Cationic Surfactant-Driven Evolution of NiFe2O4 Nanosheets for High-Performance Asymmetric Supercapacitors
Abstract
1. Introduction
2. Experimental Section
Hydrothermal Synthesis of CTAB-Assisted NiFe Electrodes
3. Sample Characterization and Electrochemical Measurements
4. Results and Discussions
4.1. XRD Elucidation
4.2. XPS Analysis
4.3. Morphological and Elemental Composition
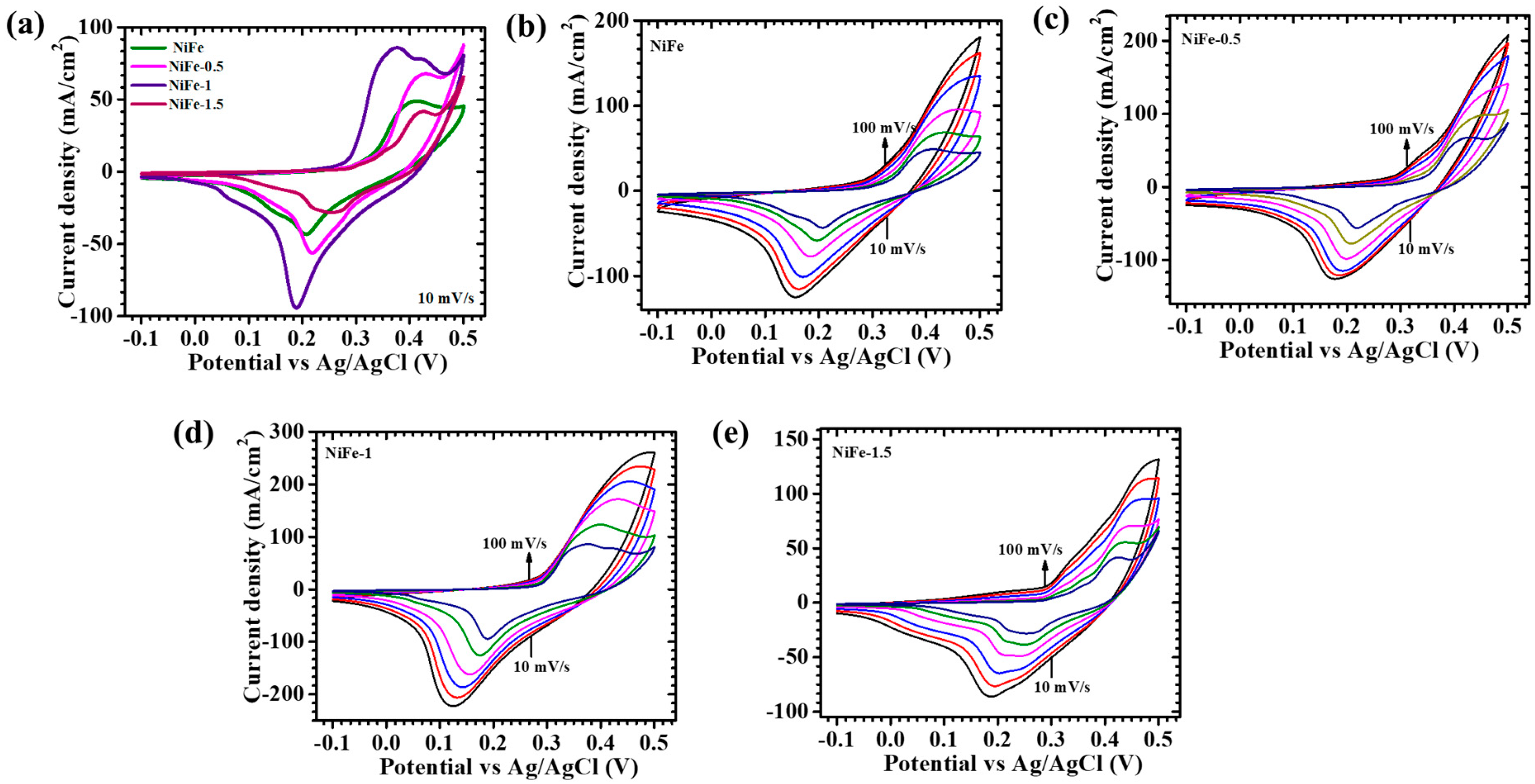
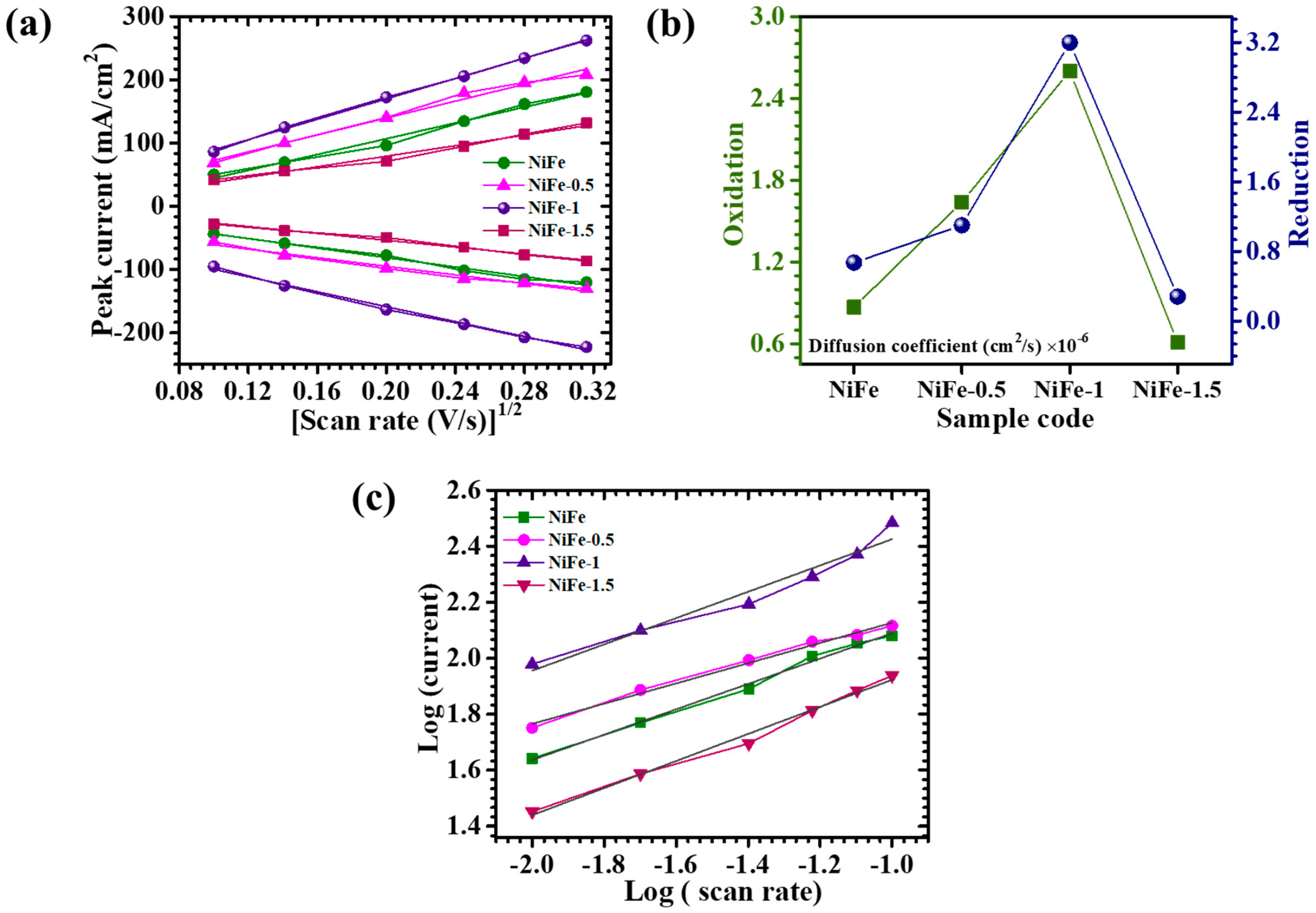
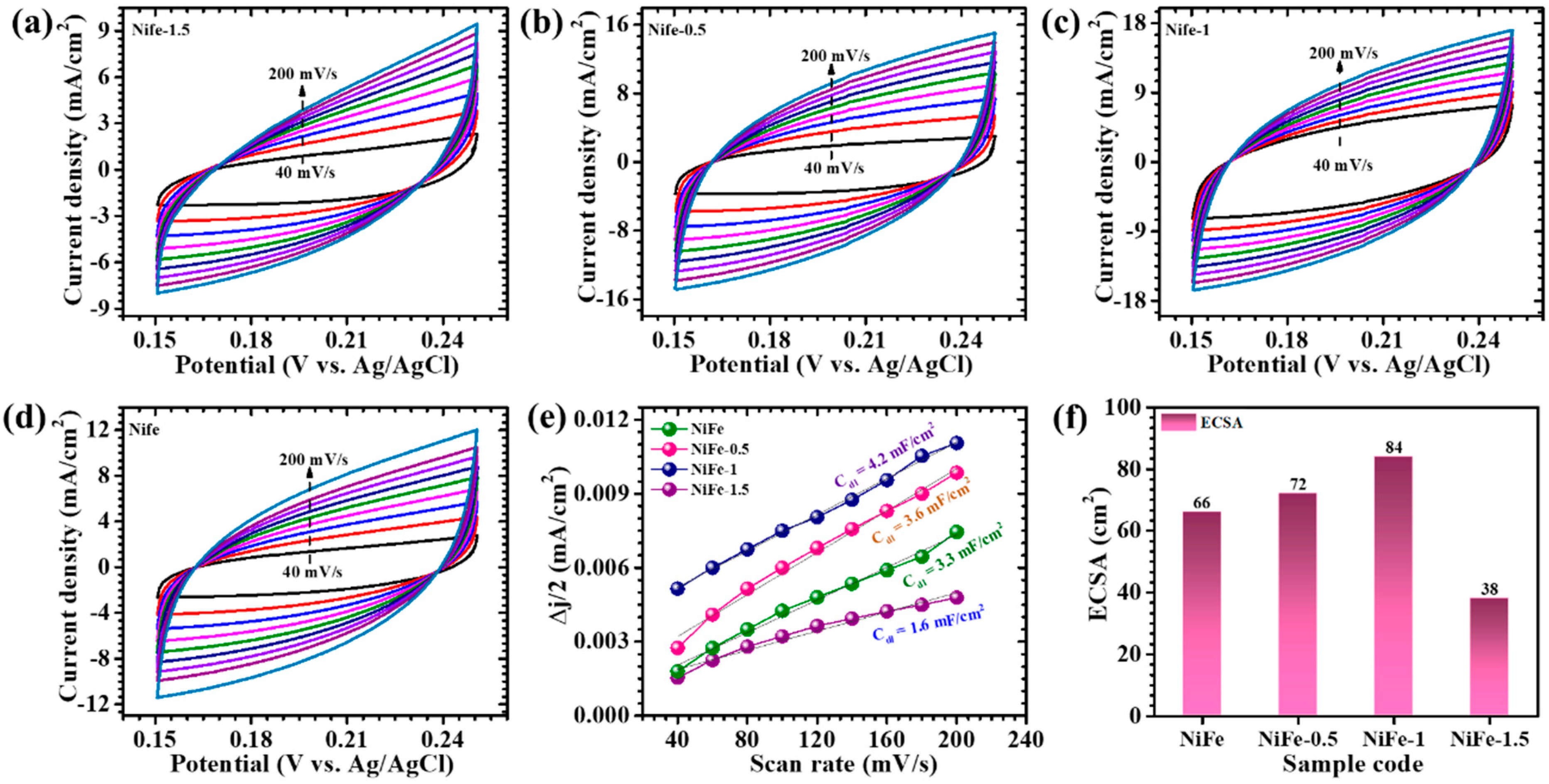
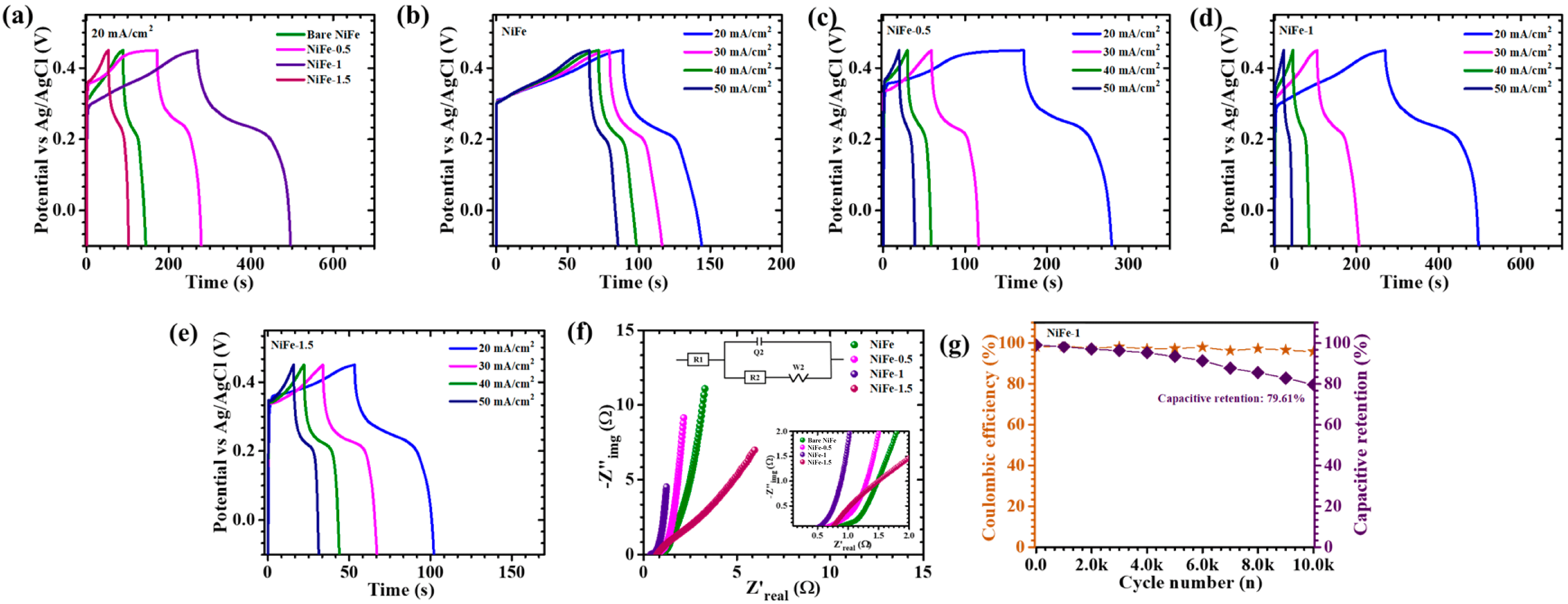
5. Electrochemical Analysis
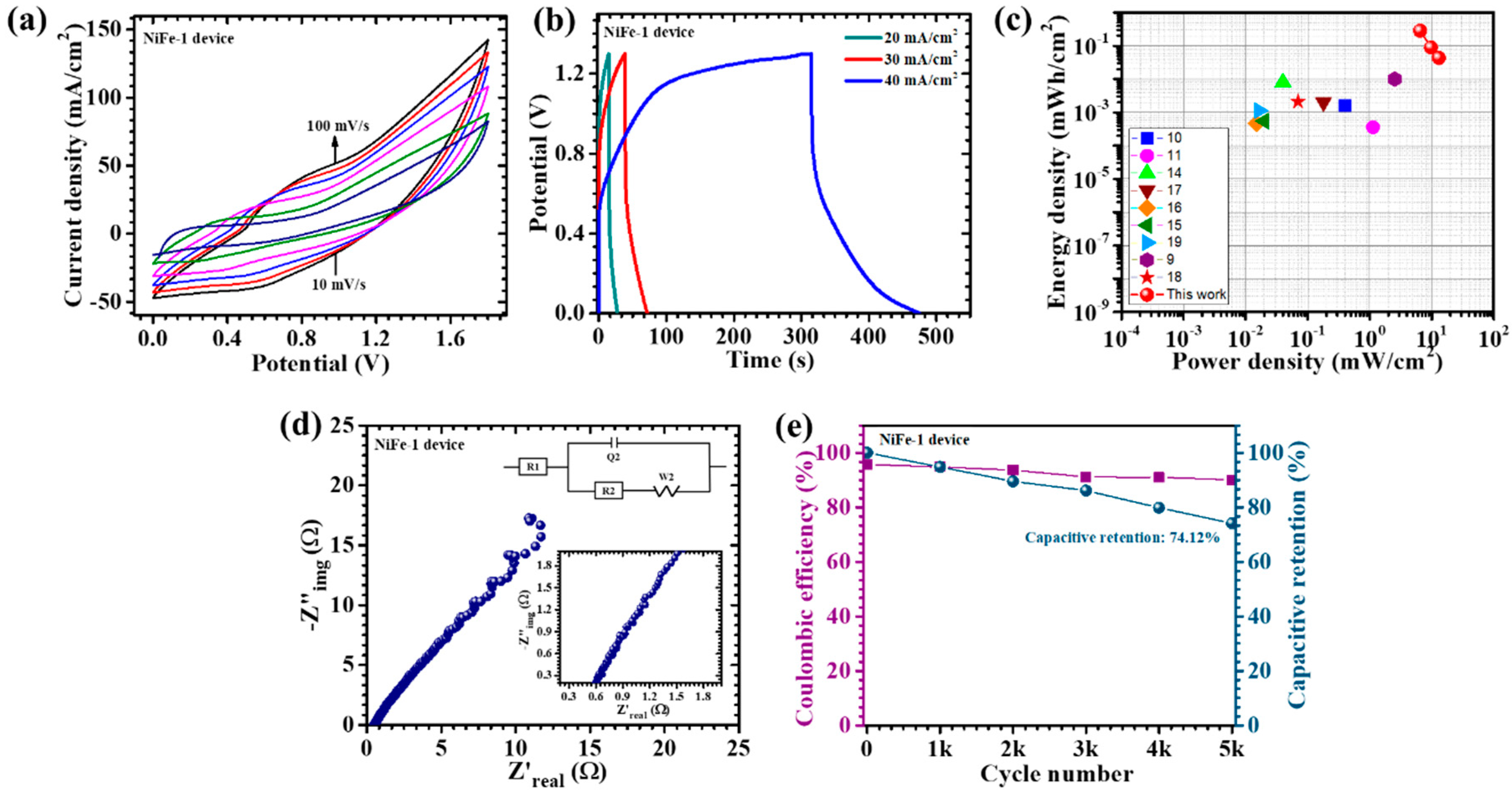
6. Electrochemical Performance of Asymmetric Supercapacitor Device
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, J.; Wang, Q.; Wei, T.; Fan, Z. Recent advances in design and fabrication of electrochemical supercapacitors with high energy densities. Adv. Energy Mater. 2014, 4, 1300816. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Schmidt, O.G.; Yan, C. Engineered nanomembranes for smart energy storage devices. Chem. Soc. Rev. 2016, 45, 1308–1330. [Google Scholar] [CrossRef] [PubMed]
- Mary, A.J.C.; Bose, A.C. Surfactant assisted ZnCo2O4 nanomaterial for supercapacitor application. Appl. Surf. Sci. 2018, 449, 105–112. [Google Scholar] [CrossRef]
- Haldorai, Y.; Huh, Y.S.; Han, Y.-K. Surfactant-assisted hydrothermal synthesis of flower-like tin oxide/graphene composites for high-performance supercapacitors. New J. Chem. 2015, 39, 8505–8512. [Google Scholar] [CrossRef]
- Ahir, N.A.; Takaloo, A.V.; Nirmal, K.A.; Kundale, S.S.; Chougale, M.Y.; Bae, J.; Kim, D.-K.; Dongale, T.D. Capacitive coupled non-zero I–V and type-II memristive properties of the NiFe2O4–TiO2 nanocomposite. Mater. Sci. Semicond. Process. 2021, 125, 105646. [Google Scholar] [CrossRef]
