Optical and Dielectric Properties of BaF2:(Er,Yb) Co-Doped Crystal
Abstract
:1. Introduction
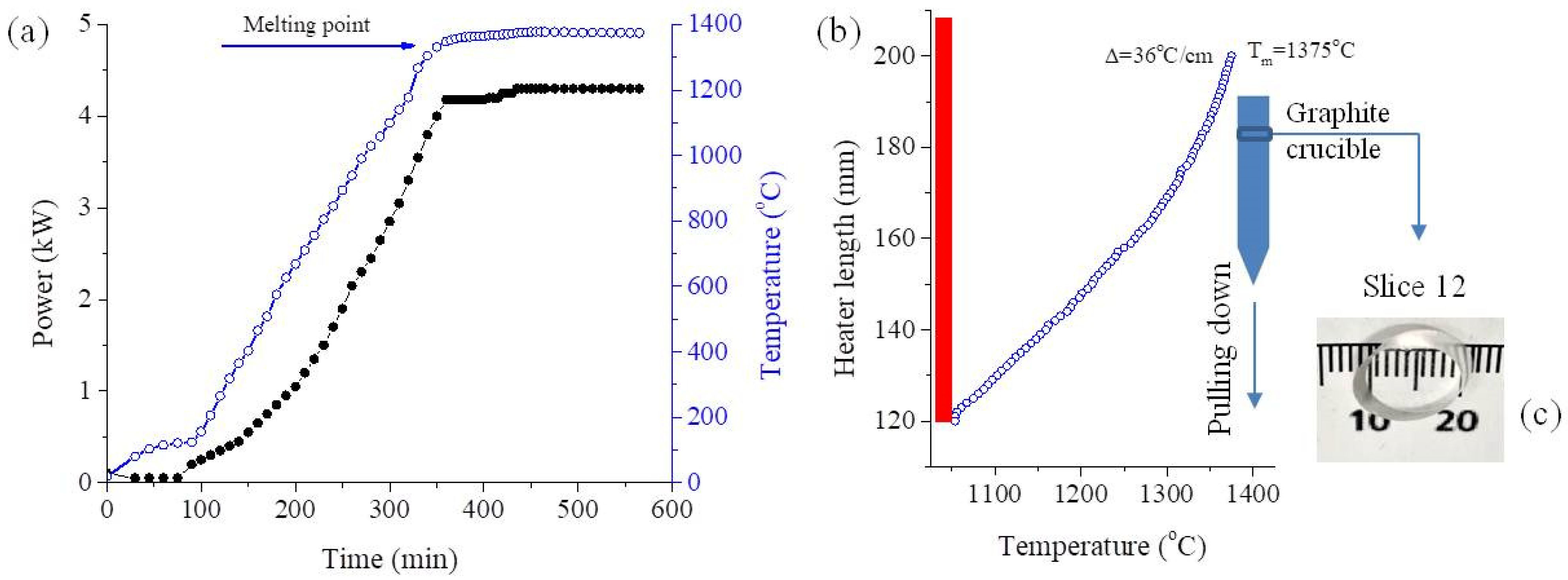
2. Materials and Methods
3. Experimental Results and Discussion
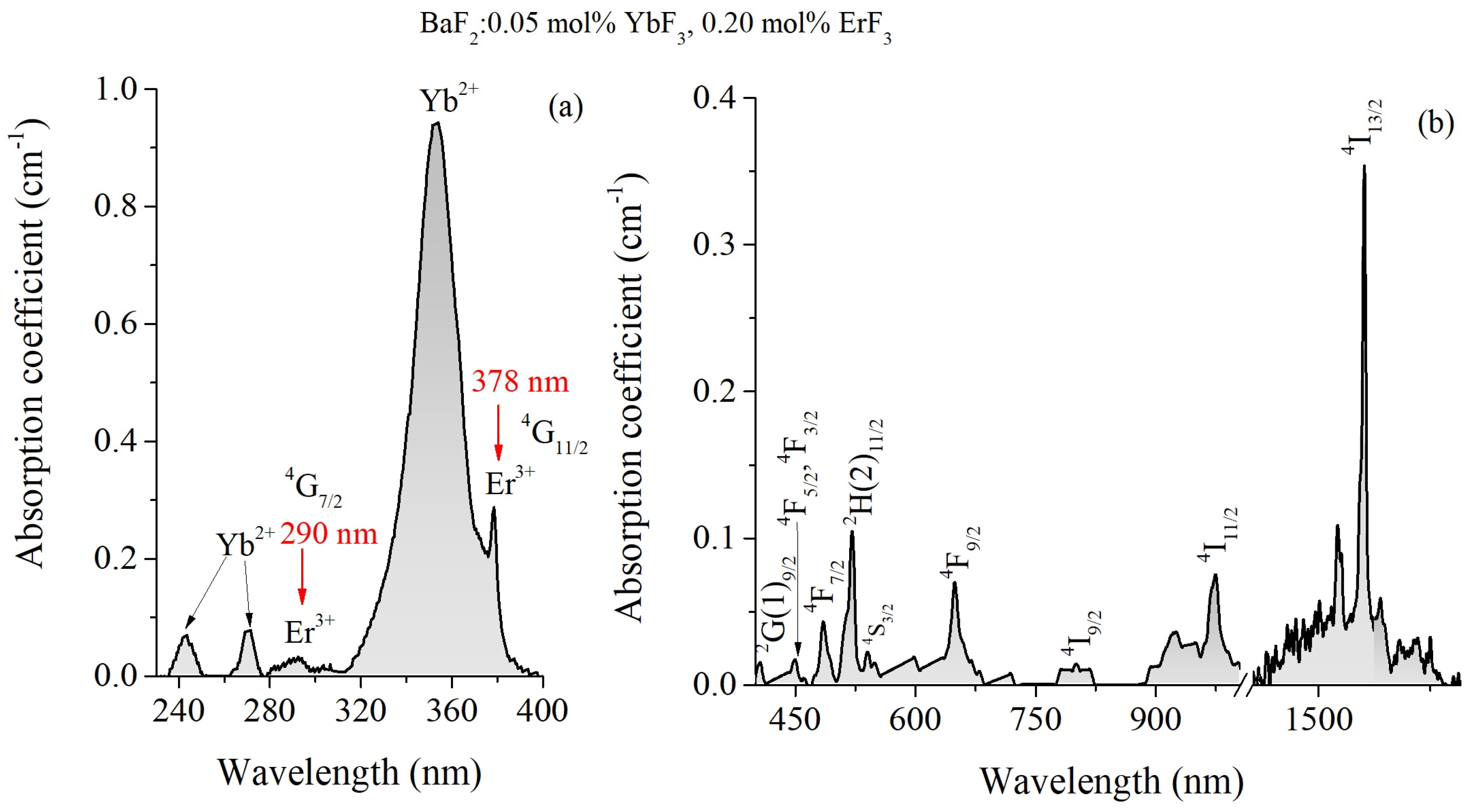
3.1. Optical Absorption Spectra
3.2. Judd–Ofelt Analysis
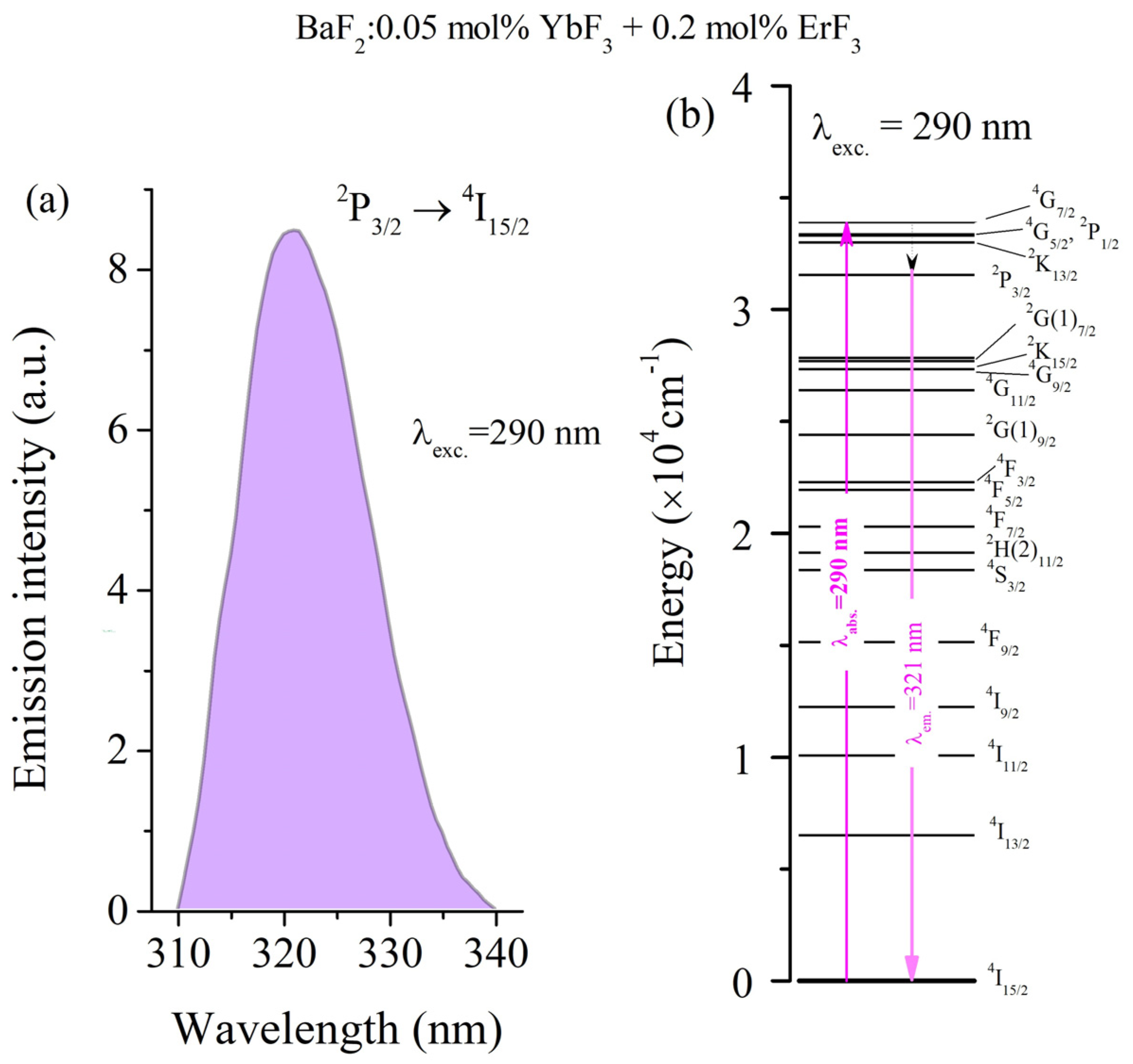
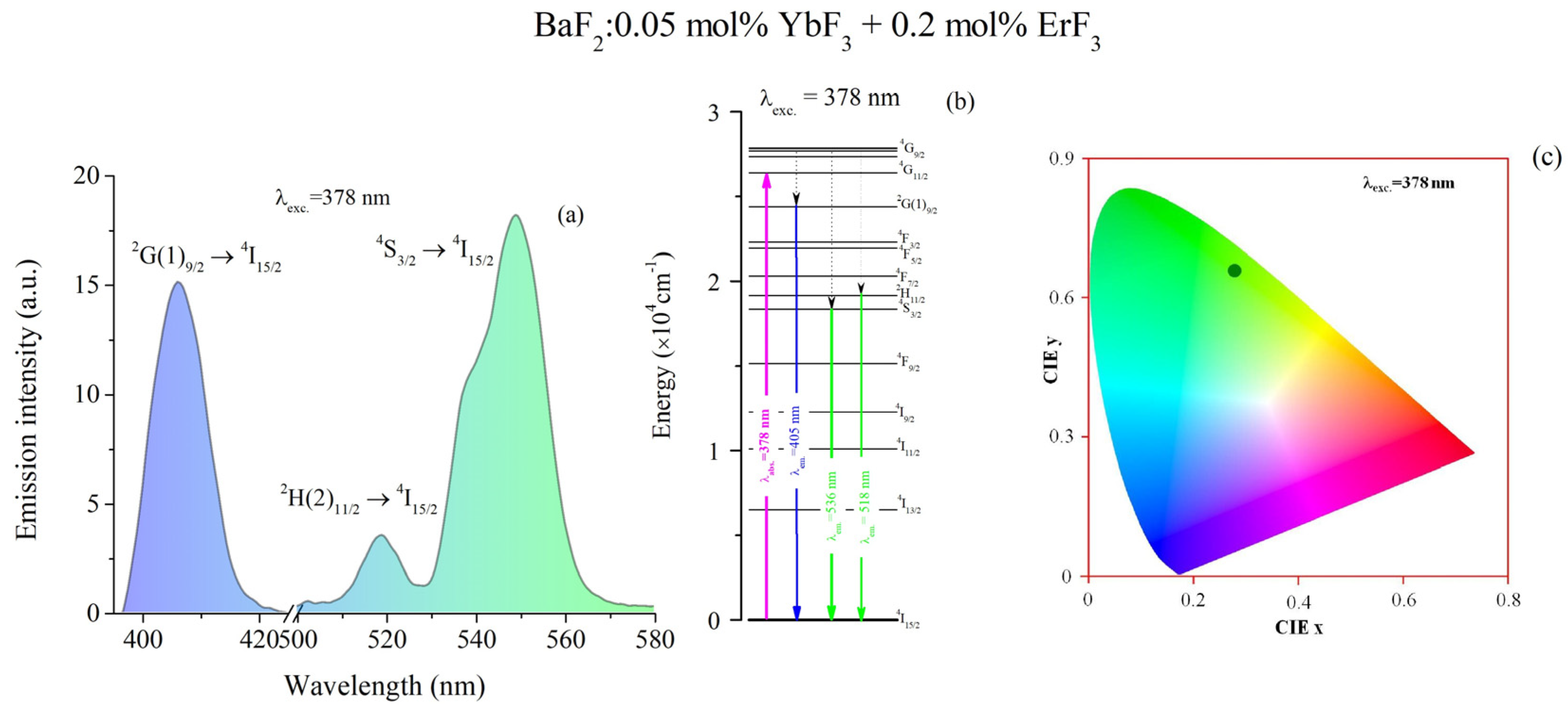
3.3. Emission Spectra
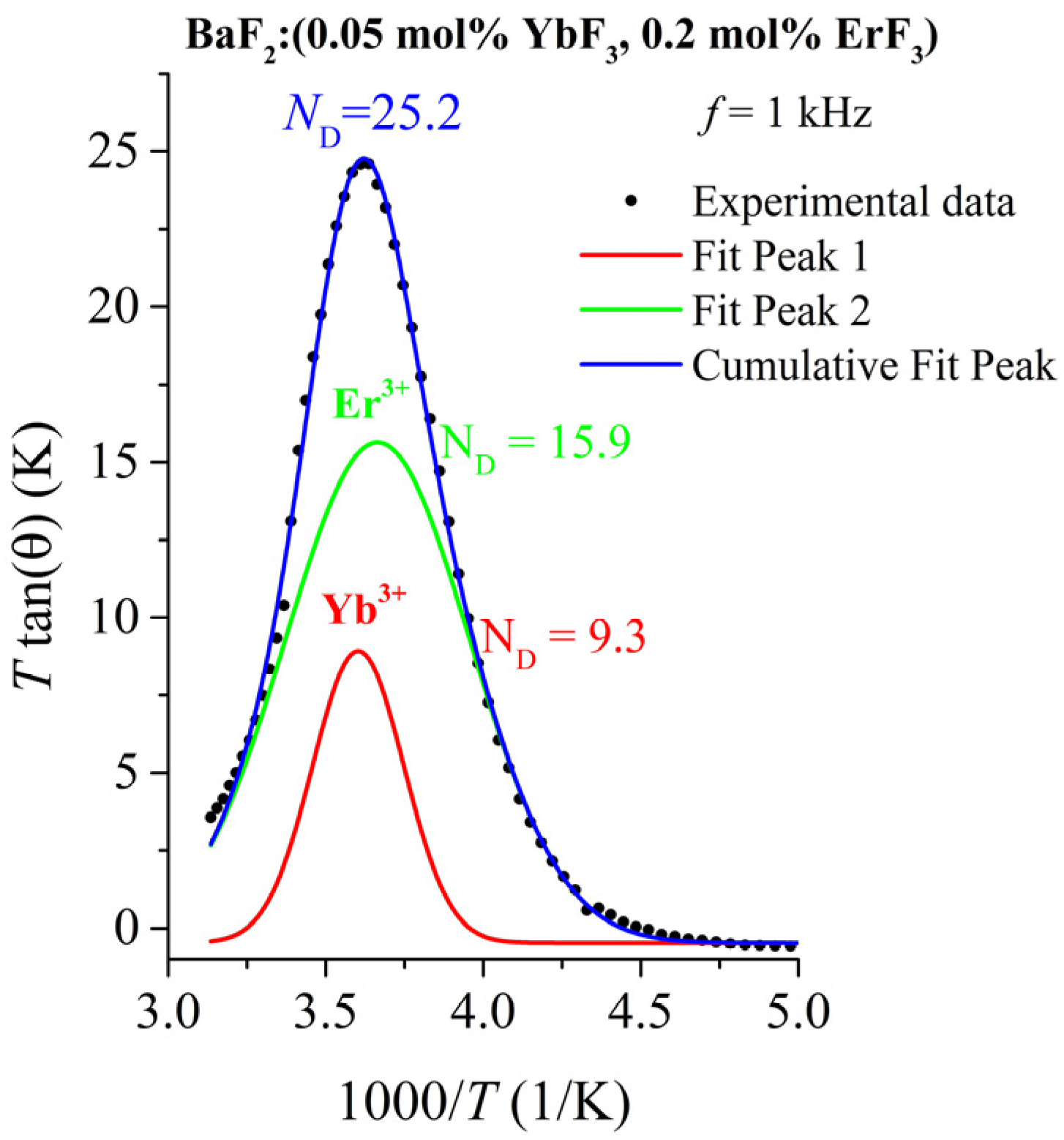
3.4. Dielectric Relaxation
| Sample | E (eV) | τ0 (10−15 s) | ND (1017 cm−3) |
|---|---|---|---|
| BaF2:0.05 mol% YbF3, 0.2 mol% ErF3 | 0.58 | 4.1 | 25.2 This work |
| BaF2:0.2 mol% ErF3 | 0.56 | 6.7 | 22.6 [26] |
| BaF2:0.05 mol% YbF3 | 0.53 | 16 | 20.95 [27] |
| BaF2:0.05 mol% GdF3 | 0.53–0.61 | 7 [47] | - |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, E.E.; Fleischman, Z.D.; McKay, J.; Dubinskii, M. Spectroscopic characterization of low-phonon Er-doped BaF2 single crystal for mid-IR lasers. Opt. Mater. Express 2021, 11, 575–584. [Google Scholar] [CrossRef]
- Tolstik, N.A.; Kurilchik, S.V.; Kisel, V.E.; Kuleshov, N.V.; Maltsev, V.V.; Pilipenko, O.V.; Koporulina, E.V.; Leonyuk, N. Efficient 1 W continuous diode-pumped Er,Yb:YAl3(BO3)4. Opt. Lett. 2007, 32, 3233–3235. [Google Scholar] [CrossRef] [PubMed]
