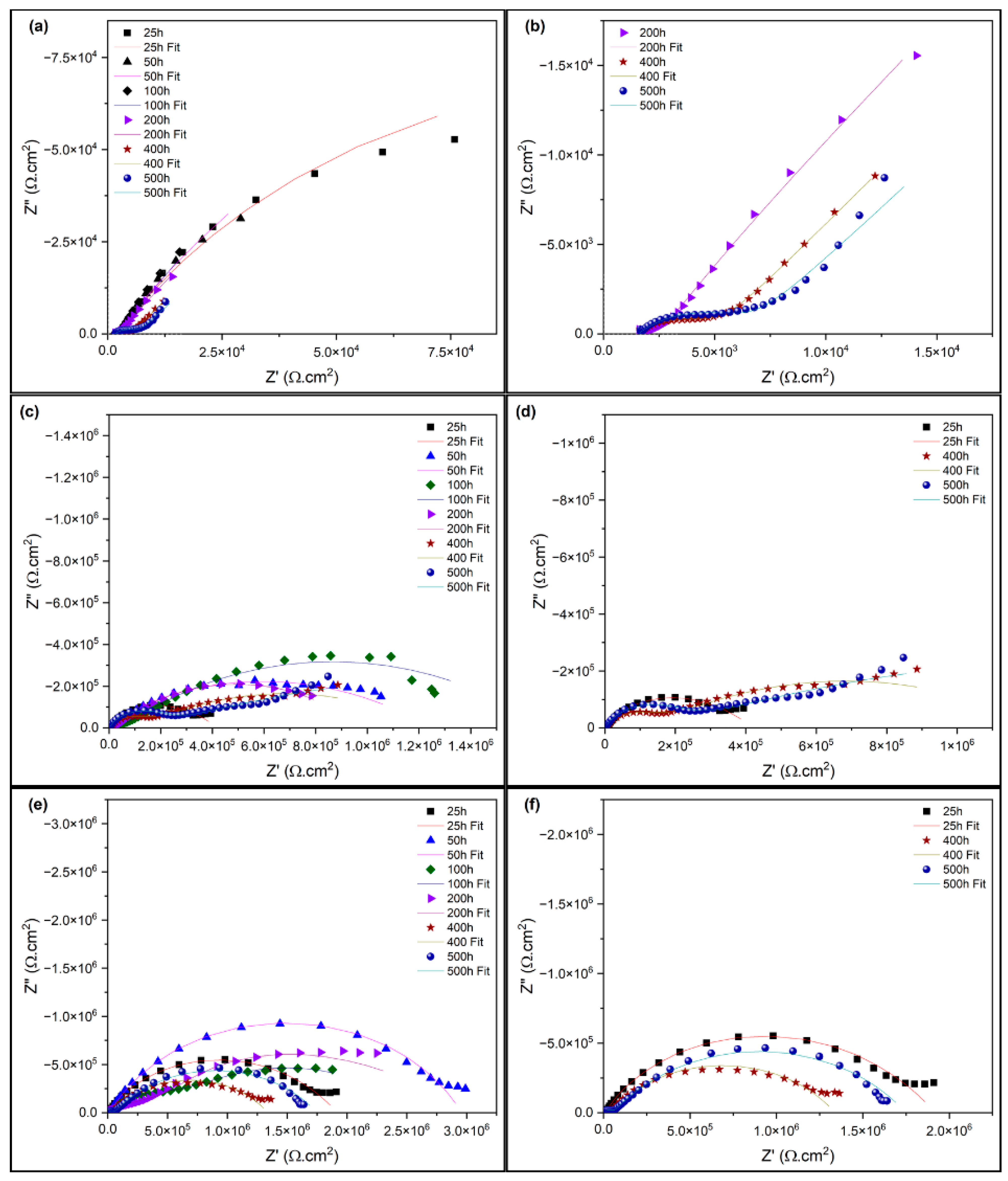

| Immersion Time | Sample | Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| Re | Qc | nc | Rc | Qdl | ndl | Rtc | ||
| 100 h | EP0 | 2000 | 3.23 × 10−10 | 0.8801 | 1.01 × 108 | 5.90 × 10−8 | 0.5514 | 7.47 × 107 |
| EP10 | 2000 | 2.55 × 10−10 | 0.8639 | 2.96 × 107 | 2.09 × 10−7 | 0.4465 | 1.97 × 108 | |
| 150 h | EP0 | 2000 | 1.78 × 10−10 | 0.9286 | 6.76 × 107 | 7.70 × 10−9 | 0.5275 | 7.27 × 107 |
| EP10 | 2000 | 2.34 × 10−10 | 0.8752 | 2.31 × 107 | 2.38 × 10−7 | 0.4238 | 6.70 × 107 | |
| 350 h | EP0 | 2000 | 1.63 × 10−10 | 0.9361 | 2.04 × 107 | 4.21 × 10−8 | 0.3765 | 1.40 × 108 |
| EP10 | 2000 | 2.50 × 10−10 | 0.8717 | 1.71 × 107 | 2.40 × 10−7 | 0.4323 | 5.44 × 107 | |
| 500 h | EP0 | 2000 | 1.53 × 10−10 | 0.9395 | 2.01 × 107 | 6.72 × 10−8 | 0.4533 | 1.10 × 108 |
| EP10 | 2000 | 2.48 × 10−10 | 0.8731 | 1.38 × 107 | 2.46 × 10−7 | 0.4276 | 3.78 × 107 | |
| 750 h | EP0 | 2000 | 1.21 × 10−10 | 0.9508 | 1.13 × 107 | 1.02 × 10−7 | 0.4447 | 1.01 × 108 |
| EP10 | 2000 | 2.80 × 10−10 | 0.8664 | 1.06 × 107 | 2.76 × 10−7 | 0.4465 | 2.17 × 107 | |
| 1000 h | EP0 | 2000 | 1.14 × 10−10 | 0.9556 | 1.23 × 107 | 9.07 × 10−8 | 0.4315 | 1.13 × 108 |
| EP10 | 2000 | 2.57 × 10−10 | 0.8790 | 6.62 × 106 | 2.37 × 10−7 | 0.4192 | 9.50 × 106 | |
| Immersion Time | Sample | Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Re | Qc | nc | Rc | Qsh | nsh | Rsh | Qdl | ndl | Rtc | ||
| 25 h | EP0 | 2000 | - | - | - | - | - | - | 4.27 × 10−5 | 0.6223 | 2.75 × 105 |
| EP10-I | 1000 | 5.79 × 10−7 | 0.4164 | 4576 | 3.42 × 10−7 | 0.7536 | 7.60 × 105 | - | - | - | |
| EP10-D | 2000 | 8.49 × 10−8 | 0.5288 | 11,505 | 7.48 × 10−8 | 0.7629 | 1.91 × 106 | - | - | - | |
| 50 h | EP0 | 2000 | - | - | - | - | - | - | 1.15 × 10−4 | 0.6254 | 3.72 × 105 |
| EP10-I | 1000 | 3.97 × 10−7 | 0.4394 | 22,372 | 4.33 × 10−8 | 0.7477 | 6.26 × 106 | - | - | - | |
| EP10-D | 2000 | 4.42 × 10−9 | 0.7119 | 12,252 | 9.04 × 10−8 | 0.7187 | 2.94 × 106 | - | - | - | |
| 100 h | EP0 | 2000 | - | - | - | - | - | - | 2.13 × 10−4 | 0.6092 | 1.52 × 105 |
| EP10-I | 1000 | 1.03 × 10−8 | 0.7494 | 214,474 | 4.90 × 10−7 | 0.4179 | 5.04 × 106 | - | - | - | |
