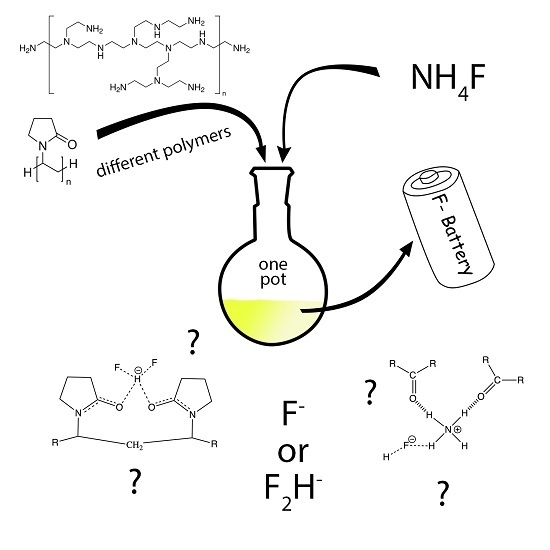
Simple One-Pot Syntheses and Characterizations of Free Fluoride- and Bifluoride-Containing Polymers Soluble in Non-Aqueous Solvents
Abstract
:1. Introduction
2. Results
2.1. Measuring Bifluoride in Solvent: An Approach Based on a Standard Fluoride Sensitive Electrode
2.2. Conductivity of the Fluoride Electrolytes and Reference Samples
2.3. NMR and IR Spectroscopic Results
2.4. Characterization with X-ray Diffraction
2.5. Optical Properties
2.6. Characterization Summary
3. Discussion
3.1. Electrochemical Stability and Impedance Spectroscopy
3.2. Preliminary Tests of Electrolytes in RT-FIB
4. Materials and Methods
4.1. Materials
4.2. Experimental Section
4.3. NH3-Evolution
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, D.W.; Icon, S.T.; Iyer, S.S. Handbook of Fluoropolymer Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Yoshio, M.; Brodd, R.J.; Kozawaka, A. Lithium-Ion Batteries Science and Technology; Springer: New York, NY, USA, 2007. [Google Scholar]
- Choi, S.W.; Fu, Y.Z.; Ahn, Y.R.; Jo, S.M.; Manthiram, A. Nafion-impregnated electrospun polyvinylidene fluoride composite membranes for direct methanol fuel cells. J. Power Sources 2008, 180, 167–171. [Google Scholar] [CrossRef]
- Hermann, A.; Chaudhuri, T.; Spagnol, P. Bipolar plates for PEM fuel cells: A review. Int. J. Hydrogen Energy 2005, 30, 1297–1302. [Google Scholar] [CrossRef]
- Liang, S.; Xiao, K.; Mo, Y.; Huang, X. A novel ZnO nanoparticle blended polyvinylidene fluoride membrane for anti-irreversible fouling. J. Membr. Sci. 2012, 394–395, 184–192. [Google Scholar] [CrossRef]
- Hester, J. Design and performance of foul-resistant poly(vinylidene fluoride) membranes prepared in a single-step by surface segregation. J. Membr. Sci. 2002, 202, 119–135. [Google Scholar] [CrossRef]
- Olah, G.A.; Li, X.-Y.; Wang, Q.; Prakash, G.K.S. Poly-4-vinylpyridinium Poly(Hydrogen Fluoride): A Solid Hydrogen Fluoride Equivalent Reagent. Synthesis 1993, 1993, 693–699. [Google Scholar] [CrossRef]
- Olah, G.A.; Li, X.-Y. Poly-4-vinylpyridinium Poly(hydrogen fluoride): A Convenient Polymeric Fluorinating Agent. Synlett 1990, 1990, 267–269. [Google Scholar] [CrossRef]
- Kim, D.W.; Jeong, H.-J.; Lim, S.T.; Sohn, M.-H. Tetrabutylammonium Tetra(tert-Butyl Alcohol)-Coordinated Fluoride as a Facile Fluoride Source. Angew. Chem. Int. Ed. 2008, 47, 8404–8406. [Google Scholar] [CrossRef] [PubMed]
- Bosch, P.; Camps, F.; Chamorro, E.; Gasol, V.; Guerrero, A. Tetrahutylammonium Bifluoride: A versatile and efficient fluorinating agent. Tetrahedron Lett. 1987, 28, 4733–4736. [Google Scholar] [CrossRef]
- Furin, G.G. Synthetic aspects of the use of organosilicon compounds under nucleophilic catalysis conditions. Tetrahedron 1983, 44, 2675–2749. [Google Scholar] [CrossRef]
