Development and Evaluation of Ethosomes Loaded with Zingiber zerumbet Linn Rhizome Extract for Antifungal Skin Infection in Deep Layer Skin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Z. zerumbet (L.) Extract
2.2.2. Ethosome Preparation
2.2.3. Physical Evaluation of Ethosomes
- Vesicle size, size distribution, and zeta potential
- Morphology
2.2.4. Entrapment Efficiency (EE) of Ethosomes
2.2.5. Antifungal Activities of Ethosomes Loaded with Z. zerumbet (L.) Rhizome Extract
2.2.6. In Vitro Skin Penetration Studies of Ethosomes Loaded with Z. zerumbet (L.) Rhizome Extract
2.2.7. In Vitro Skin Retention Studies of Ethosomes Loaded with Z. zerumbet (L.) Rhizome Extract
2.2.8. Statistical Analysis
3. Results and Discussion
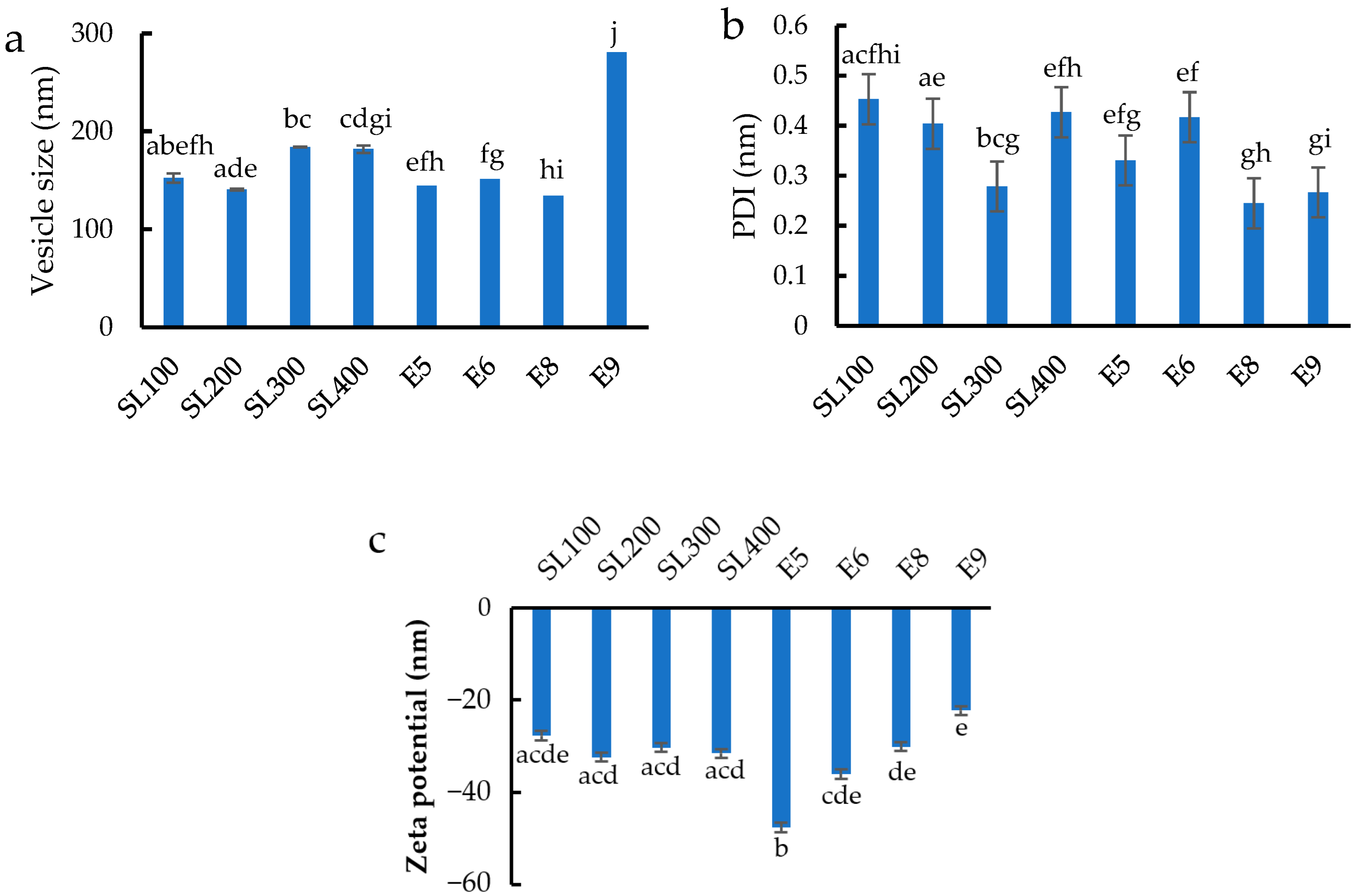
3.1. Vesicle Size, Size Distribution, and Zeta Potential
3.2. Morphology
3.3. Entrapment Efficiency of Ethosomes
3.4. Antifungal Activities of Ethosomes Loaded with Z. zerumbet (L.) Rhizome Extract
3.5. In Vitro Skin Penetration Studies of Ethosomes Loaded with Z. zerumbet (L.) Rhizome Extract
3.6. In Vitro Skin Retention Studies of Ethosomes Loaded with Z. zerumbet (L.) Rhizome Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Fungal Diseases. 2018. Available online: http://www.cdc.gov/ncezid/dfwed/mycotics (accessed on 16 January 2022).
