Preparation of Cement Composites with Ordered Microstructures via Doping with Graphene Oxide Nanosheets and an Investigation of Their Strength and Durability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of GONs
2.3. Preparation of GON/Cement Composites
2.4. Test Methods
3. Results and Discussion
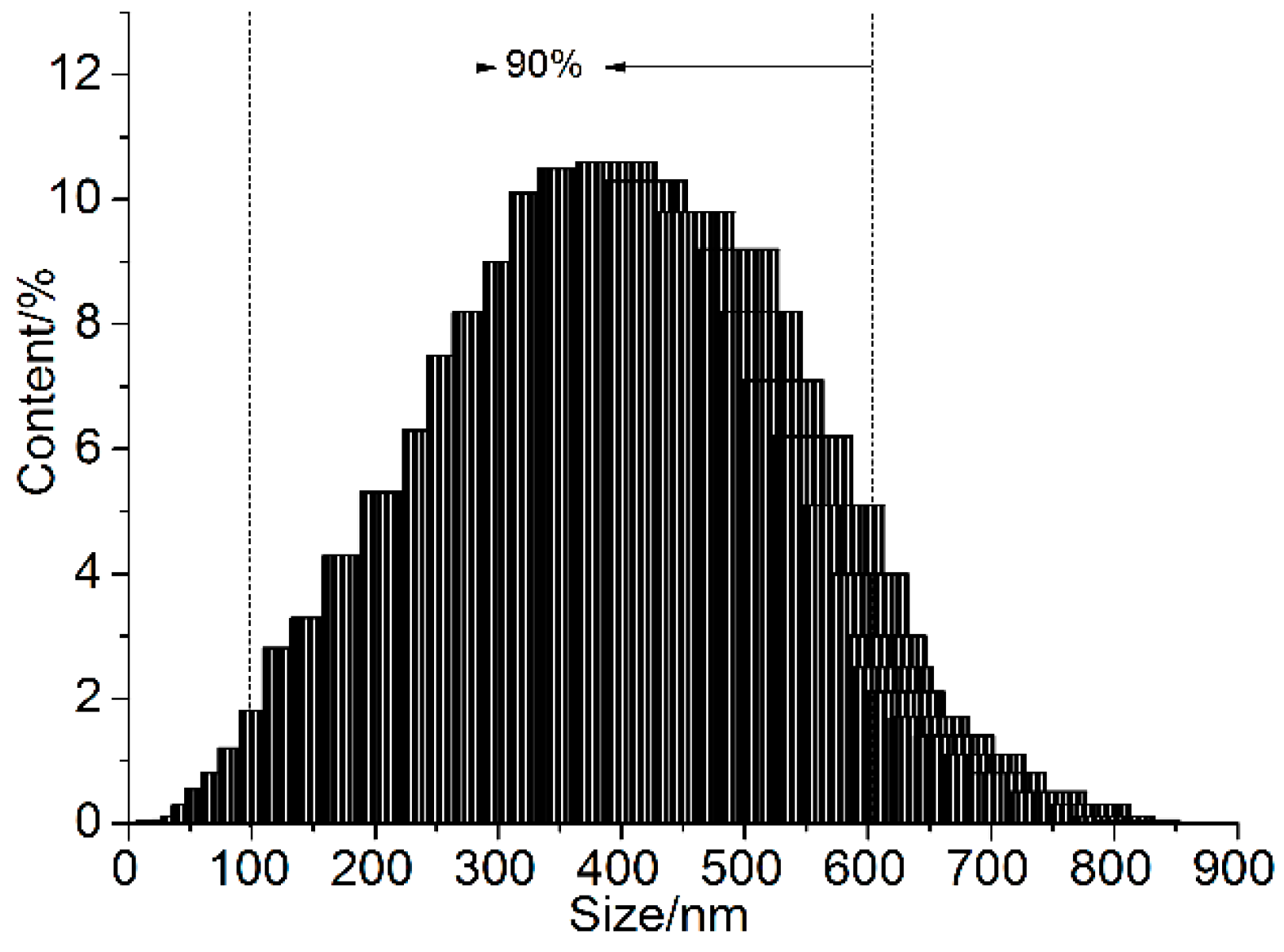
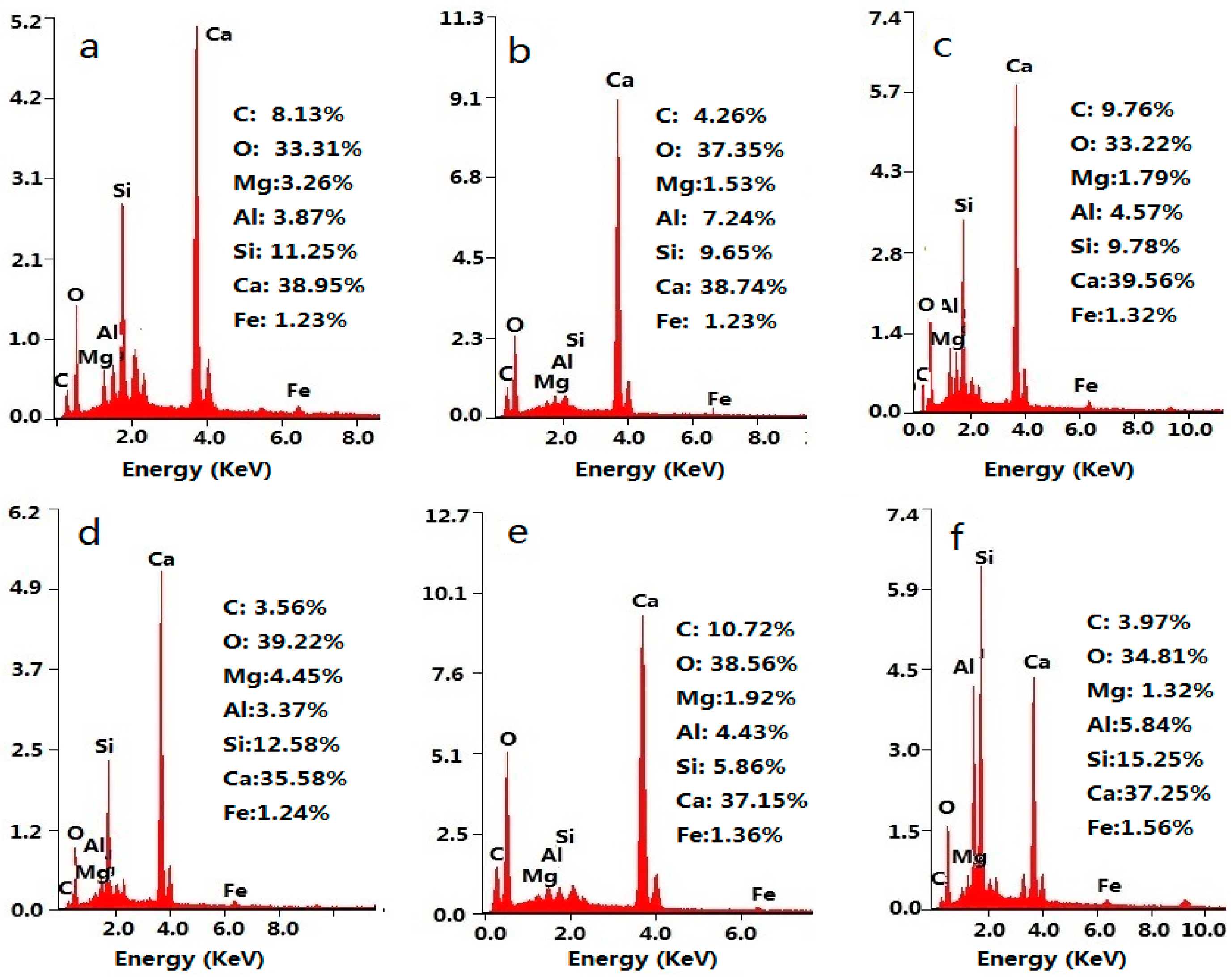
3.1. Structural Characterization of GONs
3.2. Microstructure of GON/Cement Composites
3.3. Strength and Durability of GON/Cement Composites
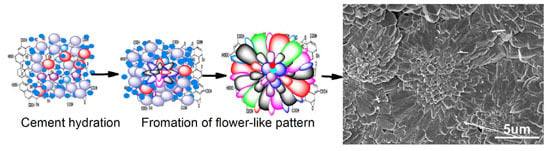
3.4. Formation Mechanism of Regular Cement Hydration Products
4. Conclusions
- (1)
- GONs can be used to control the formation of Portland cement hydration products into polyhedron-like crystals and an aggregate-forming ordered microstructure with flower-like patterns. The research results indicate that polyhedron products can transform into flower-like patterns and further form ordered microstructures with defect-free structures.
