The Influence of Tree Structural and Species Diversity on Temperate Forest Productivity and Stability in Korea
Abstract
:1. Introduction
2. Materials and Methods
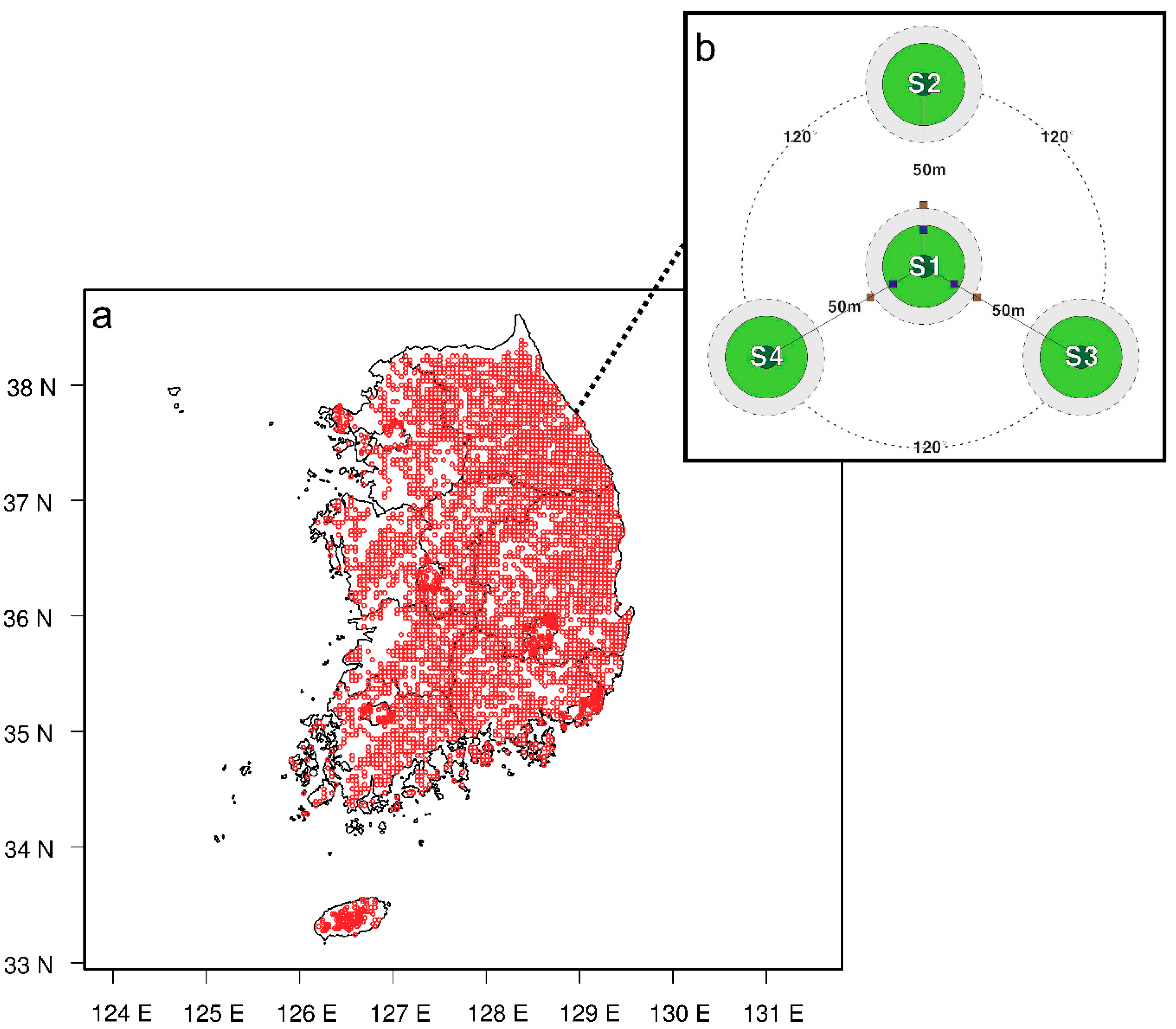
2.1. Study Area and Inventory Method