- Toghan, A.; Khairy, M.; Kamar, E.; Mousa, M. Effect of particle size and morphological structure on the physical properties of NiFe2O4 for supercapacitor application. J. Mater. Res. Technol. 2022, 19, 3521–3535. [Google Scholar] [CrossRef]
- Samuel, E.; Aldalbahi, A.; El-Newehy, M.; El-Hamshary, H.; Yoon, S.S. Nickel ferrite beehive-like nanosheets for binder-free and high-energy-storage supercapacitor electrodes. J. Alloys Compd. 2021, 852, 156929. [Google Scholar] [CrossRef]
- Guo, Z.; Qiao, J.; Liu, X.; Lin, F.; Liu, M.; Fang, M.; Huang, Z.; Zhang, X.; Min, X. Advances in Mineral-Based Composite Phase Change Materials for Energy Storage: A Review. Green Smart Min. Eng. 2024, 1, 447–473. [Google Scholar] [CrossRef]
- Xing, B.; Meng, W.; Liang, H.; Kang, W.; Zeng, H.; Zhang, C.; Egun, I.L.; Li, P.; Cao, Y.; Chen, Z. Flexible Coal-Derived Carbon Fibers via Electrospinning for Self-Standing Lithium-Ion Battery Anodes. Int. J. Min. Sci. Technol. 2024, 34, 1753–1763. [Google Scholar] [CrossRef]
- Bandgar, S.B.; Vadiyar, M.M.; Jambhale, C.L.; Kim, J.-H.; Kolekar, S.S. Superfast ice crystal-assisted synthesis of NiFe2O4 and ZnFe2O4 nanostructures for flexible high-energy density asymmetric supercapacitors. J. Alloys Compd. 2021, 853, 157129. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Chen, L.-F.; Yu, S.-H. Growth of NiFe2O4 nanoparticles on carbon cloth for high performance flexible supercapacitors. J. Mater. Chem. A 2014, 2, 10889–10894. [Google Scholar] [CrossRef]
- Patil, V.D.; Patil, D.A.; Jadhav, A.L.; Jadhav, S.L.; Kadam, A.V.; Dandwate, S.R.; Shinde, B.R. Spinel nickel ferrite (NiFe2O4) materials synthesized via spray-pyrolysis for electrochemical supercapacitor application. Discov. Electrochem. 2024, 1, 2. [Google Scholar] [CrossRef]