- (2)
- These cement hydration products are easier to grow in cracks and holes of the cement matrix; therefore, they can repair structural defects through growth. The result is the formation of regular and interpenetrating networks via the crosslinking and growth of polyhedron-like cement hydration crystals. This ordered network is a new kind of microstructure in cement composites, which can significantly enhance the strength and toughness of cement.
- (3)
- These results have major practical applications for the production of cement composites with high strength, high toughness, and long durability.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, W.W.; Ji, W.M.; Wang, Y.C.; Liu, Y.; Shen, R.X.; Xing, F. Investigation on the mechanical properties of a cement-based material containing carbon nanotube under drying and freeze–thaw conditions. Materials 2015, 8, 8780–8792. [Google Scholar] [CrossRef]
- Mokdad, F.; Chen, D.L.; Liu, Z.Y.; Xiao, B.L. Deformation and strengthening mechanisms of a carbon nanotube reinforced aluminum composite. Carbon 2016, 104, 64–77. [Google Scholar] [CrossRef]
- Liu, H.B.; Wang, X.Q.; Jiao, Y.B.; Sha, T. Experimental investigation of the mechanical and durability properties of crumb rubber concrete. Materials 2016, 9, 172. [Google Scholar] [CrossRef]
- Ahn, T.H.; Kim, H.G.; Ryou, J.S. New surface-treatment technique of concrete structures using crack repair stick with healing ingredients. Materials 2016, 9, 654. [Google Scholar] [CrossRef]
- Ghatefar, A.; El-Salakawy, E.; Bassuoni, M.T. Early-age restrained shrinkage cracking of GFRP-RC bridge deck slabs: Effect of environmental conditions. Cem. Concr. Compos. 2015, 64, 62–73. [Google Scholar] [CrossRef]
- Lameiras, R.; Barros, J.A.O.; Azenha, M. Influence of casting condition on the anisotropy of the fracture properties of steel fibre reinforced self-compacting concrete (SFRSCC). Cem. Concr. Compos. 2015, 59, 60–76. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.J.; Basheer, P.A.M.; Nanukuttan, S.V. Influence of service loading and the resulting micro-cracks on chloride resistance of concrete. Constr. Build. Mater. 2016, 108, 56–66. [Google Scholar] [CrossRef]
- Shen, D.J.; Jiang, J.L.; Shen, J.X. Influence of curing temperature on autogenous shrinkage and cracking resistance of high-performance concrete at an early age. Constr. Build. Mater. 2016, 103, 67–76. [Google Scholar] [CrossRef]
- Di Bella, C.; Wyrzykowski, M.; Griffa, M. Application of microstructurally-designed mortars for studying early-age properties:Microstructure and mechanical properties. Cem. Concr. Res. 2015, 78, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Scrivener, K.L.; Juilland, P.; Monteiro, P.J.M. Advances in understanding hydration of Portland cement. Cem. Concr. Res. 2015, 78, 38–56. [Google Scholar] [CrossRef]
- Sun, H.F.; Li, Z.S.S.; Memon, S.A.; Zhang, Q.W.; Wang, Y.C.; Liu, B.; Xu, W.T.; Xing, F. Influence of ultrafine 2CaO·SiO2 powder on hydration properties of reactive powder concrete. Materials 2015, 8, 6195–6207. [Google Scholar] [CrossRef]
- Quercia, G.; Lazaro, A.; Geus, J.W.; Brouwers, H.J.H. Characterization of morphology and texture of several amorphous nano-silica particles used in concrete. Cem. Concr. Compos. 2013, 44, 77–92. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kundu, S.P.; Roy, A.; Adhikari, B.; Majumder, S.B. Effect of jute as fiber reinforcement controlling the hydration characteristics of cement matrix. Ind. Eng. Chem. Res. 2013, 52, 1252–1260. [Google Scholar] [CrossRef]