| EP10-D | 2000 | 1.09 × 10−7 | 0.6553 | 638,130 | 1.27 × 10−6 | 0.5488 | 1.85 × 106 | - | - | - | |
| 200 h | EP0 | 2000 | 5.25 × 10−5 | 0.4317 | 1961 | - | - | - | 1.70 × 10−4 | 0.5719 | 1.54 × 108 |
| EP10-I | 1000 | 2.41 × 10−7 | 0.4583 | 35,667 | 1.01 × 10−6 | 0.5509 | 1.65 × 106 | - | - | - | |
| EP10-D | 2000 | 3.01 × 10−7 | 0.3935 | 927,800 | 3.95 × 10−7 | 0.7629 | 2.12 × 106 | - | - | - | |
| 400 h | EP0 | 2000 | 1.82 × 10−5 | 0.5012 | 3521 | - | - | - | 2.52 × 10−4 | 0.5039 | 7.35 × 104 |
| EP10-I | 1000 | 6.37 × 10−8 | 0.5885 | 129,935 | 1.46 × 10−6 | 0.4360 | 1.25 × 106 | - | - | - | |
| EP10-D | 2000 | 3.76 × 10−10 | 0.8849 | 33,789 | 1.92 × 10−7 | 0.6114 | 1.30 × 106 | - | - | - | |
| 500 h | EP0 | 2000 | 1.30 × 10−5 | 0.5380 | 4306 | - | - | - | 2.57 × 10−4 | 0.5541 | 1.10 × 105 |
| EP10-I | 1000 | 2.72 × 10−8 | 0.6472 | 169,330 | 1.97 × 10−6 | 0.5046 | 2.22 × 106 | - | - | - | |
| EP10-D | 2000 | 1.45 × 10−9 | 0.7808 | 56,978 | 1.84 × 10−7 | 0.6085 | 1.69 × 106 | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, L.H.d.O.; Fedel, M.; Cotting, F.; Campos, W.R.d.C. In Situ Evaluation of Epoxy Self-Healing Coating by Encapsulated Linseed Oil in Poly(Urea–Formaldehyde–Melamine) Microcapsules. Materials 2025, 18, 1906. https://doi.org/10.3390/ma18091906
Souza LHdO, Fedel M, Cotting F, Campos WRdC. In Situ Evaluation of Epoxy Self-Healing Coating by Encapsulated Linseed Oil in Poly(Urea–Formaldehyde–Melamine) Microcapsules. Materials. 2025; 18(9):1906. https://doi.org/10.3390/ma18091906
Chicago/Turabian StyleSouza, Lucas Henrique de Oliveira, Michele Fedel, Fernando Cotting, and Wagner Reis da Costa Campos. 2025. "In Situ Evaluation of Epoxy Self-Healing Coating by Encapsulated Linseed Oil in Poly(Urea–Formaldehyde–Melamine) Microcapsules" Materials 18, no. 9: 1906. https://doi.org/10.3390/ma18091906
APA StyleSouza, L. H. d. O., Fedel, M., Cotting, F., & Campos, W. R. d. C. (2025). In Situ Evaluation of Epoxy Self-Healing Coating by Encapsulated Linseed Oil in Poly(Urea–Formaldehyde–Melamine) Microcapsules. Materials, 18(9), 1906. https://doi.org/10.3390/ma18091906