- Racu, A.V.; Ristic, Z.; Ciric, A.; Dordevic, V.; Buse, G.; Poienar, M.; Guttmann, M.J.; Ivashko, O.; Stef, M.; Vizman, D.; et al. Analysis of site symmetries of Er3⁺ doped CaF2 and BaF2 crystals by high resolution photoluminescence spectroscopy. Opt. Mater. 2023, 136, 113337. [Google Scholar] [CrossRef]
- Kaminskii, A.A. Laser Crystals: Their Physics and Properties; Springer-Verlag: Berlin/Heidelberg, Germany, 1990. [Google Scholar]
- Perera, S.S.; Dissanayake, K.T.; Rabuffetti, F.A. Alkaline-earth fluorohalide nanocrystals for upconversion thermometry. J. Lumin. 2019, 207, 416–423. [Google Scholar] [CrossRef]
- Li, N.; Shao, H.; Qi, H.; Sheng, Y.; Yang, L.; Xie, Y.; Li, D.; Yu, W.; Ma, Q.; Dong, X. A strategy towards MF2:Yb3⁺,Er3⁺/SiO2 (M=Ba, Sr, Ca) yolk-shell nanofibers and yolk-shell nanobelts with up-conversion fluorescence. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129338. [Google Scholar] [CrossRef]
- Duhanapala, B.D.; Munasinghe, H.N.; Rabuffetti, F.A. Temperature-dependent luminescence of CaFCl:Yb,Er upconverting nanocrystals. J. Lumin. 2021, 235, 117974. [Google Scholar] [CrossRef]
- Liu, M.-H.; Li, T.-T.; Wang, X.; Liu, D.-Y.; Yuan, N.; Zhang, D.-L. Synthesis and Er3⁺ spectroscopic property of Er3⁺/Yb3⁺-codoped CaIn2O4 nanofibers for thermometry. J. Lumin. 2020, 215, 116703. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Li, G.; Yang, G.; Zhang, X.; Bai, Z.; Wang, X. Synthetic process and luminescence properties of Er3⁺, Yb3⁺ doping fluoride-based up-conversion phosphors. J. Mater. Sci. Mater. Electron. 2020, 31, 6217–6225. [Google Scholar] [CrossRef]