- Bandgar, S.B.; Vadiyar, M.M.; Ling, Y.-C.; Chang, J.-Y.; Han, S.-H.; Ghule, A.V.; Kolekar, S.S. Metal precursor dependent synthesis of NiFe2O4 thin films for high-performance flexible symmetric supercapacitor. ACS Appl. Energy Mater. 2018, 1, 638–648. [Google Scholar] [CrossRef]
- Arun, T.; Kavinkumar, T.; Udayabhaskar, R.; Kiruthiga, R.; Morel, M.J.; Aepuru, R.; Dineshbabu, N.; Ravichandran, K.; Akbari-Fakhrabadi, A.; Mangalaraja, R. NiFe2O4 nanospheres with size-tunable magnetic and electrochemical properties for superior supercapacitor electrode performance. Electrochim. Acta 2021, 399, 139346. [Google Scholar] [CrossRef]
- Gao, X.; Wang, W.; Bi, J.; Chen, Y.; Hao, X.; Sun, X.; Zhang, J. Morphology-controllable preparation of NiFe2O4 as high performance electrode material for supercapacitor. Electrochim. Acta 2019, 296, 181–189. [Google Scholar] [CrossRef]
- Mousa, M.; Khairy, M.; Shehab, M. Nanostructured ferrite/graphene/polyaniline using for supercapacitor to enhance the capacitive behavior. J. Solid State Electrochem. 2017, 21, 995–1005. [Google Scholar] [CrossRef]
- Baig, M.M.; Pervaiz, E.; Azad, M.; Jahan, Z.; Niazi, M.B.K.; Baig, S.M. NiFe2O4/SiO2 nanostructures as a potential electrode material for high rated supercapacitors. Ceram. Int. 2021, 47, 12557–12566. [Google Scholar] [CrossRef]
- Mahala, C.; Sharma, M.D.; Basu, M. 2D nanostructures of CoFe2O4 and NiFe2O4: Efficient oxygen evolution catalyst. Electrochim. Acta 2018, 273, 462–473. [Google Scholar] [CrossRef]
- Sivakumar, P.; Ramesh, R.; Ramanand, A.; Ponnusamy, S.; Muthamizhchelvan, C. Synthesis and characterization of NiFe2O4 nanoparticles and nanorods. J. Alloys Compd. 2013, 563, 6–11. [Google Scholar] [CrossRef]
