Improved Osteoblast and Chondrocyte Adhesion and Viability by Surface-Modified Ti6Al4V Alloy with Anodized TiO2 Nanotubes Using a Super-Oxidative Solution
Abstract
:1. Introduction
2. Results
2.1. Surface Characterization
2.1.1. SEM Material Characterization

2.1.2. Chemical Composition
| Sample | C (%) | N (%) | Al (%) | Ti (%) | O (%) | F (%) |
|---|---|---|---|---|---|---|
| Anodized Ti6Al4V | 3.9 | - | 5.40 | 61.91 | 25.51 | 3.28 |
| Non-anodized Ti6Al4V | 3.45 | 3.38 | 6.06 | 87.11 | - | - |
2.1.3. Surface Roughness

2.2. Biological Activity
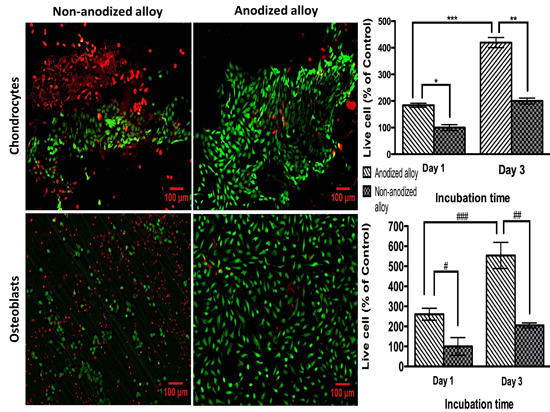
2.2.1. Biocompatibility Assay

2.2.2. Cell Adhesion

2.2.3. Cell Morphology by SEM


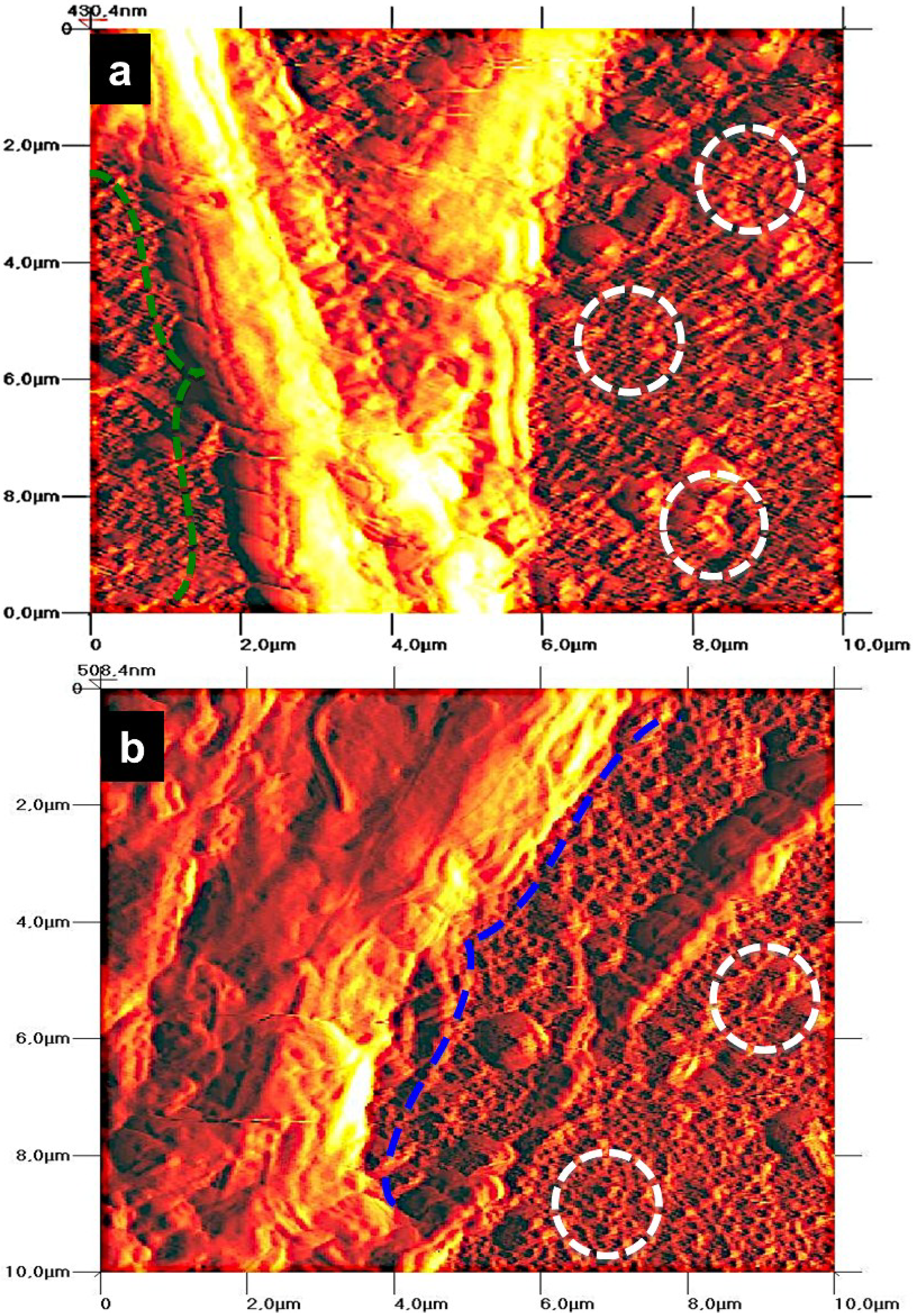
2.2.4. Cell Morphology by AFM
3. Discussion
4. Experimental Section
4.1. Synthesis of NTs
4.2. Substrate Surface Characterization
4.2.1. SEM
4.2.2. EDX
4.2.3. AFM
4.3. Biological Activity
4.3.1. Cell Culture
4.3.2. Cell Viability
4.3.3. Cell Adhesion
4.3.4. Cell Morphology by SEM
4.3.5. Cell Morphology by AFM
4.4. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.Q.; Li, S.J.; Hao, Y.L.; Zhao, Y.K.; Ai, H.J. Effect of nanotube diameters on bioactivity of a multifunctional titanium alloy. Appl. Surf. Sci. 2013, 268, 44–51. [Google Scholar] [CrossRef]
- Chen, Z.X.; Takao, Y.; Wang, W.X.; Matsubara, T.; Ren, L.M. Surface characteristics and in vitro biocompatibility of titanium anodized in a phosphoric acid solution at different voltages. Biomed. Mater. 2009, 4. [Google Scholar] [CrossRef]
- Tavangar, A.; Tan, B.; Venkatakrishnan, K. Synthesis of bio-functionalized three-dimensional titania nanofibrous structures using femtosecond laser ablation. Acta Biomater. 2011, 7, 2726–2732. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Bose, S.; Bandyopadhyay, A. TiO2 nanotubes on Ti: Influence of nanoscale morphology on bone cell-materials interaction. J Biomed. Mater. Res A 2009, 90, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Chiapasco, M.; Gatti, C. Implant-retained mandibular overdentures with immediate loading: A 3- to 8-year prospective study on 328 implants. Clin. Implant Dent. Relat Res. 2003, 5, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.L.; Beloti, M.M. Rat bone marrow cell response to titanium and titanium alloy with different surface roughness. Clin. Oral Implants Res. 2003, 14, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yu, W.; Zhang, Y.; Zhang, F. Increased preosteoblast adhesion and osteogenic gene expression on TiO2 nanotubes modified with KRSR. J Mater. Sci. Mater. Med. 2013, 24, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Saino, E.; Rimondini, L.; Pedeferri, M.P.; Visai, L.; Cigada, A.; Chiesa, R. Electrochemically induced anatase inhibits bacterial colonization on Titanium Grade 2 and Ti6Al4V alloy for dental and orthopedic devices. Colloids