- Pisarski, W.A.; Janek, J.; Pisarska, J.; Lisiecki, R.; Ryba-Romanowski, W. Influence of temperature on up-conversion luminescence in Er3⁺/Yb3⁺ doubly doped lead-free fluorogermanate glasses for optical sensing. Sens. Actuators B Chem. 2017, 253, 85–92. [Google Scholar] [CrossRef]
- Bitam, A.; Fartas, R.; Diaf, M.; Boubekri, H.; Cheddadi, A.; Martin, I.R. Investigation of Er3+-Doped BaF2 Single Crystals for Infrared Emission and Photovoltaic Efficiency Enhancement. Luminescence 2025, 40, e70102. [Google Scholar] [CrossRef]
- Stef, M.; Nicoara, I.; Racu, A.; Buse, G.; Vizman, D. Spectroscopic properties of the gamma irradiated ErF3-doped BaF2 crystals. Radiat. Phys. Chem. 2020, 176, 109024. [Google Scholar] [CrossRef]
- Racu, A.; Stef, M.; Buse, G.; Nicoara, I.; Vizman, D. Luminescence properties and Judd–Ofelt analysis of various ErF3 concentration-doped BaF2 crystals. Materials 2021, 14, 4221. [Google Scholar] [CrossRef]
- Han, X.; Wang, G.; Tsuboi, T. Growth and spectral properties of Er3+/Yb3+-codoped KY(WO4)2 crystal. J. Cryst. Growth 2002, 242, 412. [Google Scholar] [CrossRef]