- Meng, X.; Tang, Z.; Gao, P.; Zhang, Y. Mechanism Analysis of Hydrogen Mineral Phase-Transformed Iron Ore Tailings in Cementitious Materials: A Study on Hydration Kinetics, Mechanical Properties, and Microstructural Characteristics. Constr. Build. Mater. 2025, 475, 141260. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, R.; Wang, S.; Liu, S.; Olet, V.; Gu, B.; Jansz, J.; Xu, Y.; Qing, M.; Liu, L.; et al. Na, Mg, and Ca as Promoters for N2O Adsorption on Char Surface: A Theoretical Study. Chem. Eng. Sci. 2025, 304, 120957. [Google Scholar] [CrossRef]
- Saffari, F.; Kameli, P.; Rahimi, M.; Ahmadvand, H.; Salamati, H. Effects of Co-substitution on the structural and magnetic properties of NiCoxFe2−xO4 ferrite nanoparticles. Ceram. Int. 2015, 41, 7352–7358. [Google Scholar] [CrossRef]
- Bhat, T.S.; Bhogale, S.B.; Patil, S.S.; Pisal, S.H.; Phaltane, S.A.; Patil, P.S. Synthesis and Characterization of Hexagonal Zinc Oxide Nanorods for Eosin-Y Dye Sensitized Solar Cell. Mater. Today Proc. 2021, 43 Pt 4, 2800–2804. [Google Scholar] [CrossRef]
- Hua, M.; Xu, L.; Cui, F.; Lian, J.; Huang, Y.; Bao, J.; Qiu, J.; Xu, Y.; Xu, H.; Zhao, Y. Hexamethylenetetramine-assisted hydrothermal synthesis of octahedral nickel ferrite oxide nanocrystallines with excellent supercapacitive performance. J. Mater. Sci. 2018, 53, 7621–7636. [Google Scholar] [CrossRef]
- Jiang, W.; Dong, L.; Liu, S.; Zhao, S.; Han, K.; Zhang, W.; Pan, K.; Zhang, L. NiFe2O4/ketjen black composites as efficient membrane separators to suppress the shuttle effect for long-life lithium-sulfur batteries. Nanomaterials 2022, 12, 1347. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Yang, S.; Wan, L.; Xiao, F.; Wang, S. Electrodeposition of manganese oxide nanosheets on a continuous three-dimensional nickel porous scaffold for high performance electrochemical capacitors. J. Power Sources 2014, 245, 1027–1034. [Google Scholar] [CrossRef]
