Abstract
A new complex [Mg(L)2(phen)(H2O)2](phen)(H2O)2 [L= N-benzenesulphonyl-L-leucine] was synthesized by the reaction of magnesium chloride hexahydrate with N-benzenesulphonyl-L-leucine and 1,10-phenanthroline in the CH3CH2OH/H2O (v:v = 5:1). It was characterized by elemental analysis, IR and X-ray single crystal diffraction analysis. The crystal of the title complex [Mg(L)2(phen)(H2O)2](phen)(H2O)2 belongs to triclinic, space group P-1 with a = 0.72772(15) nm, b = 1.4279(3) nm, c = 1.4418(3) nm, α = 63.53(3)°, β = 79.75(3)°, γ = 81.83(3)°, V = 1.3163(5) nm3, Z =1, Dc= 1.258 μg·m−3, μ = 0.177 mm−1, F(000) = 526, and final R1 = 0.0506, ωR2 = 0.1328. The complex comprises a six-coordinated magnesium(II) center, with a N2O4 distorted octahedron coordination environment. The molecules are connected by hydrogen bonds and π-π stacking to form one dimensional chain structure. The luminescent property of the Mg(II) complex has been investigated in solid.
1. Introduction
The design and synthesis of metal complex materials with carboxylate ligands have attracted intense attention in recent years owing to their potential practical applications, such as molecule-based magnets, luminescence, biological properties [1,2,3]. Increasing investigations have been focused on the transition metal complex materials with carboxylate ligands [4,5,6]. Magnesium is an indispensable element in biology. It is involved in several biochemical processes and is an essential cofactor required for the activation of a variety of enzymes. Therefore, it is significant to study on the structure and characteristic coordination of magnesium carefully to understand the physiological and biochemical mechanisms of life. To the best of our knowledge, the magnesium(II) complex materials with carboxylate ligands have been much less extensively studied than other complexes. In this paper, we report the synthesis, X-ray crystal structure of [Mg(L)2(phen)(H2O)2]·(phen)·(H2O)2, the luminescent property of Mg(II) complex also has been investigated.
2. Results and Discussion
2.1. IR Spectra
In the infrared spectra, the νas(COOH) and νs(COOH) vibrations of the free ligand are at 1,659 and 1,436 cm−1, respectively. For the complex, the vibration observed at 1,612 cm−1 was assigned as νas(COO−) and that at 1,398 cm−1 as νs(COO−). It can be explained that the carboxylate oxygen atoms of N-benzenesulphonyl-L-leucine ligand take part in the coordination with magnesium atom [7]. The difference between the νas(COO−) and νs(COO−) band is 214 cm−1, indicating an unidentate carboxylate moiety. The ν(C = N) vibration of the free phen is at 1,589 cm−1, and it shifts to 1,556 cm−1 in the complex, indicating that the nitrogen atoms of phen take part in the coordination with magnesium atom. The bands of the -SO2-NH- groups at 3,248 cm−1, 1,320 cm−1 and 1,155 cm−1 show that there are uncoordinated atoms of the groups, because compared with the free ligand the strong absorption bands are not shifted. The new band at 421 cm−1 is assigned to the ν(Mg-O) vibration. In addition, the band at 3,423 cm−1 shows that the complex contains water molecules, which are accordance with the results of elemental analysis.