- Cheng, Z.X.; Zhang, S.J.; Song, F.; Guo, H.C.; Han, J.R.; Chen, H.C. Optical spectroscopy of Yb/Er codoped NaY(WO4)2 crystal. J. Phys. Chem. Solids 2002, 63, 2011. [Google Scholar] [CrossRef]
- Sardar, D.K.; Gruber, J.B.; Zandi, B.; Hutchinson, J.A.; Trussell, C.W. Judd-Ofelt analysis of the Er3+ (4f11) absorption intensities in phosphate glass:Er3+,Yb3+. J. Appl. Phys. 2003, 93, 2041. [Google Scholar] [CrossRef]
- Denker, B.; Galagan, B.; Ivleva, L.; Osiko, V.; Sverchkov, S.; Voronina, I.; Hellstrom, J.E.; Karlsson, G.; Laurell, F. Luminescent properties of Yb-Er:GdCa4O(BO3)3: A new crystal for eye-safe 1.5-μm lasers. Appl. Phys. B 2004, 79, 577. [Google Scholar] [CrossRef]
- Kaminskii, A.A. Laser crystals and ceramics: Recent advances. Laser Photonics Rev. 2007, 1, 93. [Google Scholar] [CrossRef]
- Buse, G.; Preda, E.; Stef, M.; Nicoara, I. Influence of Yb3+ ions on the optical properties of double-doped Er,Yb:CaF2 crystals. Phys. Scr. 2011, 83, 025604. [Google Scholar] [CrossRef]
- Chen, D.; Yu, Y.; Huang, F.; Huang, P.; Yang, A.; Wang, Z.; Wang, Y. Monodisperse upconversion Er3+/Yb3+:MFCl (M = Ca, Sr, Ba) nanocrystals synthesized via a seed-based chlorination route. Chem. Commun. 2011, 47, 11083. [Google Scholar] [CrossRef]
- Xiao, B.; Lin, Z.; Zhang, K.; Huang, Y.; Wang, G. Growth, thermal and spectral properties of Er3+-doped and Er3+/Yb3+-codoped Li3Ba2La3(WO4)8 crystals. PLoS ONE 2012, 7, e40631. [Google Scholar] [CrossRef]