- Ntafalias, E.; Koutsoukos, P.G. Spontaneous precipitation of calcium silicate hydrate in aqueous solutions. Cryst. Res. Technol. 2010, 45, 39–47. [Google Scholar] [CrossRef]
- Al-Tulaian, B.S.; Al-Shannag, M.J.; Al-Hozaimy, A.R. Recycled plastic waste fibers for reinforcing Portland cement mortar. Const. Build. Mater. 2016, 127, 102–110. [Google Scholar] [CrossRef]
- Mehran Khan, M.; Ali, M. Use of glass and nylon fibers in concrete for controlling early age micro cracking in bridge decks. Const. Build. Mater. 2016, 125, 800–808. [Google Scholar] [CrossRef]
- Deyu Kong, D.; Corr, D.J.; Hou, P.; Yang, Y.; Shah, S.P. Influence of colloidal silica sol on fresh properties of cement paste as compared to nano-silica powder with agglomerates in micron-scale. Cem. Concr. Compos. 2015, 63, 30–41. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Jiang, J.; Zhou, W.; Lai, L.; Xi, L.; Lam, Y.M.; Shen, Z.; Bahareh, K.; Yu, T. Influences of graphene oxide support on the electrochemical performances of graphene oxide–MnO2 nanocomposites. Nanoscale Res. Lett. 2011, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gil, G.A.; Sandra, M.A.; Cruz, A.; Ramalho, J.G.; Marques, A.A. Graphene oxide versus functionalized carbon nanotubes as a reinforcing agent in a PMMA/HA bone cement. Nanoscale 2012, 4, 2937–2945. [Google Scholar]
- Mujtaba, A.; Keller, M.; Ilisch, S.; Radusch, H.J.; Beiner, M.; Thurn-Albrecht, T.; Saalwachter, K. Detection of surface-immobilized components and their role in viscoelastic reinforcement of rubber-silica nanocomposites. ACS Macro Lett. 2014, 3, 481–485. [Google Scholar] [CrossRef]
- Mun, S.C.; Kim, M.; Prakashan, K.; Jung, H.J.; Son, Y.; Park, O.O. A new approach to determine rheological percolation of carbon nanotubes in microstructured polymer matrices. Carbon 2014, 67, 64–71. [Google Scholar] [CrossRef]
- Stein, J.; Lenczowski, B.; Anglaret, E.; Frety, N. Influence of the concentration and nature of carbon nanotubes on the mechanical properties of AA5083 aluminium alloy matrix composites. Carbon 2014, 77, 44–52. [Google Scholar] [CrossRef]
- Poulia, A.; Sakkas, P.M.; Kanellopoulou, D.G. Preparation of metal-ceramic composites by sonochemical synthesis of metallic nano-particles and in-situ decoration on ceramic powders. Ultrason. Sonochem. 2016, 3, 417–422. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, X.D.; Zhu, Q. Dispersion of nano-sized yttria powder using triammonium citrate dispersant for the fabrication of transparent ceramics. Ceram. Int. 2016, 42, 9737–9743. [Google Scholar] [CrossRef]
- Li, J.J.; Shao, L.S.; Zhou, X.H. Fabrication of high strength PVA/rGO composite fibers by gel spinning. RSC Adv. 2014, 4, 43612–43618. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Yu, G.; Jiang, W.; Mao, C. Self-assembly of molecule-like nanoparticle clusters directed by DNA nanocages. J. Am. Chem. Soc. 2015, 137, 4320–4323. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Jacovetty, E.; Liu, Y.; Yan, H. Encapsulation of gold nanoparticles in a DNA origami cage. Angew. Chem. Int. Ed. 2011, 50, 2041–2044. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Hosokawa, K.; Maeda, M. Rapid Aggregation of gold nanoparticles induced by non-cross-linking DNA hybridization. J. Am. Chem. Soc. 2003, 125, 8102–8103. [Google Scholar] [CrossRef] [PubMed]
- Storhofff, J.; Elghanian, R.; Mirkin, C.; Letsinger, R. Sequence dependent stability of DNA- modified gold nanoparticles. Langmuir 2002, 18, 6666–6670. [Google Scholar] [CrossRef]
