Electrochemical Corrosion Behavior of Ta2N Nanoceramic Coating in Simulated Body Fluid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coating Specimen Preparation
2.2. Phase and Microstructural Characterization
2.3. Mechanical Properties Measurements
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Microstructure and Phase Analysis
3.2. Mechanical Properties
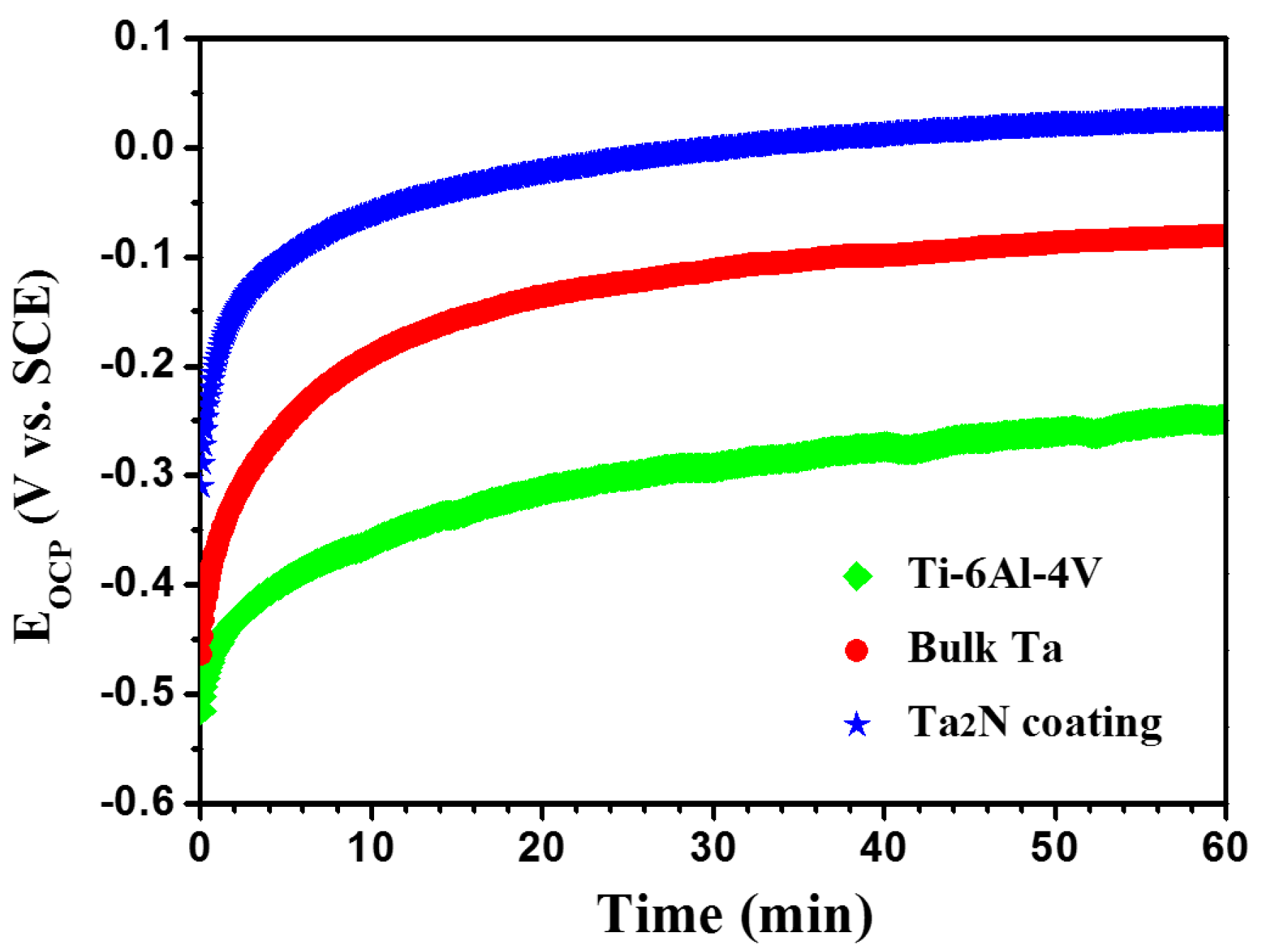
3.3. Open Circuit Potential Measurements
3.4. Potentiodynamic Polarization Tests
3.5. Electrochemical Impedance Spectroscopy (EIS) Measurements
3.6. Potentiostatic Polarization Tests
3.7. XPS Analysis of Passive Film Composition
3.8. Mott-Schottky Analysis and Point Defect Model
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpem, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Hanawa, T. Metal ion release from metal implants. Mater. Sci. Eng. C 2004, 24, 745–752. [Google Scholar] [CrossRef]
- Sun, Z.L.; Wataha, J.C.; Hanks, C.T. Effects of metal ions on osteoblast-like cell metabolism and differentiation. Biomed. J. Mater. Res. 1997, 34, 29–37. [Google Scholar] [CrossRef]
- Rao, S.; Ushida, T.; Tateishi, T.; Okazaki, Y.; Asao, S. Effect of Ti, Al and V ions on the relative growth rate of fibroblasts (L929) and osteoblasts (MC3T3-E1) cells. Bio-Med. Mater. Eng. 1996, 6, 79–86. [Google Scholar]
- Niinomi, M. Recent research and development in titanium alloys for biomedical applications and healthcare goods. Sci. Technol. Adv. Mater. 2003, 4, 445–454. [Google Scholar] [CrossRef]
- Hallab, N.J.; Jacobs, J.J. Orthopedic implant fretting corrosion. Corros. Rev. 2003, 21, 183–213. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Ching, H.A.; Choudhury, D.; Nine, M.J.; Osman, N.A.A. Effects of surface coating on reducing friction and wear of orthopaedic implants. Sci. Technol. Adv. Mater. 2014, 15, 014402. [Google Scholar] [CrossRef]
- Liu, L.L.; Xu, J.; Munroe, P.; Xu, J.K.; Xie, Z.-H. Electrochemical behavior of (Ti1–xNbx)5Si3 nanocrystalline films in simulated physiological media. Acta Biomater. 2014, 10, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hu, W.; Xu, S.; Munroe, P.; Xie, Z.-H. Electrochemical properties of a novel β-Ta2O5 nanoceramic coating exposed to simulated body solutions. ACS Biomater. Sci. Eng. 2016, 2, 73–89. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Mei, X.X.; Yang, D.Z.; Chen, F.X.; Ma, T.C.; Wang, Y.M.; Teng, F.N. Preparation, structure and properties of TaN and TaC films obtained by ion beam assisted deposition. Nucl. Instrum. Methods Phys. Res. B 1997, 127, 664–668. [Google Scholar] [CrossRef]