- Kong, Q.; Wang, J.; Dong, X.; Yu, W.; Liu, G. Synthesis and luminescence properties of Yb3+-Er3+ co-doped LaOCl nanostructures. J. Mater. Sci. 2014, 49, 2919. [Google Scholar] [CrossRef]
- Li, N.; Shao, H.; Qi, H.; Yang, L.; Sheng, Y.; Xie, Y.; Li, D.; Yu, W.; Ma, Q.; Dong, X. A neoteric approach to achieve BaF2:Yb3+, Er3+ one-dimensional nanostructures with color-tuned up-conversion luminescence. Ceram. Int. 2022, 48, 35422. [Google Scholar] [CrossRef]
- Balabhadra, S.; Reid, M.; Golovko, V.; Wells, J.-P.R. Absorption spectra, defect site distribution and upconversion excitation spectra of CaF2/SrF2/BaF2:Yb3+:Er3+ nanoparticles. J. Alloys Compd. 2020, 837, 155165. [Google Scholar] [CrossRef]
- Madirov, E.I.; Konyushkin, V.A.; Nakladov, A.N.; Fedorov, P.P.; Bergfeldt, T.; Busko, D.; Howard, I.A.; Richards, B.S.; Kuznetsov, S.V.; Turshatov, A. Upconversion luminescence in Er3+-doped BaF2 nanoceramics. J. Mater. Chem. C 2021, 9, 3493. [Google Scholar] [CrossRef]
- Nicoara, I.; Stef, M.; Buse, G.; Racu, A. Growth and characterization of ErF3 doped BaF2 crystals. J. Cryst. Growth 2020, 547, 125817. [Google Scholar] [CrossRef]
- Nicoara, I.; Stef, M. Charge Compensating Defects Study of YbF3-Doped BaF2 Crystals Using Dielectric Loss. Phys. Status Solidi B 2016, 253, 397–403. [Google Scholar] [CrossRef]