Surf. B Biointerfaces 2011, 88, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Junker, R.; Dimakis, A.; Thoneick, M.; Jansen, J.A. Effects of implant surface coatings and composition on bone integration: A systematic review. Clin. Oral Implants Res. 2009, 20, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Mosher, C.Z.; Boushell, M.K.; Lu, H.H. Engineering complex orthopaedic tissues via strategic biomimicry. Ann. Biomed. Eng. 2014. [Google Scholar] [CrossRef]
- Grenier, S.; Donnelly, P.E.; Gittens, J.; Torzilli, P.A. Resurfacing damaged articular cartilage to restore compressive properties. J. Biomech. 2014, 48, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Sundfeldt, M.; Carlsson, L.V.; Johansson, C.B.; Thomsen, P.; Gretzer, C. Aseptic loosening, not only a question of wear: A review of different theories. Acta Orthop. 2006, 77, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Wang, X.J.; Wang, L.P.; Lei, F.Y.; Wang, X.F.; Ai, H.J. Effect of fluoride-ion implantation on the biocompatibility of titanium for dental applications. Appl. Surf. Sci. 2008, 254, 6305–6312. [Google Scholar] [CrossRef]
- Advincula, M.C.; Petersen, D.; Rahemtulla, F.; Advincula, R.; Lemons, J.E. Surface analysis and biocorrosion properties of nanostructured surface sol-gel coatings on Ti6Al4V titanium alloy implants. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gao, B.; Wang, R.; Wu, J.; Zhang, L.J.; Hao, Y.L.; Tao, X.J. Improved biological performance of low modulus Ti-24Nb-4Zr-7.9Sn implants due to surface modification by anodic oxidation. Appl. Surf. Sci. 2009, 255, 5009–5015. [Google Scholar] [CrossRef]
- Zheng, C.Y.; Nie, F.L.; Zheng, Y.F.; Cheng, Y.; Wei, S.C.; Valiev, R.Z. Enhanced in vitro biocompatibility of ultrafine-grained biomedical NiTi alloy with microporous surface. Appl. Surf. Sci. 2011, 257, 9086–9093. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.J.; Textor, M.; Spencer, N.D.; Wieland, M.; Keller, B.; Sigrist, H. Immobilization of the cell-adhesive peptide Arg-Gly-Asp-Cys (RGDC) on titanium surfaces by covalent chemical attachment. J. Mater. Sci. Mater. Med. 1997, 8, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; Lee, H.-J.; Kwon, T.-Y.; Kim, K.-H. Anodic TiO2 nanotubes from stirred baths: Hydroxyapatite growth & osteoblast responses. Mater. Chem. Phys. 2011, 125, 510–517. [Google Scholar] [CrossRef]
- Cao, X.; Yu, W.Q.; Qiu, J.; Zhao, Y.F.; Zhang, Y.L.; Zhang, F.Q. RGD peptide immobilized on TiO2 nanotubes for increased bone marrow stromal cells adhesion and osteogenic gene expression. J. Mater. Sci. Mater. Med. 2012, 23, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.; Yao, C.; Webster, T.J. Increased chondrocyte adhesion on nanotubular anodized titanium. J. Biomed. Mater. Res. A 2009, 88, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Brammer, K.S.; Oh, S.; Frandsen, C.J.; Varghese, S.; Jin, S. Nanotube surface triggers increased chondrocyte extracellular matrix production. Mater. Sci. Eng. C 2010, 30, 518–525. [Google Scholar] [CrossRef]