- Tsai, M.H.; Sun, S.C.; Lee, C.P.; Chiu, H.T.; Tsai, C.E.; Chuang, S.H.; Wu, S.C. Metal-organic chemical vapor deposition of tantalum nitride barrier layers for ULSI applications. Thin Solid Films 1995, 270, 531–536. [Google Scholar] [CrossRef]
- Lovejoy, M.L.; Patrizi, G.A.; Roger, D.J.; Barbour, J.C. Thin-film tantalum-nitride resistor technology for phosphide-based optoelectronics. Thin Solid Films 1996, 290, 513–517. [Google Scholar] [CrossRef]
- Leng, Y.X.; Sun, H.; Yang, P.; Chen, J.Y.; Wang, J.; Wan, G.J.; Huang, N.; Tian, X.B.; Wang, L.P.; Chu, P.K. Biomedical properties of tantalum nitride films synthesized by reactive magnetron sputtering. Thin Solid Films 2001, 398, 471–475. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Jiang, H.G.; Rühle, M.; Lavernia, E.J. On the applicability of the X-ray diffraction line profile analysis in extracting grain size and microstrain in nanocrystalline materials. J. Mater. Res. 1999, 14, 549–559. [Google Scholar] [CrossRef]
- Moody, N.R.; Medlin, D.; Boehme, D.; Norwood, D.P. Film thickness effects on the fracture of tantalum nitride on aluminum nitride thin film systems. Eng. Fract. Mech. 1998, 61, 107–118. [Google Scholar] [CrossRef]
- Nie, X.; Leyland, A.; Matthews, A. Deposition of layered bioceramic hydroxyapatite/TiO2 coatings on titanium alloys using a hybrid technique of micro-arc oxidation and electrophoresis. Surf. Coat. Technol. 2000, 125, 407–414. [Google Scholar] [CrossRef]
- Hogmark, S.; Jacobson, S.; Larsson, M. Design and evaluation of tribological coatings. Wear 2000, 246, 20–33. [Google Scholar] [CrossRef]
- Munoz, A.I.; Mischler, S. Interactive effects of albumin and phosphate ions on the corrosion of CoCrMo implant alloy. J. Electrochem. Soc. 2007, 154, C562–C570. [Google Scholar] [CrossRef]
- Chidambaram, D.; Clayton, C.R.; Dorfman, M.R. Evaluation of the electrochemical behavior of HVOF-sprayed alloy coating. Surf. Coat. Technol. 2004, 176, 307–317. [Google Scholar] [CrossRef]
- Starosvetsky, D.; Gotman, I. Corrosion behavior of titanium nitride coated Ni-Ti shape memory surgical alloy. Biomaterials 2001, 22, 1853–1859. [Google Scholar] [CrossRef]