- Heinz, H.; Pramanik, C.; Heinz, O.; Ding, Y.; Mishra, R.K.; Marchon, D.; Flatt, R.J.; Estrela-Lopis, I.; Llop, J.; Moya, S. Nanoparticle decoration with surfactants: Molecular interactions, assembly, and applications. Surf. Sci. Rep. 2017, 72, 1–58. [Google Scholar] [CrossRef]
- Yi, G.; Ye, M.; Wu, J.; Wang, Y.; Long, Y.; Fan, G. Facile chemical blowing synthesis of interconnected N-doped carbon nanosheets coupled with Co3O4 nanoparticles as superior peroxymonosulfate activators for p-nitrophenol destruction: Mechanisms and degradation pathways. Appl. Surf. Sci. 2022, 593, 153244. [Google Scholar] [CrossRef]
- Łuczak, J.; Kroczewska, M.; Baluk, M.; Sowik, J.; Mazierski, P.; Zaleska-Medynska, A. Morphology control through the synthesis of metal-organic frameworks. Adv. Colloid Interface Sci. 2023, 314, 102864. [Google Scholar] [CrossRef]
- Yang, Y.; Chao, R.; Feng, W.-X.; Yang, Y.; Wang, J.; Guo, P.-F.; Yang, Q.-N.; Jia, Y.; Li, J.-K.; Wang, G. Hafnium Incorporation Modulating Electronic Structure of NiFe Layered Double Hydroxide for Effective Oxygen Evolution. Chem. Commun. 2025, 61, 5735–5738. [Google Scholar] [CrossRef]
- Hussain, I.; Lamiel, C.; Mohamed, S.G.; Vijayakumar, S.; Ali, A.; Shim, J.-J. Controlled synthesis and growth mechanism of zinc cobalt sulfide rods on Ni-foam for high-performance supercapacitors. J. Ind. Eng. Chem. 2019, 71, 250–259. [Google Scholar] [CrossRef]
- Amate, R.U.; Morankar, P.J.; Teli, A.M.; Beknalkar, S.A.; Chavan, G.T.; Ahir, N.A.; Dalavi, D.S.; Jeon, C.-W. Versatile electrochromic energy storage smart window utilizing surfactant-assisted niobium oxide thin films. Chem. Eng. J. 2024, 484, 149556. [Google Scholar] [CrossRef]
- Amate, R.U.; Morankar, P.J.; Chavan, G.T.; Teli, A.M.; Desai, R.S.; Dalavi, D.S.; Jeon, C.-W. Bi-functional electrochromic supercapacitor based on hydrothermal-grown 3D Nb2O5 nanospheres. Electrochim. Acta 2023, 459, 142522. [Google Scholar] [CrossRef]