- Banks, R.E.; Mohialdin-Khaffaf, S.N.; Lal, G.S.; Sharif, I.; Syvret, R.G. 1-Alkyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane salts: A novel family of electrophilic fluorinating agents. J. Chem. Soc. Chem. Commun. 1992, 595. [Google Scholar] [CrossRef]
- Landini, D.; Maia, A.; Rampoldi, A. Dramatic effect of the specific solvation on the reactivity of quaternary ammonium fluorides and poly(hydrogen fluorides), (HF)n*F−, in media of low polarity. J. Org. Chem. 1989, 54, 328–332. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.F.; Yoon, J. Fluorescence and Colorimetric Chemosensors for Fluoride-Ion Detection. Chem. Rev. 2014, 114, 5511–5571. [Google Scholar] [CrossRef] [PubMed]
- Hudnall, T.W.; Chiu, C.-W.; Gabbaï, F.P. Fluoride Ion Recognition by Chelating and Cationic Boranes. Acc. Chem. Res. 2009, 42, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Ryu, B.J.; Lee, Y.J.; Nam, K.C. Visible Colorimetric Fluoride Ion Sensors. Org. Lett. 2005, 7, 2607–2609. [Google Scholar] [CrossRef] [PubMed]
- Seppelt, K. Does the Naked Fluoride Ion Exist? Angew. Chem. Int. Ed. 1992, 31, 292–293. [Google Scholar] [CrossRef]
- Miller, J.M.; Clark, J.H. “Naked fluoride” masked: N.M.R. evidence for tight ion pairs in KF–crown ether. J. Chem. Soc. Chem. Commun. 1982, 1318–1319. [Google Scholar] [CrossRef]
- Kornath, A.; Neumann, F.; Oberhammer, H. Tetramethylphosphonium Fluoride: “Naked” Fluoride and Phosphorane. Inorg. Chem. 2003, 42, 2894–2901. [Google Scholar] [CrossRef] [PubMed]
- Emsley, J. Very strong hydrogen bonding. Chem. Soc. Rev. 1980, 9, 91–124. [Google Scholar] [CrossRef]
- Gschwind, F.; Zao-Karger, Z.; Fichtner, M. A fluoride-doped PEG matrix as an electrolyte for anion transportation in a room-temperature fluoride ion battery. J. Mater. Chem. A 2014, 2, 1214–1218. [Google Scholar] [CrossRef]
- Gschwind, F.; Bastien, J. Parametric investigation of room-temperature fluoride-ion batteries: Assessment of electrolytes, Mg-based anodes, and BiF3-cathodes. J. Mater. Chem. A 2015, 3, 5628–5634. [Google Scholar] [CrossRef]
- Anji Reddy, M.; Fichtner, M. Batteries based on fluoride shuttle. J. Mater. Chem. 2011, 21, 17059–17062. [Google Scholar] [CrossRef]
- Gschwind, F.; Rodriguez-Garcia, G.; Sandbeck, D.J.S.; Gross, A.; Weil, M.; Fichtner, M.; Hörmann, N. Fluoride ion batteries: Theoretical performance, safety, toxicity, and a combinatorial screening of new electrodes. J. Fluor. Chem. 2016, 182, 76–90. [Google Scholar] [CrossRef]
- Clark, J.H. Fluoride Ion as a Base in Organic Synthesis. Chem. Rev. 1980, 80, 429–452. [Google Scholar] [CrossRef]
- Dick, C.R.; Ham, G.E. Characterization of Polyethylenimine. J. Macromol. Sci. Part A—Chem. 1970, 4, 1301–1314. [Google Scholar] [CrossRef]