- Creus, J.; Mazille, H.; Idrissi, H. Porosity evaluation of protective coatings onto steel, through electrochemical techniques. Surf. Coat. Technol. 2000, 130, 224–232. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical polarization I. A theoretical analysis of the shapes of polarization curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Pacha-Olivenza, M.A.; Gallardo-Moreno, A.M.; Vadillo-Rodríguez, V.; González-Martín, M.L.; Péres-Giraldo, C.; Galván, J.C. Electrochemical analysis of the UV treated bactericidal Ti6Al4V surfaces. Mater. Sci. Eng. C 2013, 33, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Córdoba-Torres, P.; Mesquita, T.J.; Devos, O.; Tribollet, B.; Roche, V.; Nogueira, R.P. On the intrinsic coupling between constant-phase element parameters α and Q in electrochemical impedance spectroscopy. Electrochim. Acta 2012, 72, 172–178. [Google Scholar] [CrossRef]
- Potucek, R.K., Jr.; Rateick, R.G.; Birss, V.I. Impedance characterization of anodic barrier Al oxide film beneath porous oxide layer. J. Electrochem. Soc. 2006, 153, B304–B310. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Jiang, P.; Lin, L.; Zhang, F.; Dong, X.; Ren, L.; Lin, C. Electrochemical construction of micro-nano spongelike structure on titanium substrate for enhancing corrosion resistance and bioactivity. Electrochim. Acta 2013, 107, 16–25. [Google Scholar] [CrossRef]
- Gray, J.J.; Orme, C.A. Electrochemical impedance spectroscopy study of the passive films of alloy 22 in low pH nitrate and chloride environments. Electrochim. Acta 2007, 52, 2370–2375. [Google Scholar] [CrossRef]
- Labjar, N.; Lebrini, M.; Bentiss, F.; Chihib, N.-E.; Hajjaji, S.E.; Jama, C. Corrosion inhibition of carbon steel and antibacterial properties of aminotris-(methylenephosphonic) acid. Mater. Chem. Phys. 2010, 119, 330–336. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Urquidi-Macdonald, M. Theory of steady-state passive films. J. Electrochem. Soc. 1990, 137, 2395–2402. [Google Scholar] [CrossRef]
- Lakatos-Varsányi, M.; Falkenberg, F.; Olefjord, I. The influence of phosphate on repassivation of 304 stainless steel in neutral chloride solution. Electrochim. Acta 1998, 43, 187–197. [Google Scholar] [CrossRef]
- Chang, C.-C.; Jeng, J.S.; Chen, J.S. Microstructural and electrical characteristics of reactively sputtered Ta-N thin films. Thin Solid Films 2002, 413, 46–51. [Google Scholar] [CrossRef]
- Lamour, P.; Fioux, P.; Ponche, A.; Nardin, M.; Vallat, M.-F.; Dugay, P.; Brun, J.-P.; Moreaud, N.; Pinvidic, J.-M. Direct measurement of the nitrogen content by XPS in self-passivated TaNx thin films. Surf. Interface Anal. 2008, 40, 1430–1437. [Google Scholar] [CrossRef]