2.2. Structure Description
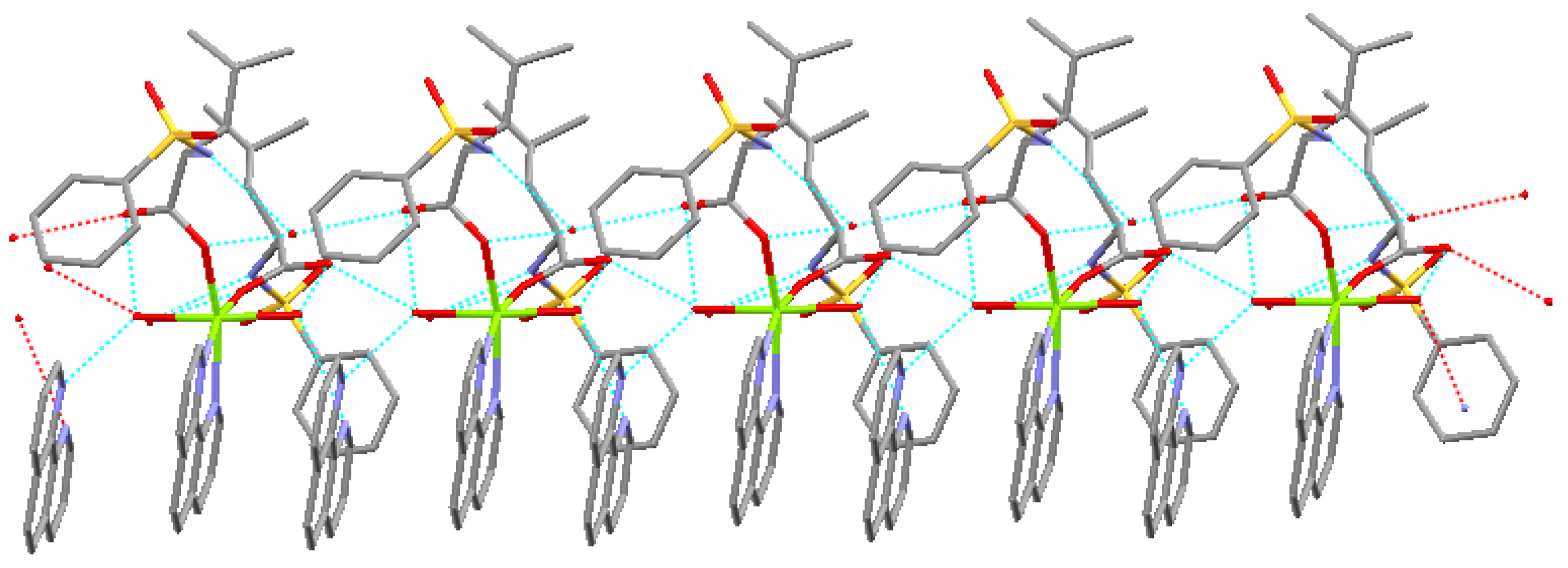
Perspective view of the molecule and molecular packing arrangement are shown in Figure 1 and Figure 2, respectively. It can be seen that the coordination environment of the Mg(II) atom consists of two oxygen atoms from the N-benzenesulphonyl-L-leucine ligand, two oxygen atoms from the coordinated water molecules and two nitrogen atoms from the 1,10-phenanthroline ligand, making up a distorted octahedral environment. The coordination atoms with O(1), O(5), N(4)and N(3) atoms are situated equatorial plane and O(1w), O(2w) atoms are situated axial place. The axial bond angle O(1)-Mg-N(4) [165.95(12)°] is consistent with that of the literature structure [165.5(2)°] [8]. The distances of the Mg-O bonds are in the range of 2.020(2)~2.091(2) Å, and that of Mg-N bonds are 2.193(3) Å and 2.204(3) Å, respectively, which are similar to the Mg-O bond and Mg-N bond lengths reported previously [8]. In addition, a free 1,10-phenanthroline molecule exists in the crystal structure, and it is very important in the construction of the final structure.
The complex forms one dimensional chain structure along by intramolecule and intermolecule hydrogen bonds and π-π stacking (Figure 3).
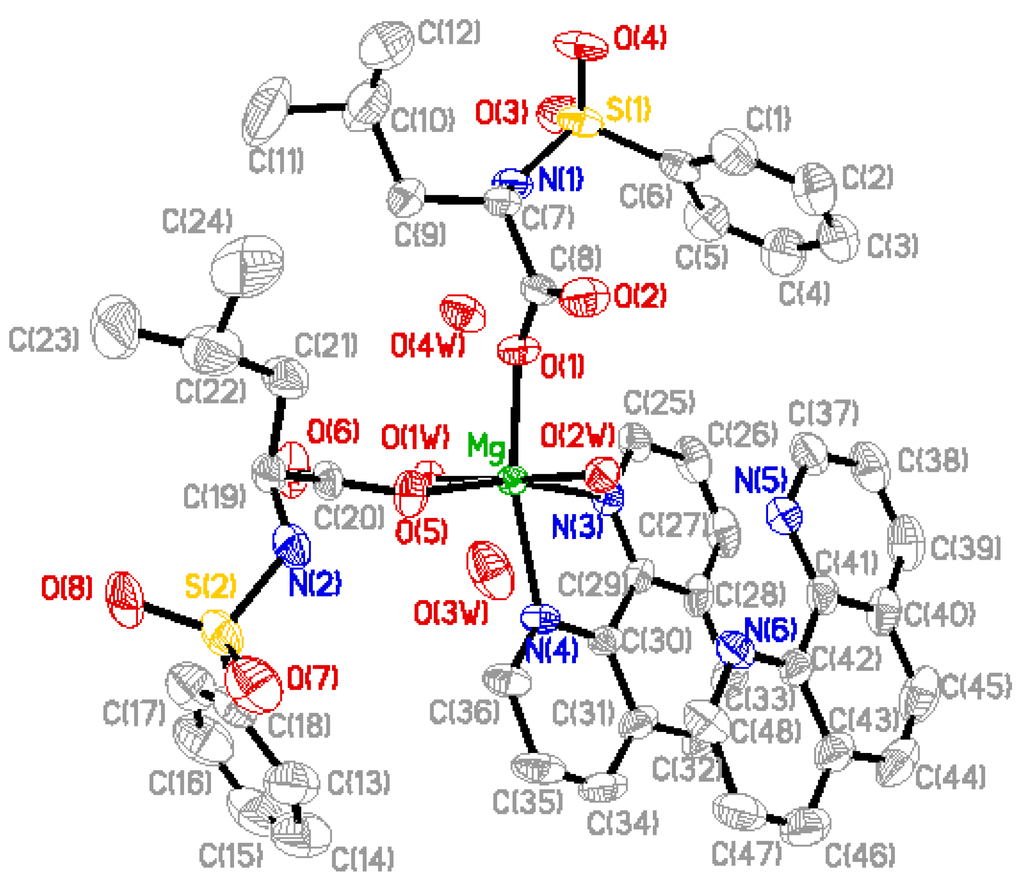
Figure 1.