- Ahsan, M.T.; Usman, M.; Ali, Z.; Javed, S.; Ali, R.; Farooq, M.U.; Akram, M.A.; Mahmood, A. 3D hierarchically mesoporous zinc-nickel-cobalt ternary oxide (Zn0.6Ni0.8Co1.6O4) nanowires for high-performance asymmetric supercapacitors. Front. Chem. 2020, 8, 487. [Google Scholar] [CrossRef] [PubMed]
- Teli, A.M.; Beknalkar, S.A.; Amte, R.U.; Morankar, P.J.; Yewale, M.A.; Burungale, V.V.; Jeon, C.-W.; Efstathiadis, H.; Shin, J.C. Investigating into the intricacies of charge storage kinetics in NbMn-oxide composite electrodes for asymmetric supercapacitor and HER applications. J. Alloys Compd. 2023, 965, 171305. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Xu, J.; Guo, H.; Hao, Y.; Tian, J.; Liu, Y.; Ren, H.; Yang, W. NiFe2O4 quantum dots anchored on flower-like Ni-MOF with enhanced electrochemical performance for supercapacitors. J. Mater. Chem. C 2023, 11, 15624–15637. [Google Scholar] [CrossRef]
- Xu, J.; Gai, S.; He, F.; Niu, N.; Gao, P.; Chen, Y.; Yang, P. A sandwich-type three-dimensional layered double hydroxide nanosheet array/graphene composite: Fabrication and high supercapacitor performance. J. Mater. Chem. A 2014, 2, 1022–1031. [Google Scholar] [CrossRef]
- Morankar, P.J.; Amate, R.U.; Teli, A.M.; Beknalkar, S.A.; Jeon, C.-W. Synergistic Effects of Niobium Phosphate/Tungsten Oxide Core–Shell Nanocomposites for Asymmetric Supercapacitor. Surf. Interfaces 2025, 56, 105639. [Google Scholar] [CrossRef]
- Amulya, M.S.; Nagaswarupa, H.; Kumar, M.A.; Ravikumar, C.; Kusuma, K.; Prashantha, S. Evaluation of bifunctional applications of CuFe2O4 nanoparticles synthesized by a sonochemical method. J. Phys. Chem. Solids 2021, 148, 109756. [Google Scholar] [CrossRef]