- Boral, H.; Metin, B.; Döğen, A.; Seyedmousavi, S.; Ilkit, M. Overview of selected virulence attributes in Aspergillus fumigatus, Candida albicans, Cryptococcus neoformans, Trichophyton rubrum, and Exophiala dermatitidis. Fungal Genet. Biol. 2018, 111, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Bouz, G.; Doležal, M. Advances in Antifungal Drug Development: An Up-To-Date Mini Review. Pharmaceuticals 2021, 14, 1312. [Google Scholar] [CrossRef]
- Felton, T.; Troke, P.F.; Hope, W.W. Tissue penetration of antifungal agents. Clin. Microbiol. Rev. 2014, 27, 68–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rattanachitthawat, N.; Sriamornsak, P.; Puri, V.; Singh, I.; Huanbutta, K.; Sangnim, T. Bioactivity assessment of Zingiber zerumbet Linn rhizome extract for topical treatment of skin diseases. J. Appl. Pharm. Sci. 2022. [Google Scholar] [CrossRef]
- Roskov, Y.; Orrell, T.; Abucay, L.; Paglinawan, L.; Culham, A.; Bailly, N.; Kirk, P.; Bourgoin, T.; Baillargeon, G.; Decock, W.; et al. (Eds.) Species 2000 & ITIS Catalogue of Life, 2014 Annual Checklist; Naturalis: Leiden, The Netherlands, 2000; Available online: www.catalogueoflife.org/annual-checklist/2014 (accessed on 3 February 2022).