Molecular structure of the complex, where the thermal ellipsoids were drawn at 30% possibility.
Figure 1.
Molecular structure of the complex, where the thermal ellipsoids were drawn at 30% possibility.
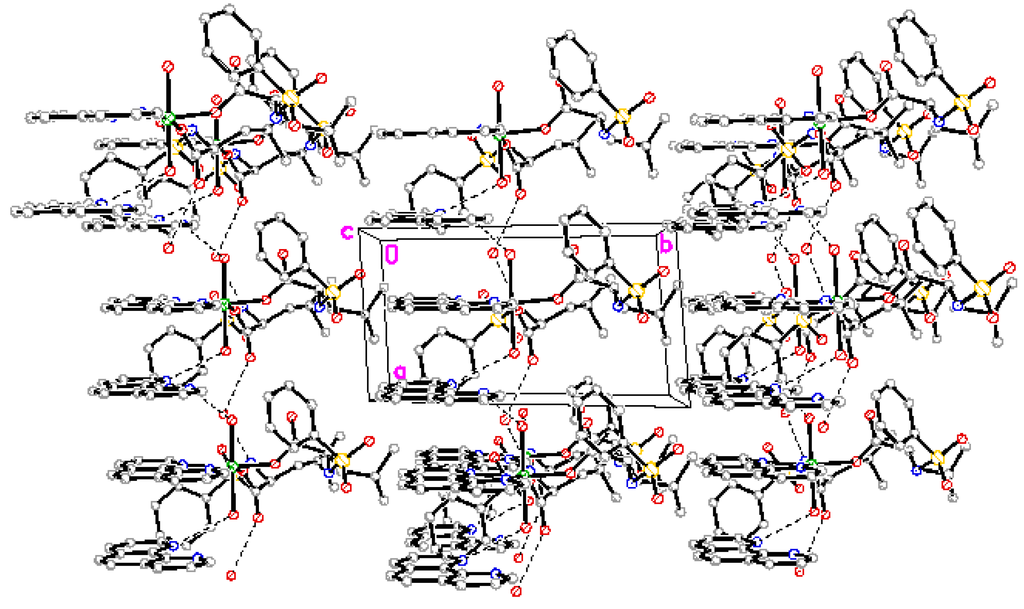
Figure 2.
Packing of the complex.
Figure 2.
Packing of the complex.