- Bitam, A.; Khiari, S.; Diaf, M.; Boubekri, H.; Boluma, E.; Bensalem, C.; Guerbous, L.; Jouart, J.P. Spectroscopic Investigation of Er3⁺ Doped BaF2 Crystal. Opt. Mater. 2018, 82, 104–110. [Google Scholar] [CrossRef]
- Labbe, C.; Doualan, J.L.; Moncorgé, R.; Braud, A.; Camy, P. Excited-State Absorption and Fluorescence Dynamics in Er:CaF2. J. Lumin. 2018, 200, 74–80. [Google Scholar] [CrossRef]
- Patel, D.N.; Reddy, R.B.; Stevenson, K.N. Diode-Pumped Violet Energy Upconversion in BaF2:Er3+. Appl. Opt. 1998, 37, 7805–7810. [Google Scholar] [CrossRef]
- Liu, H.-W.; Sun, J.-L.; Wang, Y.-F.; Ma, X.-Y.; Li, B.; Bai, Z.-H.; Zhang, X.-Y. Dual Mode Conversion Luminescent Properties of PbF2:Er3⁺,Yb3⁺ Phosphors. Funct. Mater. Lett. 2019, 12, 1950033. [Google Scholar] [CrossRef]
- Qiu, J.; Jiao, Q.; Zhou, D.; Yang, Z. Recent Progress on Upconversion Luminescence Enhancement in Rare-Earth Doped Transparent Glass-Ceramics. J. Rare Earths 2016, 34, 341–356. [Google Scholar] [CrossRef]
- Kaczmarek, A.M.; Kaczmarek, M.K.; Deun, R. Er3⁺-to-Yb3⁺ and Pr3⁺-to-Yb3⁺ Energy Transfer for Highly Efficient Near-Infrared Cryogenic Optical Temperature Sensing. Nanoscale 2019, 11, 833–842. [Google Scholar] [CrossRef]