- Verma, P.; Pathak, K. Therapeutic and cosmeceutical potential of ethosomes: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paolino, D.; Mancuso, A.; Cristiano, M.C.; Froiio, F.; Lammari, N.; Celia, C.; Fresta, M. Nanonutraceuticals: The New Frontier of Supplementary Food. Nanomaterials 2021, 11, 792. [Google Scholar] [CrossRef] [PubMed]
- Natsheh, H.; Vettorato, E.; Touitou, E. Ethosomes for dermal administration of natural active molecules. Curr. Pharm. Des. 2019, 25, 2338–2348. [Google Scholar] [CrossRef]
- Rahman, H.S.; Rasedee, A.; How, C.W.; Abdul, A.B.; Zeenathul, N.A.; Othman, H.H.; Saeed, M.I.; Yeap, S.K. Zerumbone-loaded nanostructured lipid carriers: Preparation, characterization, and antileukemic effect. Int. J. Nanomed. 2013, 8, 2769. [Google Scholar] [CrossRef] [Green Version]
- Anyanwu, C. Investigation of in vitro antifungal activity of honey. J. Med. Plants Res. 2012, 6, 3512–3516. [Google Scholar] [CrossRef]
- Subongkot, T.; Sirirak, T. Development and skin penetration pathway evaluation of microemulsions for enhancing the dermal delivery of celecoxib. Colloids Surf. B Biointerfaces 2020, 193, 111103. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Lima, S.A.C.; Reis, S. Development of methotrexate loaded fucoidan/chitosan nanoparticles with anti-inflammatory potential and enhanced skin permeation. Int. J. Biol. Macromol. 2019, 124, 1115–1122. [Google Scholar] [CrossRef]
- Duangjit, S.; Pamornpathomkul, B.; Opanasopit, P.; Rojanarata, T.; Obata, Y.; Takayama, K.; Ngawhirunpat, T. Role of the charge, carbon chain length, and content of surfactant on the skin penetration of meloxicam-loaded liposomes. Int. J. Nanomed. 2014, 9, 2005. [Google Scholar] [CrossRef] [Green Version]
- Subongkot, T.; Ngawhirunpat, T.; Opanasopit, P. Development of ultradeformable liposomes with fatty acids for enhanced dermal rosmarinic acid delivery. Pharmaceutics 2021, 13, 404. [Google Scholar] [CrossRef]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.K.; Abou Assi, R.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279. [Google Scholar] [CrossRef] [Green Version]
- Puri, R.; Jain, S. Ethogel topical formulation for increasing the local bioavailability of 5-fluorouracil: A mechanistic study. Anti-Cancer Drugs 2012, 23, 923–934. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Liu, J.; He, Z.; Ding, C.; Huang, G.; Zhou, W.; Zhou, L. Preparation of a ligustrazine ethosome patch and its evaluation in vitro and in vivo. Int. J. Nanomed. 2011, 6, 241. [Google Scholar] [CrossRef] [Green Version]
- Paolino, D.; Lucania, G.; Mardente, D.; Alhaique, F.; Fresta, M. Ethosomes for skin delivery of ammonium glycyrrhizinate: In vitro percutaneous permeation through human skin and in vivo anti-inflammatory activity on human volunteers. J. Control. Release 2005, 106, 99–110. [Google Scholar] [CrossRef]
- Limsuwan, T.; Amnuaikit, T. Development of ethosomes containing mycophenolic acid. Procedia Chem. 2012, 4, 328–335. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, S. DLS and zeta potential–what they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Shah, R.; Eldridge, D.; Palombo, E.; Harding, I. Optimisation and stability assessment of solid lipid nanoparticles using particle size and zeta potential. J. Phys. Sci. 2014, 25, 59–75. [Google Scholar]
- Paolino, D.; Ventura, C.A.; Nistico, S.; Puglisi, G.; Fresta, M. Lecithin microemulsions for the topical administration of ketoprofen: Percutaneous adsorption through human skin and in vivo human skin tolerability. Int. J. Pharm. 2002, 244, 21–31. [Google Scholar] [CrossRef]