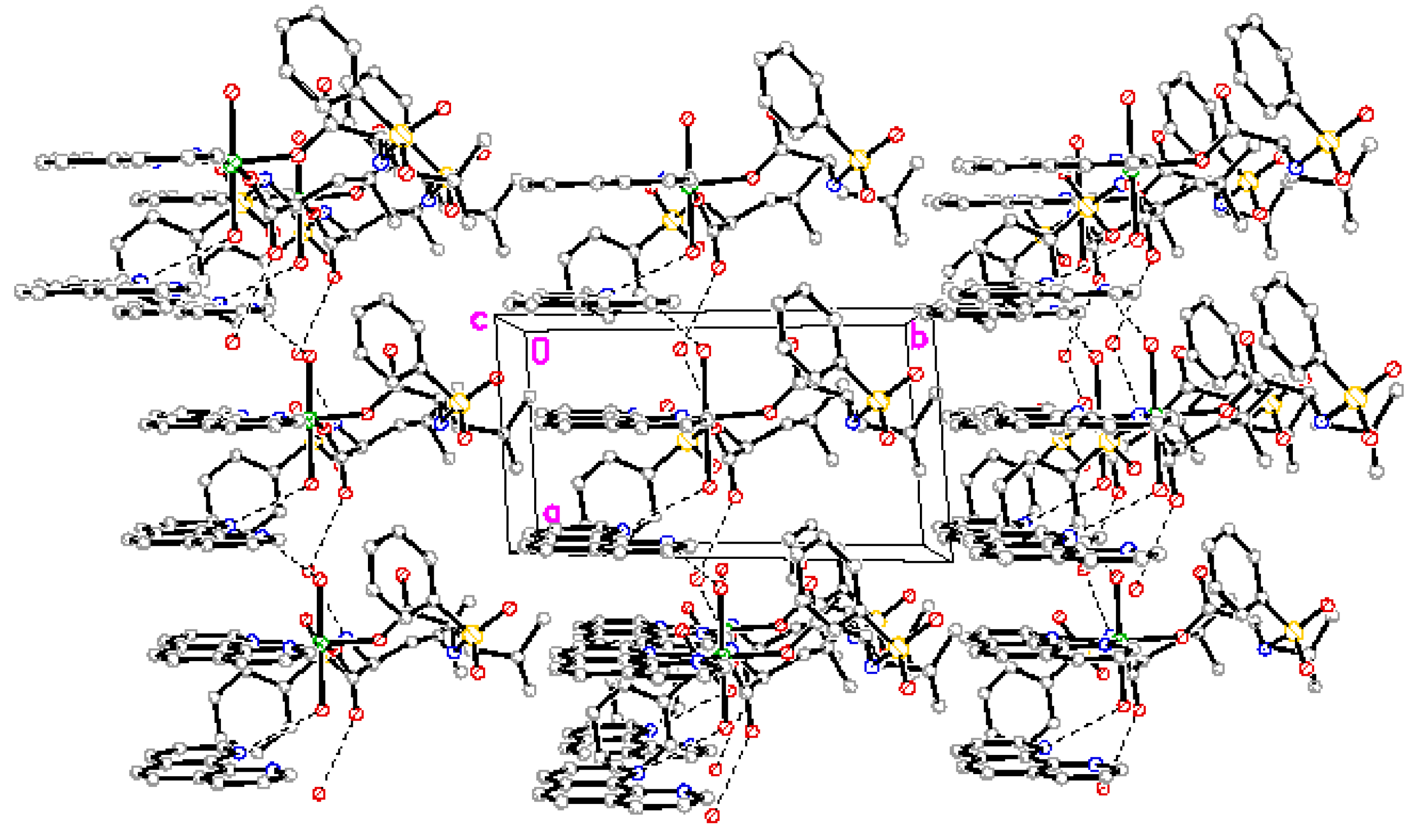
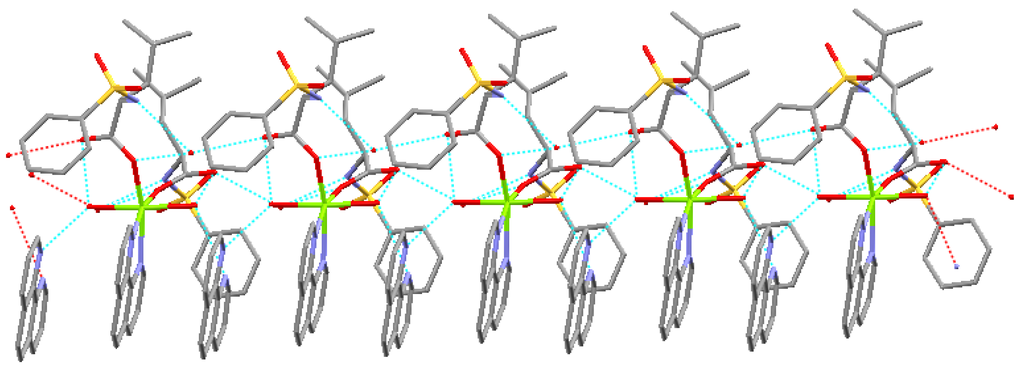
Figure 3.
One dimensional chain structure.
Figure 3.
One dimensional chain structure.