- Martin, J.S.; Fujiwara, F.Y. High Resolution Nuclear Magnetic Resonance Spectra of Bifluoride Ion and Its Homologues. Can. J. Chem. 1971, 49, 3071–3073. [Google Scholar] [CrossRef]
- Whittlesey, M.K.; Perutz, R.N.; Greener, B.; Moore, M.H. Synthesis, molecular structure and NMR spectroscopy of a transition-metal bifluoride complex: Formation via C–F activation or reaction with Et3N·3HF. Chem. Commun. 1997, 187–188. [Google Scholar] [CrossRef]
- Gerken, M.; Boatz, J.A.; Kornath, A.; Haiges, R.; Schneider, S.; Schroer, T.; Christe, K.O. The NMR shifts are not a measure for the nakedness of the fluoride anion. J. Fluor. Chem. 2002, 116, 49–58. [Google Scholar] [CrossRef]
- Fluorine NMR Database. Available online: http://www.chem.wisc.edu/areas/reich/handouts/nmr/f-data.htm (accessed on 16 September 2016).
- Holland, B. The thermal degradation of poly(vinyl acetate) measured by thermal analysis–Fourier transform infrared spectroscopy. Polymer 2002, 43, 2207–2211. [Google Scholar] [CrossRef]
- Lakard, S.; Herlem, G.; Lakard, B.; Fahys, B. Theoretical study of the vibrational spectra of polyethylenimine and polypropylenimine. J. Mol. Struct. 2004, 685, 83–87. [Google Scholar] [CrossRef]
- Ibrahim, M.; Alaam, M.; El-Haes, H.; Jalbout, A.F.; de Leon, A. Analysis of the structure and vibrational spectra of glucose and fructose. Eclét. Quím. 2006, 31, 15–21. [Google Scholar] [CrossRef]
- Friestag, G.K.; Branchaud, B.P. Ammonium Tetrafluoroborate, Encyclopedia of Reagents for Organic Synthesis. EEROS 2001. [Google Scholar] [CrossRef]
- Schlemper, E.O.; Hamilton, W.C. On the Structure of Trigonal Ammonium Flourosilicate. J. Chem. Phys. 1966, 45, 408–409. [Google Scholar] [CrossRef]
- Linares, J.M.; Powell, D.; Bowman-James, K. Ammonium based anion receptors. Coord. Chem. Rev. 2003, 240, 57–75. [Google Scholar] [CrossRef]
- Kubo, Y.; Yamamoto, M.; Ikeda, M.; Takeuchi, M.; Shinkai, S.; Yamaguchi, S.; Tamao, K. A Colorimetric and Ratiometric Fluorescent Chemosensor with Three Emission Changes: Fluoride Ion Sensing by a Triarylborane—Porphyrin Conjugate. Angew. Chem. Int. Ed. 2003, 42, 2036–2040. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ji, Y.; Huang, X.; Yang, X.; Gouda, P.-I.; Dudley, M. Fabrication and characterization of polycrystalline WO3 nanofibers and their application for ammonia sensing. J. Phys. Chem. B 2006, 110, 23777–23782. [Google Scholar] [CrossRef] [PubMed]
- Dubas, S.T.; Pimpan, V. Green synthesis of silver nanoparticles for ammonia sensing. Talanta 2008, 76, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.H.; Ku, H.-Y.; Kim, Y.; Kim, S.-G.; Kim, Y.K.; Son, H.S.; Ku, J.K. Fluorescence Sensing of Ammonium and Organoammonium Ions with Tripodal Oxazoline Receptors. Org. Lett. 2003, 5, 1419–1422. [Google Scholar] [CrossRef] [PubMed]