- Narayanan, R.; Mukherjee, P.; Seshadri, S.K. Synthesis, corrosion and wear of anodic oxide coatings on Ti-6Al-4V. J. Mater. Sci. Mater. Med. 2007, 18, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Christenson, E.M.; Anseth, K.S.; van den Beucken, J.J.; Chan, C.K.; Ercan, B.; Jansen, J.A.; Laurencin, C.T.; Li, W.J.; Murugan, R.; Nair, L.S.; et al. Nanobiomaterial applications in orthopedics. J. Orthop. Res. 2007, 25, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Macak, J.M.; Muller, L.; Kunze, J.; Muller, F.; Greil, P.; Virtanen, S.; Schmuki, P. Hydroxyapatite growth on anodic TiO2 nanotubes. J. Biomed. Mater. Res. A 2006, 77, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, Y.; Li, J.; Fu, X.; Li, H.; Wang, H.; Xin, S.; Zhou, L.; Liang, C.; Li, C. Influence of nanostructures on the biological properties of Ti implants after anodic oxidation. J. Mater. Sci. Mater. Med. 2014, 25, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Webster, T.J. Mechanical properties of dispersed ceramic nanoparticles in polymer composites for orthopedic applications. Int. J. Nanomed. 2010, 5, 299–313. [Google Scholar]
- Oh, S.; Brammer, K.S.; Li, Y.S.; Teng, D.; Engler, A.J.; Chien, S.; Jin, S. Stem cell fate dictated solely by altered nanotube dimension. Proc. Natl. Acad. Sci. USA 2009, 106, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- Brammer, K.S.; Oh, S.; Cobb, C.J.; Bjursten, L.M.; van de Heyde, H.; Jin, S. Improved bone-forming functionality on diameter-controlled TiO2 nanotube surface. Acta Biomater. 2009, 5, 3215–3223. [Google Scholar] [CrossRef] [PubMed]
- Faix, J.; Breitsprecher, D.; Stradal, T.E.B.; Rottner, K. Filopodia: Complex models for simple rods. Int. J. Biochem. Cell Biol. 2009, 41, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Altmann, B.; Löchner, A.; Swain, M.; Kohal, R.-J.; Giselbrecht, S.; Gottwald, E.; Steinberg, T.; Tomakidi, P. Differences in morphogenesis of 3D cultured primary human osteoblasts under static and microfluidic growth conditions. Biomaterials 2014, 35, 3208–3219. [Google Scholar] [CrossRef] [PubMed]
- Mellor, H. The role of formins in filopodia formation. Biochim. Biophys. Acta. 2010, 1803, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, T.T.; Zhang, Z.; Li, G. Fabrication of highly ordered TiO2 nanotube arrays via anodization of Ti-6Al-4V alloy sheet. J. Nanosci. Nanotechnol. 2010, 10, 8312–8321. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Slamovich, E.B.; Webster, T.J. Enhanced osteoblast functions on anodized titanium with nanotube-like structures. J. Biomed. Mater. Res. A 2008, 85, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Durán, A. Eficacia y seguridad del uso de solución de superoxidación en la prevención de infecciones relacionadas con diálisis. Diálisis Trasplante 2013, 34, 160–165. (In Spanish) [Google Scholar] [CrossRef]