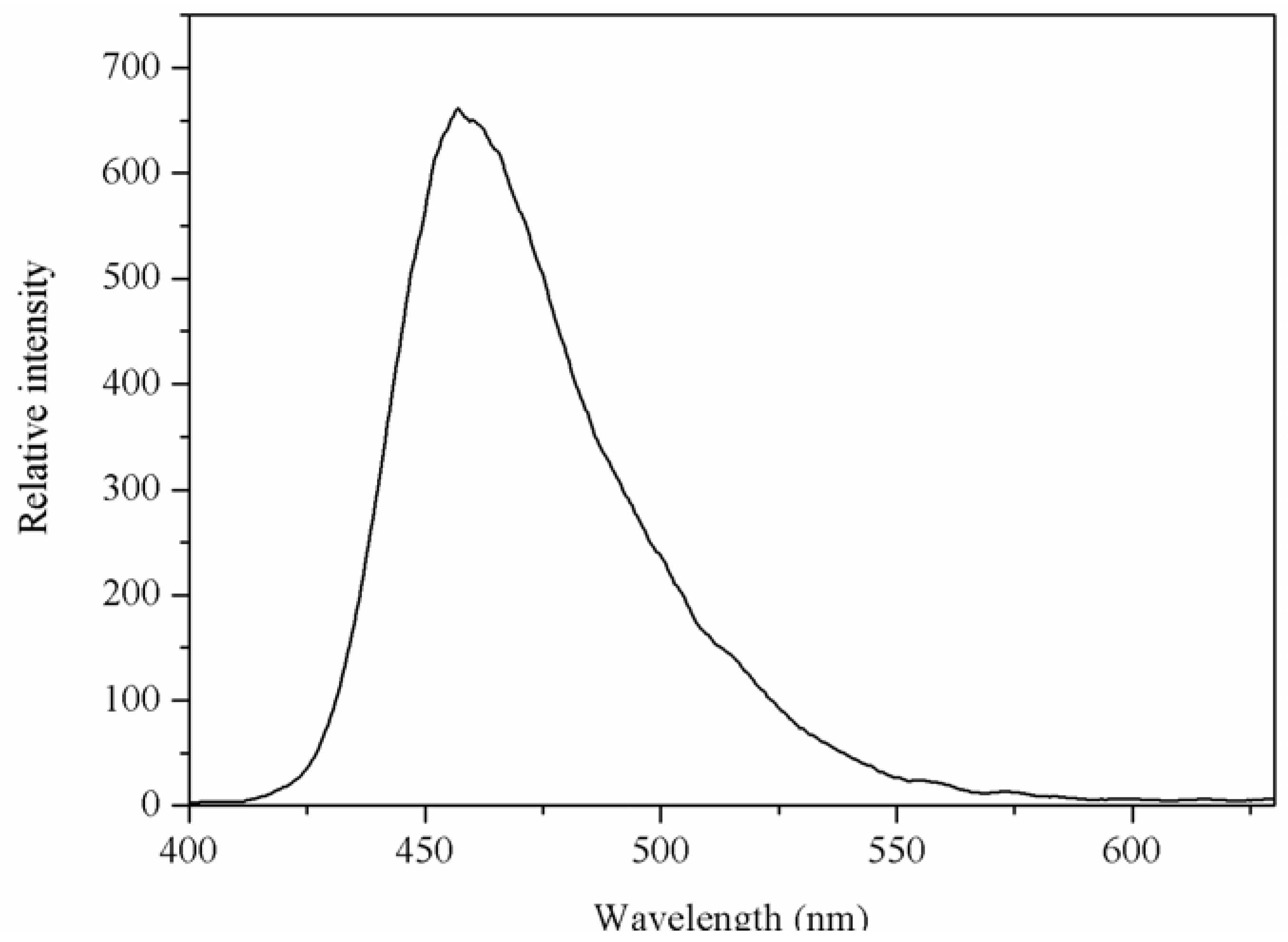
2.3. Luminescent Properties
The luminescent spectrum of the Mg(II) complex in solid-state was measured at room temperature. The emission spectrum of the Mg(II) complex is shown in Figure 4. From Figure 4, it can be seen that the Mg(II) complex displays a luminescent emission maximum at 457 nm upon excitation at 326 nm. The emission spectrum of 1,10-phenanthroline is at 367 nm upon excitation at 267 nm [9], and the N-benzenesulphonyl-L-leucine ligand does not display luminescent emission maximum. Compared with the emission of 1,10-phenanthroline, the emission maximum of Mg(II) complex was red shifted. This may be the energy gap between the triplet levels of ligand and the emitting level of Mg(II) favor to the energy transfer process for Mg(II).