- Atanassova, E.; Spassov, D. X-ray photoelectron spectroscopy of thermal thin Ta2O5 films on Si. Appl. Surf. Sci. 1998, 135, 71–82. [Google Scholar] [CrossRef]
- Olefjord, I.; Wegrelius, L. The fluence of nitrogen on the passivation of stainless steels. Corros. Sci. 1996, 38, 1203–1220. [Google Scholar] [CrossRef]
- Chun, W.-J.; Ishikawa, A.; Fujisawa, H.; Takata, T.; Kondo, J.N.; Hara, M.; Kawai, M.; Matsumoto, Y.; Domen, K. Conduction and valence band positions of Ta2O5, TaON, and Ta3N5 by UPS and electrochemical methods. J. Phys. Chem. B 2003, 107, 1798–1803. [Google Scholar] [CrossRef]
- Escrivà-Cerdán, C.; Blasco-Tamarit, E.; García-García, D.M.; García-Antón, J.; Guenbour, A. Effect of potential formation on the electrochemical behaviour of a highly alloyed austenitic stainless steel in contaminated phosphoric acid at different temperatures. Electrochim. Acta 2012, 80, 248–256. [Google Scholar] [CrossRef]
- Jovic, V.D.; Barsoum, M.W. Corrosion behavior and passive film characteristics formed on Ti, Ti3SiC2, and Ti4AlN3 in H2SO4 and HCl. J. Electrochem. Soc. 2004, 151, B71–B76. [Google Scholar] [CrossRef]
- Morrison, S.R. Electrochemistry at Semiconductor and Oxidized Metal Electrodes; Plenum Press: New York, NY, USA, 1980. [Google Scholar]
- Kerrec, O.; Devilliers, D.; Groult, H.; Chemla, M. Dielectric properties of anodic films on tantalum. Electrochim. Acta 1995, 40, 719–724. [Google Scholar] [CrossRef]
- Milošev, I.; Metikoš-Huković, M.; Strehblow, H.-H. Passive film on orthopaedic TiAlV alloy formed in physiological solution investigated by X-ray photoelectron spectroscopy. Biomaterials 2000, 21, 2103–2113. [Google Scholar] [CrossRef]
- Martini, E.M.A.; Muller, I.L. Characterization of the film formed on iron in borate solution by electrochemical impedance spectroscopy. Corros. Sci. 2000, 42, 443–454. [Google Scholar] [CrossRef]
- Li, D.G.; Wang, J.D.; Chen, D.R. Influence of potentiostatic aging, temperature and pH on the diffusivity of a point defect in the passive film on Nb in an HCl solution. Electrochim. Acta 2012, 60, 134–146. [Google Scholar] [CrossRef]
- Jović, V.D.; Jović, B.M. Properties of ZrO2 passive film formed onto Zr electrode in 1 m NaOH at low voltage. J. Electrochem. Soc. 2008, 155, C183–C188. [Google Scholar] [CrossRef]