- Gonzalez-Espinosa, D.; Perez-Romano, L.; Guzman-Soriano, B.; Arias, E.; Bongiovanni, C.M.; Gutierrez, A.A. Effects of pH-neutral, super-oxidised solution on human dermal fibroblasts in vitro. Int. Wound J. 2007, 4, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Brammer, K.S.; Frandsen, C.J.; Jin, S. TiO2 nanotubes for bone regeneration. Trends Biotechnol. 2012, 30, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Balasundaram, G.; Webster, T.J. A perspective on nanophase materials for orthopedic implant applications. J. Mater. Chem. 2006, 16, 3737–3745. [Google Scholar] [CrossRef]
- Minagar, S.; Wang, J.; Berndt, C.C.; Ivanova, E.P.; Wen, C. Cell response of anodized nanotubes on titanium and titanium alloys. J. Biomed. Mater. Res. A 2013, 101, 2726–2739. [Google Scholar] [CrossRef] [PubMed]
- Stan, M.S.; Memet, I.; Fratila, C.; Krasicka-Cydzik, E.; Roman, I.; Dinischiotu, A. Effects of titanium-based nanotube films on osteoblast behavior in vitro. J. Biomed. Mater. Res. A 2014, 103, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Saharudin, K.A.; Sreekantan, S.; Abd Aziz, S.N.; Hazan, R.; Lai, C.W.; Mydin, R.B.; Mat, I. Surface modification and bioactivity of anodic Ti6Al4V alloy. J. Nanosci. Nanotechnol. 2013, 13, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Swami, N.; Cui, Z.W.; Nair, L.S. Titania nanotubes: Novel nanostructures for improved osseointegration. J. Heat Trans.-Trans. ASME 2011, 133. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, Y.K.; Park, S., II; Jin, G.C.; Bae, T.S.; Lee, M.H. Effect of alkali and heat treatments for bioactivity of TiO2 nanotubes. Appl. Surf. Sci. 2014, 321, 412–419. [Google Scholar] [CrossRef]
- Blau, A. Cell adhesion promotion strategies for signal transduction enhancement in microelectrode array in vitro electrophysiology: An introductory overview and critical discussion. Curr. Opin. Colloid Interface Sci. 2013, 18, 481–492. [Google Scholar] [CrossRef]
- Tan, A.W.; Ismail, R.; Chua, K.H.; Ahmad, R.; Akbar, S.A.; Pingguan-Murphy, B. Osteogenic potential of in situ TiO2 nanowire surfaces formed by thermal oxidation of titanium alloy substrate. Appl. Surf. Sci. 2014, 320, 161–170. [Google Scholar] [CrossRef]
- Tan, A.W.; Pingguan-Murphy, B.; Ahmad, R.; Akbar, S.A. Review of titania nanotubes: Fabrication and cellular response. Ceram. Int. 2012, 38, 4421–4435. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, K.B.; Jeon, H.S.; Park, H.K. Effects of surface nano-topography on human osteoblast filopodia. Anal. Sci. 2011, 27. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, C.J.; Brammer, K.S.; Noh, K.; Johnston, G.; Jin, S. Tantalum coating on TiO2 nanotubes induces superior rate of matrix mineralization and osteofunctionality in human osteoblasts. Mater. Sci. Eng. C 2014, 37, 332–341. [Google Scholar] [CrossRef]