- Dar, M.; Nam, S.; Abdo, H.; Almajid, A.; Kim, D.; Qurashi, A.; Kim, W. Self-assembled Co3O4 nanoplatelets into micro-spheres via a simple solvothermal route: Structural and electrochemical properties. J. Alloys Compd. 2017, 695, 329–336. [Google Scholar] [CrossRef]
- Oliva, G.; Fiorillo, A.S.; Laganà, F.; Shuvo, M.M.H.; Islam, S.K.; Pullano, S.A. Development of a Sensor Based on a Thin Layer of Zeolite to Monitor Plant Health Through VOC Analysis. In Sensors and Microsystems (AISEM 2024); Lecture Notes in Electrical Engineering; Springer: Cham, Switzerland, 2025; Volume 1334, pp. 76–80. [Google Scholar]
- Liang, Q.; Liu, X.; Zeng, G.; Liu, Z.; Tang, L.; Shao, B.; Zeng, Z.; Zhang, W.; Liu, Y.; Cheng, M. Surfactant-assisted synthesis of photocatalysts: Mechanism, synthesis, recent advances and environmental application. Chem. Eng. J. 2019, 372, 429–451. [Google Scholar] [CrossRef]

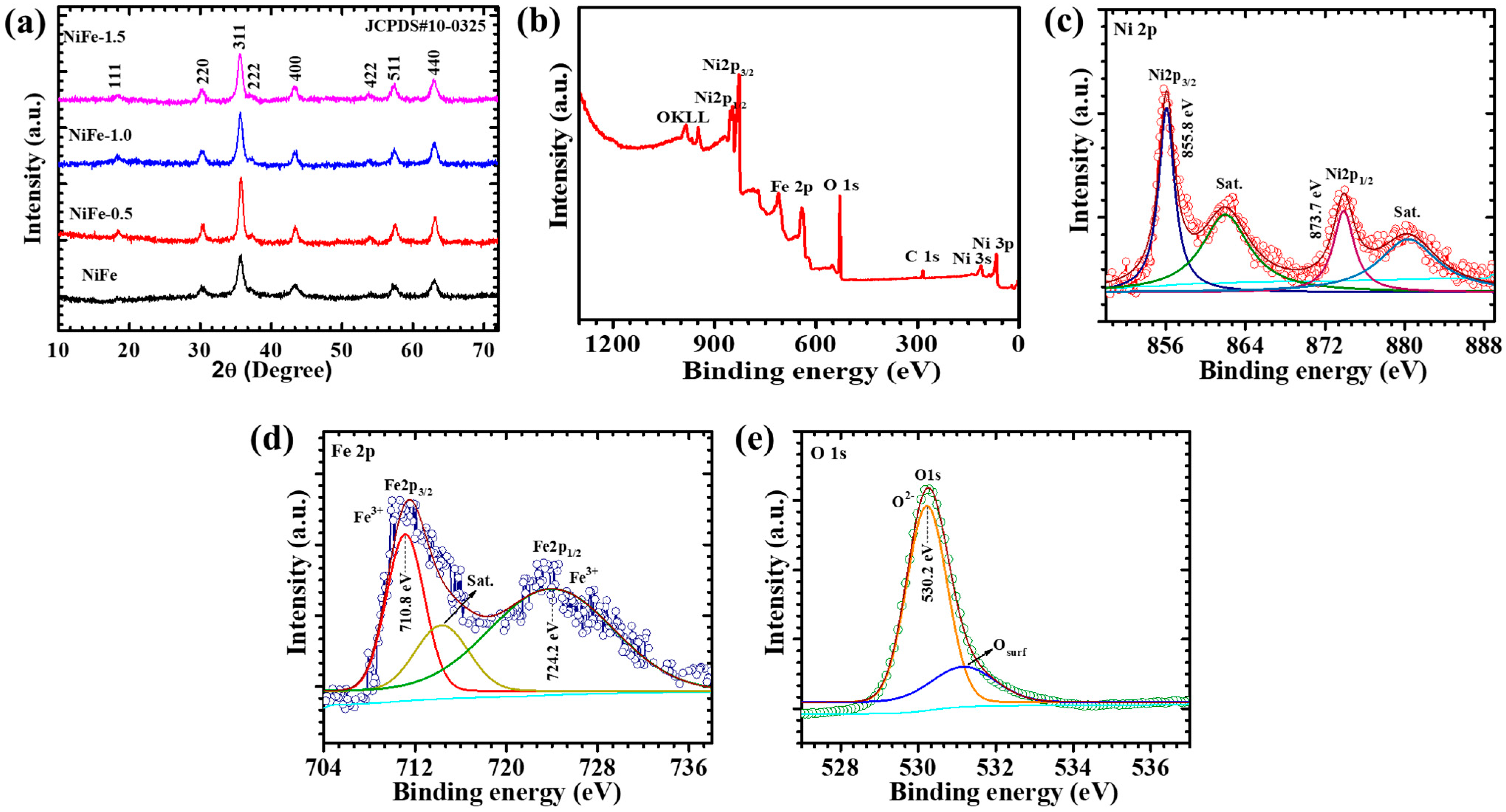
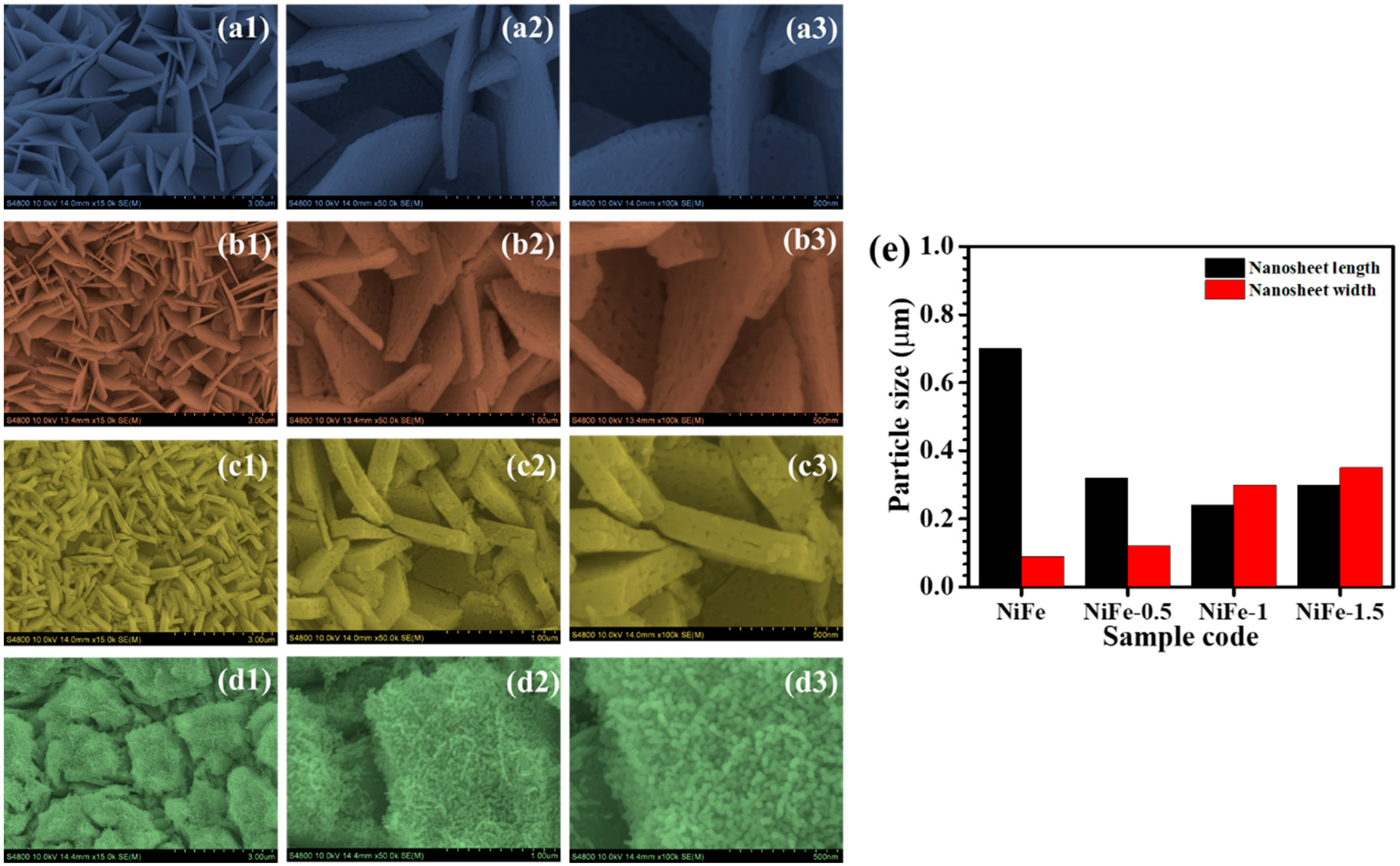


| Sample Code | Crystallite Size (D, nm) | Lattice Strain | Dislocation Density (line/m2) × 1015 |
|---|---|---|---|
| NiFe | 10.5 | 0.01123 | 9.07 |
| NiFe-0.5 | 13.2 | 0.00889 | 5.83 |
| NiFe-1 | 11.25 | 0.01052 | 5.73 |
| NiFe-1.5 | 11.3 | 0.01042 | 7.9 |
| Sample Code | Diffusion Coefficient (cm2/s) × 10−6 | b-Value | |
|---|---|---|---|
| Oxidation | Reduction | ||
| NiFe | 0.87 | 0.67 | 0.77 |
| NiFe-0.5 | 1.64 | 1.1 | 0.6 |
| NiFe-1 | 2.6 | 3.2 | 0.42 |
| NiFe-1.5 | 0.61 | 0.28 | 0.66 |
| Sample Code | I (mA) | CA (F/cm2) | C (mAh/cm2) | ED (mWh/cm2) | PD (mW/cm2) |
|---|---|---|---|---|---|
| NiFe | 20 | 1.96 | 0.5455 | 0.0825 | 5.5 |
| 30 | 1.8 | 0.5253 | 0.0749 | 8.25 | |
| 40 | 1.78 | 0.4949 | 0.0665 | 11 | |
| 50 | 1.72 | 0.4798 | 0.0535 | 13.75 | |
| NiFe-0.5 | 20 | 2.03 | 0.5657 | 0.0856 | 5.5 |
| 30 | 1.96 | 0.5455 | 0.0825 | 8.25 | |