- Li, D.G. Effect of ultrasonic cavitation on the diffusivity of a point defect in the passive film on formed Nb in 0.5 M HCl solution. Ultrason. Sonochem. 2015, 27, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Xu, J.; Lu, X.L.; Munroe, P.; Xie, Z.-H. Electrochemical corrosion behavior of nanocrystalline β-Ta coating for biomedical applications. ACS Biomater. Sci. Eng. 2016, 2, 579–594. [Google Scholar] [CrossRef]
- Macdonald, D.D. Passivity–the key to our metals-based civilization. Pure Appl. Chem. 1999, 71, 951–978. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Azambuja, D.S.; Martini, E.M.A. Semiconductive properties of titanium anodic oxide films in McIlvaine buffer solution. Corros. Sci. 2006, 48, 2901–2912. [Google Scholar] [CrossRef]
- Harrington, S.P.; Devine, T.M. Impedance study of alloy 22 in hydrochloric acid using a semiconductor model. ECS Trans. 2009, 19, 131–148. [Google Scholar]
- Katkar, V.A.; Gunasekaran, G.; Rao, A.G.; Koli, P.M. Effect of the reinforced boron carbide particulate content of AA6061 alloy on formation of the passive film in seawater. Corros. Sci. 2011, 53, 2700–2712. [Google Scholar] [CrossRef]
- Kang, J.; Yang, Y.; Jiang, X.; Shao, H. Semiconducting properties of passive films formed on electroplated Ni and Ni-Co alloys. Corros. Sci. 2008, 50, 3576–3580. [Google Scholar] [CrossRef]
- Metikoš-Huković, M.; Grubač, Z. Characterization of electronic and dielectric properties of anodic oxide films on bismuth by electrochemical impedance spectroscopy. J. Phys. Chem. B 1998, 102, 7406–7412. [Google Scholar] [CrossRef]
- Martin, F.J.; Cheek, G.T.; O’Grady, W.E.; Natishan, P.M. Impedance studies of the passive film on aluminium. Corros. Sci. 2005, 47, 3187–3201. [Google Scholar] [CrossRef]
- Kong, D.-S.; Lu, W.-H.; Feng, Y.-Y.; Yu, Z.-Y.; Wu, J.-X.; Fan, W.-J.; Liu, H.-Y. Studying on the point-defect-conductive property of the semiconducting anodic oxide films on titanium. J. Electrochem. Soc. 2009, 156, C39–C44. [Google Scholar] [CrossRef]
- Macdonald, D.D. The history of the Point Defect Model for the passive state: A brief review of film growth aspects. Electrochim. Acta 2011, 56, 1761–1772. [Google Scholar] [CrossRef]
- Jargelius-Pettersson, R.F.A. Electrochemical investigation of the influence of nitrogen alloying on pitting corrosion of austenitic stainless steels. Corros. Sci. 1999, 41, 1639–1664. [Google Scholar] [CrossRef]








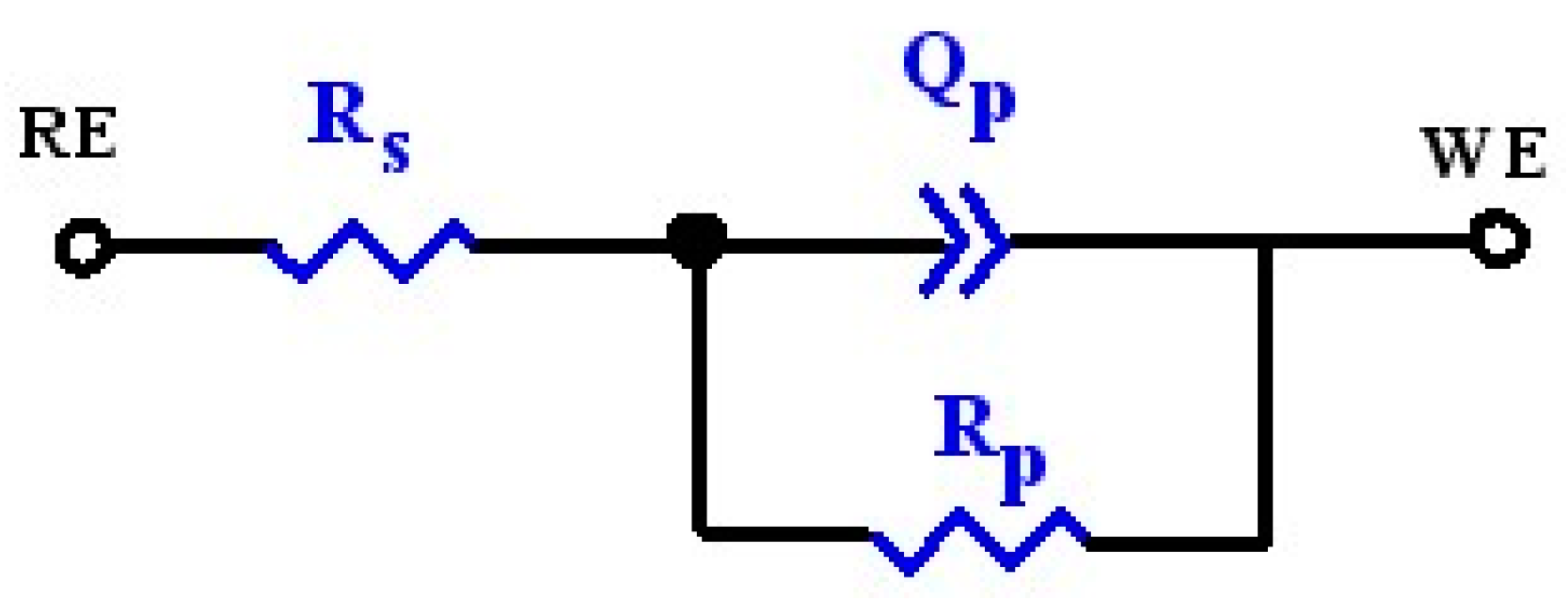