- Chen, W.; Soucie, W. Modification of surface charges of soy protein by phospholipids. J. Am. Oil Chem. Soc. 1985, 62, 1686–1689. [Google Scholar] [CrossRef]
- Tiwari, R.; Tiwari, G.; Wal, P.; Wal, A.; Maurya, P. Development, characterization and transdermal delivery of dapsone and an antibiotic entrapped in ethanolic liposomal gel for the treatment of lapromatous leprosy. Open Nanomed. J. 2018, 5, 1–15. [Google Scholar] [CrossRef]
- Rahman, H.S.; Rasedee, A.; Yeap, S.K.; Othman, H.H.; Chartrand, M.S.; Namvar, F.; Abdul, A.B.; How, C.W. Biomedical properties of a natural dietary plant metabolite, zerumbone, in cancer therapy and chemoprevention trials. BioMed Res. Int. 2014, 2014, 920742. [Google Scholar] [CrossRef] [Green Version]
- Iizhar, S.A.; Syed, I.A.; Satar, R.; Ansari, S.A. In vitro assessment of pharmaceutical potential of ethosomes entrapped with terbinafine hydrochloride. J. Adv. Res. 2016, 7, 453–461. [Google Scholar] [CrossRef]
- Lin, M.; Qi, X.-R. Purification method of drug-loaded liposome. In Liposome-Based Drug Delivery Systems; Springer: Berlin/Heidelberg, Germany, 2021; pp. 111–121. [Google Scholar] [CrossRef]
- Samad, A.; Sultana, Y.; Aqil, M. Liposomal drug delivery systems: An update review. Curr. Drug Deliv. 2007, 4, 297–305. [Google Scholar] [CrossRef]
- Kesharwani, S.S.; Bhat, G.J. Formulation and Nanotechnology-Based Approaches for Solubility and Bioavailability Enhancement of Zerumbone. Medicina 2020, 56, 557. [Google Scholar] [CrossRef]
- Choi, M.; Maibach, H. Liposomes and niosomes as topical drug delivery systems. Ski. Pharmacol. Physiol. 2005, 18, 209–219. [Google Scholar] [CrossRef]







| Ingredients | Formulations | |||||||
|---|---|---|---|---|---|---|---|---|
| SL100 | SL200 | SL300 | SL400 | E5 | E6 | E8 | E9 | |
| Soya lecithin (mg) | 100 | 200 | 300 | 400 | 200 | 200 | 200 | 200 |
| Ethanol (mL) | 7 | 7 | 7 | 7 | 5 | 6 | 8 | 9 |
| Polyethylene glycol 4000 (mg) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Water qs (mL) | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Z. zerumbet (L.) Rhizome Extract Concentration (µg/mL) | AUC | %Zerumbone Entrapment |
|---|---|---|
| 156.25 | 1,375,327 | 24.42 ± 0.04 |
| 312.5 | 2,435,877 | 31.58 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huanbutta, K.; Rattanachitthawat, N.; Luangpraditkun, K.; Sriamornsak, P.; Puri, V.; Singh, I.; Sangnim, T. Development and Evaluation of Ethosomes Loaded with Zingiber zerumbet Linn Rhizome Extract for Antifungal Skin Infection in Deep Layer Skin. Pharmaceutics 2022, 14, 2765. https://doi.org/10.3390/pharmaceutics14122765
Huanbutta K, Rattanachitthawat N, Luangpraditkun K, Sriamornsak P, Puri V, Singh I, Sangnim T. Development and Evaluation of Ethosomes Loaded with Zingiber zerumbet Linn Rhizome Extract for Antifungal Skin Infection in Deep Layer Skin. Pharmaceutics. 2022; 14(12):2765. https://doi.org/10.3390/pharmaceutics14122765
Chicago/Turabian StyleHuanbutta, Kampanart, Napapat Rattanachitthawat, Kunlathida Luangpraditkun, Pornsak Sriamornsak, Vivek Puri, Inderbir Singh, and Tanikan Sangnim. 2022. "Development and Evaluation of Ethosomes Loaded with Zingiber zerumbet Linn Rhizome Extract for Antifungal Skin Infection in Deep Layer Skin" Pharmaceutics 14, no. 12: 2765. https://doi.org/10.3390/pharmaceutics14122765
APA StyleHuanbutta, K., Rattanachitthawat, N., Luangpraditkun, K., Sriamornsak, P., Puri, V., Singh, I., & Sangnim, T. (2022). Development and Evaluation of Ethosomes Loaded with Zingiber zerumbet Linn Rhizome Extract for Antifungal Skin Infection in Deep Layer Skin. Pharmaceutics, 14(12), 2765. https://doi.org/10.3390/pharmaceutics14122765