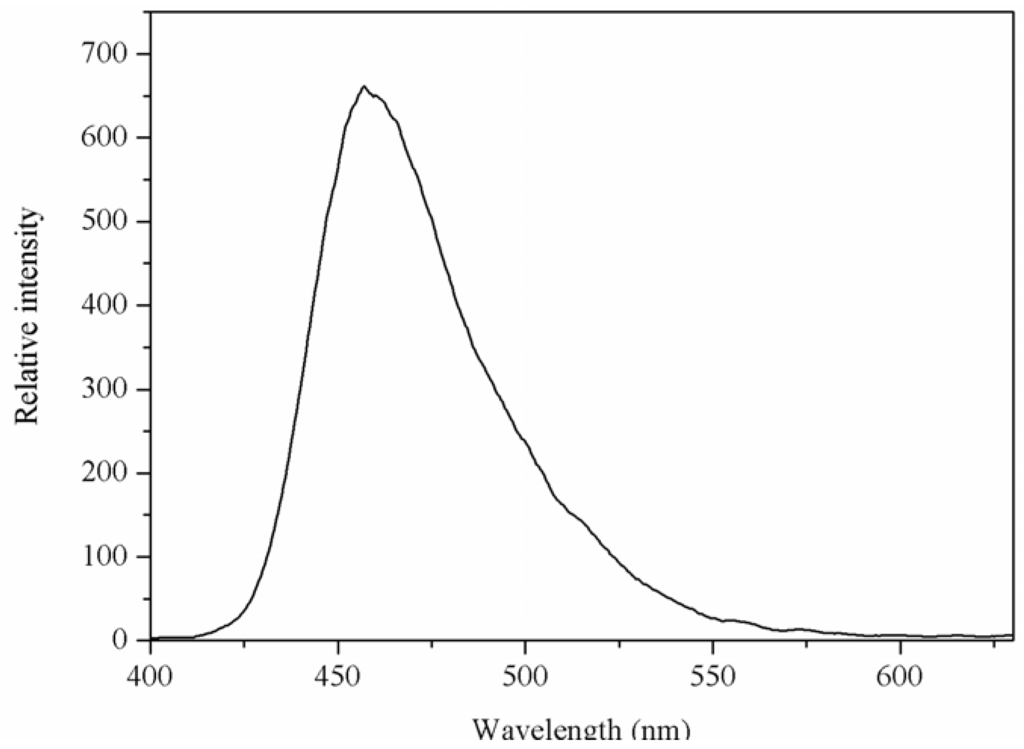
Figure 4.
The emission spectrum of Mg(II) complex. The excitation and emission slit widths were 2.5 nm.
Figure 4.
The emission spectrum of Mg(II) complex. The excitation and emission slit widths were 2.5 nm.
3. Experimental Section
3.1. Materials and Methods
The following A.R. grade chemicals were used for the preparation of the studied compound: magnesium chloride hexahydrate, benzene sulfonyl chloride, L-leucine, sodium hydroxide, 1,10-phenanthroline.
The carbon, hydrogen and nitrogen content in the newly synthesized compound were determined on a Elementar Vario III EL elemental analyzer. Infrared spectrum (4,000–400 cm−1) was recorded with KBr optics on a Nicolet AVATAR 360 FTIR spectrophotometer. The luminescent measurements were made on a PE LS-55 spectrometer. The crystal data was collected on a Bruker smart CCD Area Detector.
3.2. Synthesis of the Ligand
10 mmol (1.311 g) of L-leucine and 20 mmol (0.8 g) of sodium hydroxide were dissolved in 100 mL of water at room temperature, and added drop by drop 10 mmol (1.766 g) of benzene sulfonyl chloride by stirring at room temperature. The reaction solution was kept running for 6 h, then acidified with the solution of hydrochloric acid (V:V = 1:1) to pH = 2. The white solid precipitation were collected by filtration, washed and dried under vacuum. Yield may reach up to over 58%. Elementary analysis: calcd for C12H17NSO4:C, 53.14; H, 6.27; N, 5.17%; found: C, 42.98; H, 6.52; N, 5.37%. IR νmax (cm−1):νas(COOH):1659 cm−1, νs(COOH):1436 cm−1, ν(N-H):3248 cm−1.
3.3. Synthesis of Mg(II) Complex
1.0 mmol (0.271 g) of N-benzenesulphonyl-L-leucine and 1.0 mmol (0.04 g) of sodium hydroxide were added to the 10 mL of CH3CH2OH/H2O (v:v = 5:1) solution. After being dissolved, 0.5 mmol (0.1015 g) of magnesium chloride hexahydrate was added to the solution. The mixture was continuously stirred for 1 h at refluxing temperature, then 0.5 mmol (0.09 g) of 1,10-phenanthroline was added to the mixture by stirring 3 h at refluxing temperature. The mixture was cooled at room temperature, and was collected by filtration. By evaporation in air at room temperature, the single crystal suitable for X-ray determination was obtained from methanol solution after 10 days. Elementary analysis: calcd for C48H56MgN6O12S2:C, 57.75; H, 5.61; N, 8.42%; found: C, 57.58; H, 5.99; N, 8.72%. IR νmax (cm−1):νas(COO−):1612 cm−1, νs(COO−):1398 cm−1, ν(N-H):3248 cm−1, ν(H2O):3423 cm−1, ν(Mg-O):421 cm−1.