- Kasprowicz, D.; Jaroszewski, K.; Gluchowski, P.; Michalski, E.; Majchrowski, A. Efficient Yb3⁺ Sensitized Er3⁺ Emission of Bi2ZnOB2O₆:Yb3⁺/Er3⁺ Single Crystal. J. Alloys Compd. 2021, 873, 159772. [Google Scholar] [CrossRef]
- Nicoara, D.; Nicoara, I. An Improved Bridgman-Stockbarger Crystal-Growth System. Mater. Sci. Eng. A 1988, 102, L1–L4. [Google Scholar] [CrossRef]
- Judd, B.R. Optical Absorption Intensities of Rare-Earth Ions. Phys. Rev. 1962, 127, 750–761. [Google Scholar] [CrossRef]
- Ofelt, G.S. Intensities of Crystal Spectra of Rare-Earth Ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Li, H.H. Refractive Index of Alkaline Earth Halides and Its Wavelength and Temperature Derivatives. J. Phys. Chem. Ref. Data 1980, 9, 161–289. [Google Scholar] [CrossRef]
- Kaminskii, A.A. Crystalline Lasers: Physical Processes and Operating Schemes; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar] [CrossRef]
- Zhmykhov, V.; Dobretsova, E.; Tsvetkov, V.S.; Nikova, M.; Chikulina, I.; Vakalov, D.; Tarala, V.; Pyrkov, Y.; Kuznetsov, S.; Tsvetkov, V. Judd-Ofelt Analysis of High Erbium Content Yttrium-Aluminum and Yttrium-Scandium-Aluminum Garnet Ceramics. Inorganics 2022, 10, 170. [Google Scholar] [CrossRef]
- GoCIE V2; Justin Thomas, K.R.; Department of Chemistry, Indian Institute of Technology Roorkee, India. 2009. Available online: https://faculty.iitr.ac.in/~krjt8fcy/gocie.html (accessed on 14 February 2023).