| Samples | Ti-6Al-4V | Pure Ta | Ta2N Coating |
|---|---|---|---|
| Ecorr (V vs. SCE) | −0.24 | −0.21 | −0.12 |
| βa (mV/decade) | 158.08 | 249.25 | 305.16 |
| -βc (mV/decade) | 116.48 | 118.04 | 120.63 |
| icorr (A·cm−2) | 4.20 × 10−7 | 2.99 × 10−7 | 6.76 × 10−9 |
| ipass 1 (A·cm−2) | 9.86 × 10−6 | 3.63 × 10−6 | 4.55 × 10−8 |
| Rp (Ω·cm2) | 6.93 × 104 | 1.16 × 105 | 5.55 × 106 |
| p (%) | – | – | 0.22 |
| Samples | Ti-6Al-4V | Pure Ta | Ta2N Coating |
|---|---|---|---|
| Rs (Ω·cm2) | 26.25 ± 0.17 | 25.21 ± 0.20 | 34.74 ± 0.35 |
| Qp (Ω−1·cm−2·sn) | (1.15 ± 0.01) × 10−5 | (1.26 ± 0.01) × 10−5 | (5.72 ± 0.05) × 10−6 |
| n | 0.911 ± 0.001 | 0.919 ± 0.001 | 0.941 ± 0.002 |
| Rp (Ω·cm2) | (5.75 ± 0.15) × 105 | (1.21 ± 0.04) × 106 | (4.70 ± 0.31) × 106 |
| Cp (μF·cm−2) | 8.43 | 6.19 | 3.35 |
| τ (s) | 4.85 | 7.49 | 15.75 |
| χ2 | 9.35 × 10−4 | 5.61 × 10−4 | 6.33 × 10−4 |
| Samples | Ti-6Al-4V | Pure Ta | Ta2N Coating | |
|---|---|---|---|---|
| 0.4 V | Nd (×1019 cm−3) | 11.13 | 17.26 | 1.48 |
| Efb (V) | −1.15 | −1.66 | −1.50 | |
| δsc (nm) | 9.61 | 5.74 | 18.85 | |
| 0.6 V | Nd (×1019 cm−3) | 5.92 | 13.75 | 1.10 |
| Efb (V) | −0.90 | −1.57 | −1.49 | |
| δsc (nm) | 12.96 | 6.60 | 22.93 | |
| 0.8 V | Nd (×1019 cm−3) | 3.42 | 11.53 | 0.80 |
| Efb (V) | −0.80 | −1.53 | −1.47 | |
| δsc (nm) | 17.61 | 7.47 | 28.02 | |
| 1.0 V | Nd (×1019 cm−3) | 2.20 | 10.81 | 0.62 |
| Efb (V) | −0.78 | −1.52 | −1.48 | |
| δsc (nm) | 23.16 | 8.02 | 33.27 | |
| ω2 (×1019 cm−3) | 1.08 | 9.62 | 0.15 | |
| iss (×10−6A·cm−2) | 9.50 | 3.49 | 0.10 | |
| Do (×10−16 cm2/s) | 27.33 | 1.13 | 1.94 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Xu, J.; Liu, L.L.; Jiang, S. Electrochemical Corrosion Behavior of Ta2N Nanoceramic Coating in Simulated Body Fluid. Materials 2016, 9, 772. https://doi.org/10.3390/ma9090772
Cheng J, Xu J, Liu LL, Jiang S. Electrochemical Corrosion Behavior of Ta2N Nanoceramic Coating in Simulated Body Fluid. Materials. 2016; 9(9):772. https://doi.org/10.3390/ma9090772
Chicago/Turabian StyleCheng, Jian, Jiang Xu, Lin Lin Liu, and Shuyun Jiang. 2016. "Electrochemical Corrosion Behavior of Ta2N Nanoceramic Coating in Simulated Body Fluid" Materials 9, no. 9: 772. https://doi.org/10.3390/ma9090772
APA StyleCheng, J., Xu, J., Liu, L. L., & Jiang, S. (2016). Electrochemical Corrosion Behavior of Ta2N Nanoceramic Coating in Simulated Body Fluid. Materials, 9(9), 772. https://doi.org/10.3390/ma9090772