3.4. X-ray Crystallography
A colorless block single crystal with dimensions of 0.18 mm × 0.14 mm × 0.10 mm was placed on a glass fiber and mounted on a CCD area detector. Diffraction data were collected by φ~ω scan mode using a graphite-monochromatic Mo Kα radiation (λ = 0.71073 Å) at 293(2) K. A total of 12,805 reflections were collected in the range 3.03–27.48°, of which 9,769 were unique (Rint = 0.0472) and 7,533 were observed with I > 2σ(I). The data were corrected for Lp factors. The structure was solved by direct methods and refined by full-matrix least-squares techniques on F2. The structure was solved by direct methods [10] using SHELXL-97 and expanded using Fourier techniques. All non-hydrogen atoms and hydrogen atoms were refined anisotropically and isotropically, respectively. The final refinement by full-matrix least squares method was converged at R = 0.0506, and wR = 0.1328 (w = 1/[δ2(Fo2) + (0.0949P)2 + 0.1200P], P = (Fo2 + 2Fc2)/3, S = 1.052, (Δ/σ)max = 0.000). The largest peak in the final difference Fourier map is 0.741 e/Å3 and the minimum peak is −0.286 e/Å3. Molecular graphics were drawn with the program package SHELXTL-97 crystallographic software package [11]. The most relevant crystal data for complex are quoted in Table 1, and the selected bond distances and angles are listed in Table 2.

Table 1.
Crystallographic data for Mg(II) complex.
| Crystallographic parameter | Crystallographic data |
|---|---|
| Formula | C48H56MgN6O12S2 |
| Formula weight | 997.42 |
| Crystal system | Triclinic |
| Space group | P−1 |
| a(Å) | 7.2772(15) |
| b(Å) | 14.279(3) |
| c(Å) | 14.418(3) |
| α(°) | 63.53(3) |
| β(°) | 79.75(3) |
| γ(°) | 81.83(3) |
| Z | 1 |
| F(000) | 526 |
| Temperature (K) | 293(2) |
| V (Å3) | 1,316.3(5) |
| Calculated density (μg·m−3) | 1.258 |
| Crystal size (mm3) | 0.18 × 0.14 × 0.10 |
| μ (mm−1) | 0.177 |
| Limiting indices | −9 ≤ h ≤ 9, −18 ≤ k≤ 16, −18 ≤ l ≤ 18 |
| Reflections collected/unique | 9769/7533 |
| R1, wR2 [all data] | 0.0710, 0.1643 |
| R1, wR2 [I > 2σ(I)] | 0.0506, 0.1328 |
| Largest diff.peak and hole (e·Å−3) | 0.741, −0.286 |

Table 2.
Selected bond lengths (Å) and angles (°) for Mg(II) complex.
| Bonds | Bond parameter | Bonds | Bond parameter |
|---|---|---|---|
| Mg-O5 | 2.020(2) | S1-O3 | 1.429(3) |
| Mg-O1 | 2.035(2) | S1-O4 | 1.426(3) |
| Mg-O1W | 2.081(2) | S1-N1 | 1.594(3) |
| Mg-O2W | 2.091(2) | S1-C6 | 1.750(5) |
| Mg-N3 | 2.193(3) | N1-C7 | 1.474(5) |
| Mg-N4 | 2.204(3) | ||
| O5-Mg-O1 | 100.07(12) | O1W-Mg-N4 | 98.97(11) |
| O5-Mg-O1W | 89.62(11) | O2W-Mg-N4 | 84.09(11) |
| O1-Mg-O1W | 88.44(10) | N3-Mg-N4 | 75.36(11) |
| O2W-Mg-O5 | 89.76(10) | O3-S1-O4 | 119.1(2) |
| O2W-Mg-O1 | 88.69(10) | O3-S1-N1 | 106.5(2) |
| O1W-Mg-O2W | 176.90(10) | O4-S1-N1 | 108.1(2) |
| O5-Mg-N3 | 165.58(12) | O3-S1-C6 | 107.3(2) |
| O1-Mg-N3 | 93.44(12) | O4-S1-C6 | 106.8(2) |
| O1W-Mg-N3 | 85.72(10) | N1-S1-C6 | 108.67(16) |
| O2W-Mg-N3 | 95.60(11) | O7-S2-O8 | 119.3(2) |
| O5-Mg-N4 | 91.95(12) | O7-S2-N2 | 106.5(2) |
| O1-Mg-N4 | 165.95(12) | N2-S2-O8 | 108.3(2) |
| N1-C7-C9 | 109.9(3) |
4. Conclusions
In summary, a new complex [Mg(L)2(phen)(H2O)2](phen)(H2O)2[L = N-benzenesulphonyl-L-leucine] has been synthesized and structurally characterized. The complex comprises a six-coordinated magnesium(II) center, with a N2O4 distorted octahedron coordination environment. The molecules are connected by hydrogen bonds and π-π stacking to form one dimensional chain structure. The luminescent property of the Mg(II) complex also has been investigated in solid-state.