- Liu, P.; Yao, Z.; Zhou, J.; Yang, Z.; Kong, L.B. Small magnetic Co-doped NZn ferrite/grapheme nanocomposites and their dual-region microwave absorption performance. J. Mater. Chem. C 2016, 4, 9738–9749. [Google Scholar] [CrossRef]
- Liu, F.; Liu, S.; Cui, X.; Cheng, L.; Li, H.; Wang, J.; Rao, W. Ordered domanin and microwave properties of sub-micron structured Ba(Zn1/3Ta2/3)O3 ceramics obtained by spark plasma sintering. Materials 2019, 12, 638. [Google Scholar] [CrossRef]
- Nicoara, I.; Munteanu, M.; Pecingina-Girjoaba, N.; Stef, M.; Lighezan, L. Dielectric spectrum of rare-earth-doped calcium fluoride crystals. J. Crystal Growth 2006, 287, 234–238. [Google Scholar] [CrossRef]
- Andeen, C. Cluster-Associated Relaxations in Rare-Earth-Doped Calcium Fluoride. Phys. Rev. B 1977, 16, 3762–3769. [Google Scholar] [CrossRef]
- Campos, V.B.; Leal Ferreira, G.F. Dipolar studies in CaF2 with Ce3+. J. Phys. Chem. Solids 1974, 35, 905–908. [Google Scholar] [CrossRef]
- Edgar, A.; Welsh, H.K. Dielectric relaxation and EPR studies of Gd3⁺-Fᵢ− dipoles in strontium and barium fluoride. J. Phys. C: Solid State Phys. 1979, 12, 703–711. [Google Scholar] [CrossRef]

| Trasition, 4I15/2 → | λmean (nm) | Wavelength Range (nm) | (λ) (×10−20 cm2·nm) | (×10−20 cm2) | (×10−20 cm2) |
|---|---|---|---|---|---|
| 4I13/2 | 1530 | 1450–1600 | 14.353 | 0.405 | 0.444 |
| 4F9/2 | 648 | 620–695 | 1.576 | 0.309 | 0.330 |
| 2H(2)11/2 | 518 | 502–533 | 2.471 | 0.604 | 0.603 |
| 4F7/2 | 486 | 476–498 | 1.271 | 0.331 | 0.226 |
| Ωt (10−20 cm2) | BaF2: 0.2 mol% ErF3 + 0.05 mol% YbF3 (This Paper) | BaF2: 0.15 mol% ErF3 [13] | BaF2: 0.2 mol% ErF3 [12] | BaF2:Er3+ [1] | BaF2: 1.96 mol% ErF3, 2.91 mol% YbF3 [25] | YSAG: Er3+ [40] |
|---|---|---|---|---|---|---|
| Ω2 | 0.58983 ± 0.08026 | 0.6749 | 0.932 | 1.98 | 0.95 | 0.4681 |
| Ω4 | 0.38250 ± 0.05751 | 0.1118 | 0.153 | 1.18 | 0.49 | 0.8378 |
| Ω6 | 0.27115 ± 0.12981 | 0.5525 | 1.074 | 1.20 | 1.45 | 0.6741 |
| χ = Ω4/Ω6 | 1.41 | 0.20 | 0.14 | 0.98 | 0.34 | 1.24 |
| Transition | λem. [nm] | AJJ′ (s−1) | β | τrad (ms) This Work | τexp (ms) |
|---|---|---|---|---|---|
| 4S3/2→4I15/2 | 536 | 191.8 | 0.66 | 3.4 | 1.9 [13] |
| 0.96 [12] | |||||
| 0.38 [19] | |||||
| 0.56 [28] | |||||
| 2H(2)11/2→4I15/2 | 518 | 733.4 | 0.88 | 1.9 | 1.351 [13] |
| 0.96 [12] | |||||
| 0.39 [19] | |||||
| 2G(1)9/2→4I15/2 | 405 | 209.1 | 0.42 | 2.0 | - |
| 2P3/2→4I15/2 | 321 | 79.2 | 0.09 | 1.1 | 0.906 [13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stef, M.; Schornig, C.; Buse, G. Optical and Dielectric Properties of BaF2:(Er,Yb) Co-Doped Crystal. Materials 2025, 18, 1915. https://doi.org/10.3390/ma18091915
Stef M, Schornig C, Buse G. Optical and Dielectric Properties of BaF2:(Er,Yb) Co-Doped Crystal. Materials. 2025; 18(9):1915. https://doi.org/10.3390/ma18091915
Chicago/Turabian StyleStef, Marius, Carla Schornig, and Gabriel Buse. 2025. "Optical and Dielectric Properties of BaF2:(Er,Yb) Co-Doped Crystal" Materials 18, no. 9: 1915. https://doi.org/10.3390/ma18091915
APA StyleStef, M., Schornig, C., & Buse, G. (2025). Optical and Dielectric Properties of BaF2:(Er,Yb) Co-Doped Crystal. Materials, 18(9), 1915. https://doi.org/10.3390/ma18091915