- Jose, D.A.; Kumar, D.K.; Ganguly, B.; Das, A. Efficient and Simple Colorimetric Fluoride Ion Sensor Based on Receptors Having Urea and Thiourea Binding Sites. Org. Lett. 2004, 6, 3445–3448. [Google Scholar] [CrossRef] [PubMed]
- Thiampanya, P.; Muangsin, N.; Pulpoka, B. Azocalix[4]arene Strapped Calix[4]pyrrole: A Confirmable Fluoride Sensor. Org. Lett. 2012, 14, 4050–4053. [Google Scholar] [CrossRef] [PubMed]
- Poonia, N.S.; Bajaj, A.V. Coordination chemistry of alkali and alkaline earth cations. Chem. Rev. 1979, 79, 1–57. [Google Scholar] [CrossRef]









| Theoretical Amount of F− Present in the Test Solution (ppm) | pH | Measured F− Amount (ppm) | Percentage of Monitored F− (%) |
|---|---|---|---|
| 1000 | 3.9 | 497 | 50 |
| 885 | 7.35 | 832 | 94 |
| 664 | 9.54 | 576 | 86 |
| Electrolyte | F− Concentration (ppm) |
|---|---|
| PVAC-F | 19 |
| PVP-F | 69 |
| PEI-F | 68 |
| TEPA-F | 2.4 |
| Xy-F Filtrate | 3.7 |
| Malto-F precipitate | 14 |
| Reference TGBF | 12.5 |
| Reference KF in DMSO (saturated) | 8 |
| Electrolyte | Conductivity µS/cm (0.1 mg in 4 mL MeCN) |
|---|---|
| PVAC-F | 229 |
| PVP-F | 417 |
| PEI-F | 176 * |
| TEPA-F | 120 * |
| Xy-F Filtrate | 5972 |
| Malto-F precipitate | 350 * |
| Malto-F filtrate | 6300 |
| Reference TGBF | 273 |
| Reference PVCA | 8 |
| Reference PEI | 41.5 |
| Reference TEPA | 65.5 |
| Reference Xylitol | 7.7 * |
| Reference Maltodextrin | 10.3 * |
| Reference KF in DMSO (saturated) | 8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinle, D.; Friedrich, L.; Bevilacqua, N.; Von Hauff, E.; Gschwind, F. Simple One-Pot Syntheses and Characterizations of Free Fluoride- and Bifluoride-Containing Polymers Soluble in Non-Aqueous Solvents. Materials 2016, 9, 965. https://doi.org/10.3390/ma9120965
Steinle D, Friedrich L, Bevilacqua N, Von Hauff E, Gschwind F. Simple One-Pot Syntheses and Characterizations of Free Fluoride- and Bifluoride-Containing Polymers Soluble in Non-Aqueous Solvents. Materials. 2016; 9(12):965. https://doi.org/10.3390/ma9120965
Chicago/Turabian StyleSteinle, Dominik, Laura Friedrich, Nico Bevilacqua, Elizabeth Von Hauff, and Fabienne Gschwind. 2016. "Simple One-Pot Syntheses and Characterizations of Free Fluoride- and Bifluoride-Containing Polymers Soluble in Non-Aqueous Solvents" Materials 9, no. 12: 965. https://doi.org/10.3390/ma9120965
APA StyleSteinle, D., Friedrich, L., Bevilacqua, N., Von Hauff, E., & Gschwind, F. (2016). Simple One-Pot Syntheses and Characterizations of Free Fluoride- and Bifluoride-Containing Polymers Soluble in Non-Aqueous Solvents. Materials, 9(12), 965. https://doi.org/10.3390/ma9120965