- Adams, S.L.; Cohen, A.J.; Lassova, L. Integration of signaling pathways regulating chondrocyte differentiation during endochondral bone formation. J. Cell. Physiol. 2007, 213, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Kalamegam, G.; Musumeci, G.; Batt, M.E. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas 2014, 78, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.W.; Dalilottojari, A.; Pingguan-Murphy, B.; Ahmad, R.; Akbar, S. In vitro chondrocyte interactions with TiO2 nanofibers grown on Ti-6Al-4V substrate by oxidation. Ceram. Int. 2014, 40, 8301–8304. [Google Scholar] [CrossRef]
- Smith, B.S.; Yoriya, S.; Johnson, T.; Popat, K.C. Dermal fibroblast and epidermal keratinocyte functionality on titania nanotube arrays. Acta Biomater. 2011, 7, 2686–2696. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Yu, M.; Weng, W.; Cheng, K.; Wang, H.; Lin, J. Light-induced cell detachment for cell sheet technology. Biomaterials 2013, 34, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Sasagawa, T.; Shimizu, T.; Sekiya, S.; Haraguchi, Y.; Yamato, M.; Sawa, Y.; Okano, T. Design of prevascularized three-dimensional cell-dense tissues using a cell sheet stacking manipulation technology. Biomaterials 2010, 31, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, K.S.; Han, I.; Kim, M.H.; Jung, M.H.; Park, H.K. Quantitative and qualitative analysis of the antifungal activity of allicin alone and in combination with antifungal drugs. PLoS One 2012, 7. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrán-Partida, E.; Moreno-Ulloa, A.; Valdez-Salas, B.; Velasquillo, C.; Carrillo, M.; Escamilla, A.; Valdez, E.; Villarreal, F. Improved Osteoblast and Chondrocyte Adhesion and Viability by Surface-Modified Ti6Al4V Alloy with Anodized TiO2 Nanotubes Using a Super-Oxidative Solution. Materials 2015, 8, 867-883. https://doi.org/10.3390/ma8030867
Beltrán-Partida E, Moreno-Ulloa A, Valdez-Salas B, Velasquillo C, Carrillo M, Escamilla A, Valdez E, Villarreal F. Improved Osteoblast and Chondrocyte Adhesion and Viability by Surface-Modified Ti6Al4V Alloy with Anodized TiO2 Nanotubes Using a Super-Oxidative Solution. Materials. 2015; 8(3):867-883. https://doi.org/10.3390/ma8030867
Chicago/Turabian StyleBeltrán-Partida, Ernesto, Aldo Moreno-Ulloa, Benjamín Valdez-Salas, Cristina Velasquillo, Monica Carrillo, Alan Escamilla, Ernesto Valdez, and Francisco Villarreal. 2015. "Improved Osteoblast and Chondrocyte Adhesion and Viability by Surface-Modified Ti6Al4V Alloy with Anodized TiO2 Nanotubes Using a Super-Oxidative Solution" Materials 8, no. 3: 867-883. https://doi.org/10.3390/ma8030867