- Lv, S.H.; Ma, Y.J.; Qiu, C.C.; Sun, T.; Liu, J.J.; Zhou, Q.F. Effect of graphene oxide nanosheets of microstructure and mechanical properties of cement composites. Constr. Build. Mater. 2013, 49, 121–127. [Google Scholar] [CrossRef]
- Lv, S.H.; Ma, Y.J.; Qiu, C.C.; Zhou, Q.F. Regulation of GO on cement hydration crystals and its toughening effect. Mag. Concr. Res. 2013, 65, 1246–1254. [Google Scholar] [CrossRef]
- Lv, S.H.; Deng, L.J.; Yang, W.Q.; Zhou, Q.F.; Cui, Y.Y. Fabrication of polycarboxylate/graphene oxide nanosheet composites using copolymerization, for reinforcing and toughening cement composites. Cem. Concr. Compos. 2016, 66, 1–9. [Google Scholar] [CrossRef]
- Lv, S.H.; Sun, T.; Liu, J.J.; Zhou, Q.F. Use of graphene oxide nanosheets to regulate the microstructure of hardened cement paste to increase its strength and toughness. Cryst. Eng. Commun. 2014, 16, 8508–8516. [Google Scholar] [CrossRef]
- Pan, Z.; He, L.; Qiu, L.; Korayem, A.H. Mechanical properties and microstructure of a graphene oxide–cement composite. Cem. Concr. Compos. 2015, 58, 140–147. [Google Scholar] [CrossRef]
- Horszczaruk, I.; Mijowska, E.; Kalenczu, R.J. Nanocomposite of cement/graphene oxide–Impact on hydration kinetics and Young’s modulus. Constr. Build. Mater. 2015, 78, 234–242. [Google Scholar] [CrossRef]









| Component | SiO2 | Al2O3 | MgO | CaO | Na2O | K2O | SO3 | Fe2O3 | P2O5 | TiO2 | MnO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 21.25 | 4.21 | 2.90 | 65.16 | 0.50 | 0.97 | 0.72 | 3.35 | 0.10 | 0.21 | 0.07 |
| Specimens | Total Pore Area (m2/g) | Median Pore Diameter (nm) | Average Pore Diameter (nm) | Porosity (%) |
|---|---|---|---|---|
| Control sample | 25.64 | 28.43 | 19.92 | 23.56 |
| Sample 1 | 13.38 | 12.27 | 10.66 | 9.32 |
| Sample 2 | 12.45 | 12.25 | 11.27 | 9.56 |
| Sample 3 | 13.36 | 11.68 | 10.67 | 8.34 |
| Sample 4 | 12.78 | 12.03 | 11.54 | 8.69 |
| Specimens | Compressive Strength (MPa) | Flexural Strength (MPa) | ||||
|---|---|---|---|---|---|---|
| 3 Days | 7 Days | 28 Days | 3 Days | 7 Days | 28 Days | |
| Control sample | 26.52 | 45.67 | 57.42 | 3.18 | 6.84 | 8.33 |
| GON/cement composites | 35.65 | 62.56 | 86.75 | 4.72 | 12.37 | 15.42 |
| Specimens | Penetration Resistance | (Freeze Thaw Cycles * 100 Times) | Carbonation Depth (mm) | ||||
|---|---|---|---|---|---|---|---|
| Osmotic Pressure (MPa) | Seepage Height (mm) | m0 (g) | mloss (g) | p (%) | 7 Days | 28 Days | |
| Control samples | 3.5 | 13.6 | 9818 | 0.04 | 71.2 | 2.8 | 3.5 |
| GON/cement composites | 3.5 | 3.8 | 9860 | 0 | 93.6 | 0.6 | 1.2 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, S.; Zhang, J.; Zhu, L.; Jia, C. Preparation of Cement Composites with Ordered Microstructures via Doping with Graphene Oxide Nanosheets and an Investigation of Their Strength and Durability. Materials 2016, 9, 924. https://doi.org/10.3390/ma9110924
Lv S, Zhang J, Zhu L, Jia C. Preparation of Cement Composites with Ordered Microstructures via Doping with Graphene Oxide Nanosheets and an Investigation of Their Strength and Durability. Materials. 2016; 9(11):924. https://doi.org/10.3390/ma9110924
Chicago/Turabian StyleLv, Shenghua, Jia Zhang, Linlin Zhu, and Chunmao Jia. 2016. "Preparation of Cement Composites with Ordered Microstructures via Doping with Graphene Oxide Nanosheets and an Investigation of Their Strength and Durability" Materials 9, no. 11: 924. https://doi.org/10.3390/ma9110924
APA StyleLv, S., Zhang, J., Zhu, L., & Jia, C. (2016). Preparation of Cement Composites with Ordered Microstructures via Doping with Graphene Oxide Nanosheets and an Investigation of Their Strength and Durability. Materials, 9(11), 924. https://doi.org/10.3390/ma9110924