| 40 | 1.58 | 0.4394 | 0.0749 | 11 | |
| 50 | 1.45 | 0.4040 | 0.0611 | 13.75 | |
| NiFe-1 | 20 | 8.21 | 2.2828 | 0.3453 | 5.5 |
| 30 | 4.1 | 1.1364 | 0.1719 | 8.25 | |
| 40 | 3.2 | 0.8889 | 0.1344 | 11 | |
| 50 | 2.09 | 0.5303 | 0.0802 | 13.75 | |
| NiFe-1.5 | 20 | 1.16 | 0.3232 | 0.0489 | 5.5 |
| 30 | 1.14 | 0.3182 | 0.0481 | 8.25 | |
| 40 | 1.06 | 0.3030 | 0.0458 | 11 | |
| 50 | 0.91 | 0.2525 | 0.0382 | 13.75 |
| Sample Code | I (mA) | CA (F/cm2) | C (mAh/cm2) | ED (mWh/cm2) | PD (mW/cm2) |
|---|---|---|---|---|---|
| NiFe-1 device | 20 | 1.215 | 0.3376 | 0.2853 | 6.5 |
| 30 | 0.380 | 0.1058 | 0.0894 | 9.75 | |
| 40 | 0.184 | 0.0513 | 0.0433 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morankar, P.J.; Amate, R.U.; Teli, A.M.; Bhosale, M.K.; Beknalkar, S.A.; Jeon, C.-W. Cationic Surfactant-Driven Evolution of NiFe2O4 Nanosheets for High-Performance Asymmetric Supercapacitors. Materials 2025, 18, 1987. https://doi.org/10.3390/ma18091987
Morankar PJ, Amate RU, Teli AM, Bhosale MK, Beknalkar SA, Jeon C-W. Cationic Surfactant-Driven Evolution of NiFe2O4 Nanosheets for High-Performance Asymmetric Supercapacitors. Materials. 2025; 18(9):1987. https://doi.org/10.3390/ma18091987
Chicago/Turabian StyleMorankar, Pritam J., Rutuja U. Amate, Aviraj M. Teli, Mrunal K. Bhosale, Sonali A. Beknalkar, and Chan-Wook Jeon. 2025. "Cationic Surfactant-Driven Evolution of NiFe2O4 Nanosheets for High-Performance Asymmetric Supercapacitors" Materials 18, no. 9: 1987. https://doi.org/10.3390/ma18091987
APA StyleMorankar, P. J., Amate, R. U., Teli, A. M., Bhosale, M. K., Beknalkar, S. A., & Jeon, C.-W. (2025). Cationic Surfactant-Driven Evolution of NiFe2O4 Nanosheets for High-Performance Asymmetric Supercapacitors. Materials, 18(9), 1987. https://doi.org/10.3390/ma18091987