Acknowledgments
The authors would like to thank the National Natural Science Foundation of China (No. 21171132 and 20671073), the Promotive Research Fund for Excellent Young and Middle-aged Scientisits of Shandong Province (2010BSA07004) and Science Foundation of Weifang University.
Supplementary Material
Crystallographic data for the structure reported in this paper has been deposited with the Cambridge Crystallographic Data Centre as supplementary publication No.CCDC 860718. Copy of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (E-Mail: deposit@ccdc.cam.ac.uk; Fax: +44-1223-336-033).
References
- Lu, W.G.; Jiang, L.; Feng, X.L.; Lu, T.B. Three 3D coordination polymers constructed by Cd(II) and Zn(II) with imidazole-4,5-dicarboxylate and 4,4’-bipyridyl building blocks. Cryst. Growth Des. 2006, 6, 564–571. [Google Scholar]
- Tian, L.J.; Yang, H.J.; Zheng, X.L.; Ni, Z.H.; Yan, D.M.; Tu, L.L.; Jiang, J.Z. Synthesis, structural characterization and cytotoxic activity of diorganotin(IV) complexes of N-(5-halosalicylidene)tryptophane. Appl. Organomet. Chem. 2009, 23, 24–31. [Google Scholar]
- Wang, L.F.; Hu, Y.X.; Zhang, W.W.; Ren, X.M. Solvothermal synthesis, crystal structure and photoluminescence property of a coordination polymer based on 1,1’-ethynebenzene-3,3’,5,5’-tetracarboxylate. China J. Inorg. Chem. 2011, 27, 542–546. [Google Scholar]
- Gao, S.; Zhang, Z.Y.; Huo, L.H. Synthesis, Crystal structure and thermal behavior of 1D chain coordination polymer [Ca(2-OPA)2(H2O)2]n constructed by 2-oxo-1(4H)-pyridineacetate ligand. China J. Inorg. Chem. 2005, 21, 771–774. [Google Scholar]
- Tai, X.S.; Feng, Y.M.; Wang, L.T.; Tan, M.Y. Synthesis, structure characterization and luminescent properties of ternary zinc acetate complex with 4-aminohippuric acid and 1,10-phenanthroline ligand. Pol. J. Chem. 2009, 83, 1099–1104. [Google Scholar]
- Zhang, A.J.; Ye, C.; Hu, H.M.; Zhu, B.B. A novel Nickel (II) complex adopting a cis-configuration: Solvothermal synthesis and crystal structure of [NiL2(H2O)4](L = 1,4-dihydropyrazine-2,3-dione-5,6-dicarboxylate). Eur. J. Inorg. Chem. 2002, 7, 1595–1598. [Google Scholar]
- Nakamoto, K. Infrared and Ramen Spectra of Inorganic and Coordination Compounds, 3rd ed.; John Wiley and Sons: New York, NY, USA, 1978; Volume 1, pp. 359–368. [Google Scholar]
- Tai, X.S.; Du, L.C.; Zhao, Z.B. Synthesis, crystal structure and antibacterial activity of Magnesium(II) complex with N-benzenesulphonyl-L-phenylalanine and 1,10-phenanthroline. China J. Inorg. Chem. 2011, 27, 575–579. [Google Scholar]
- Guo, D.F.; Ye, Y.; Zeng, Z.Z. Interaction between ternary rare earth complexes of cinnamic acid and phenanthroline with DNA by spectroscopy. J. Rare Earth. 2005, 23, 162–166. [Google Scholar]
- Sheldrick, G.M. SHELXL-97, Program for Crystal Structure Solution; University of GÖttingen: GÖttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXTL-97, Program for Crystal Structure Refinement; University of GÖttingen: GÖttingen, Germany, 1997. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