2.2. Forest Productivity Estimation
2.3. Tree Species and Size Diversity
2.4. Environmental Conditions
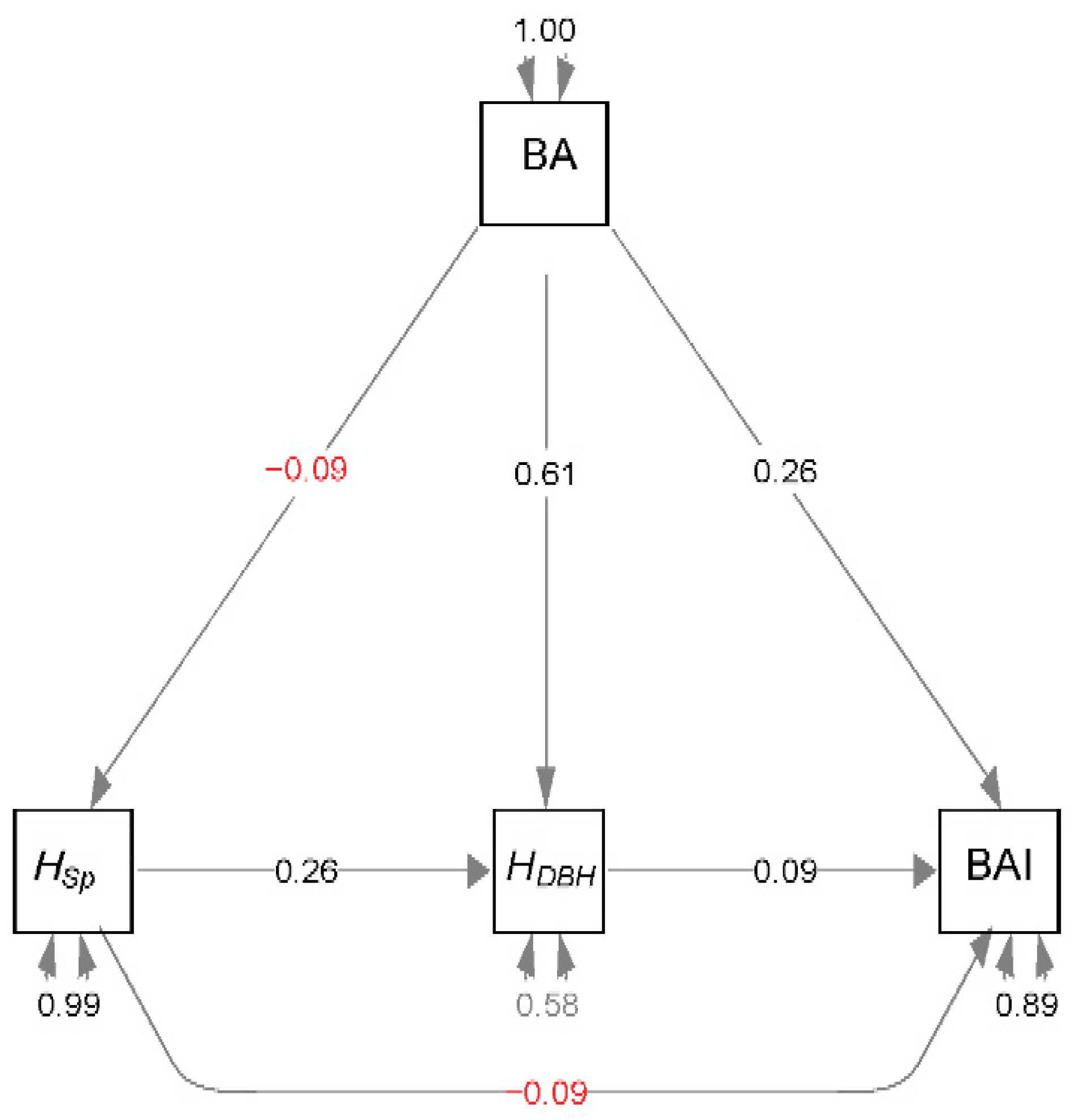
2.5. Structural Equation Modelling
2.6. Forest Stability
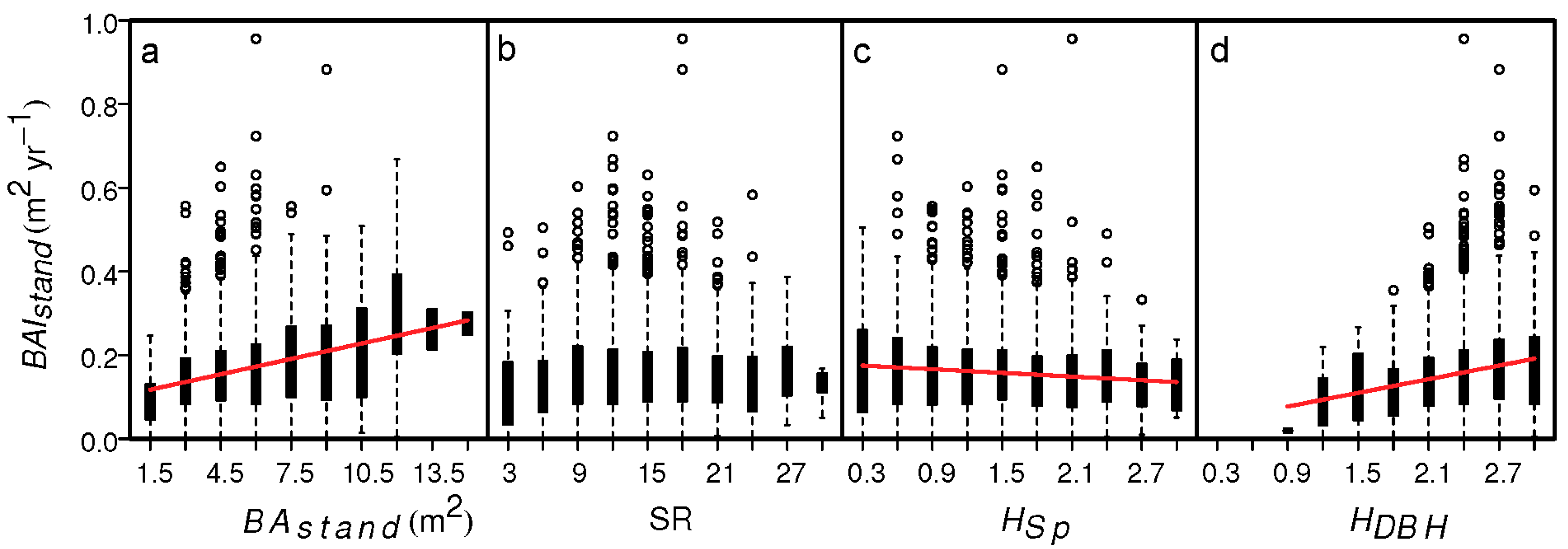
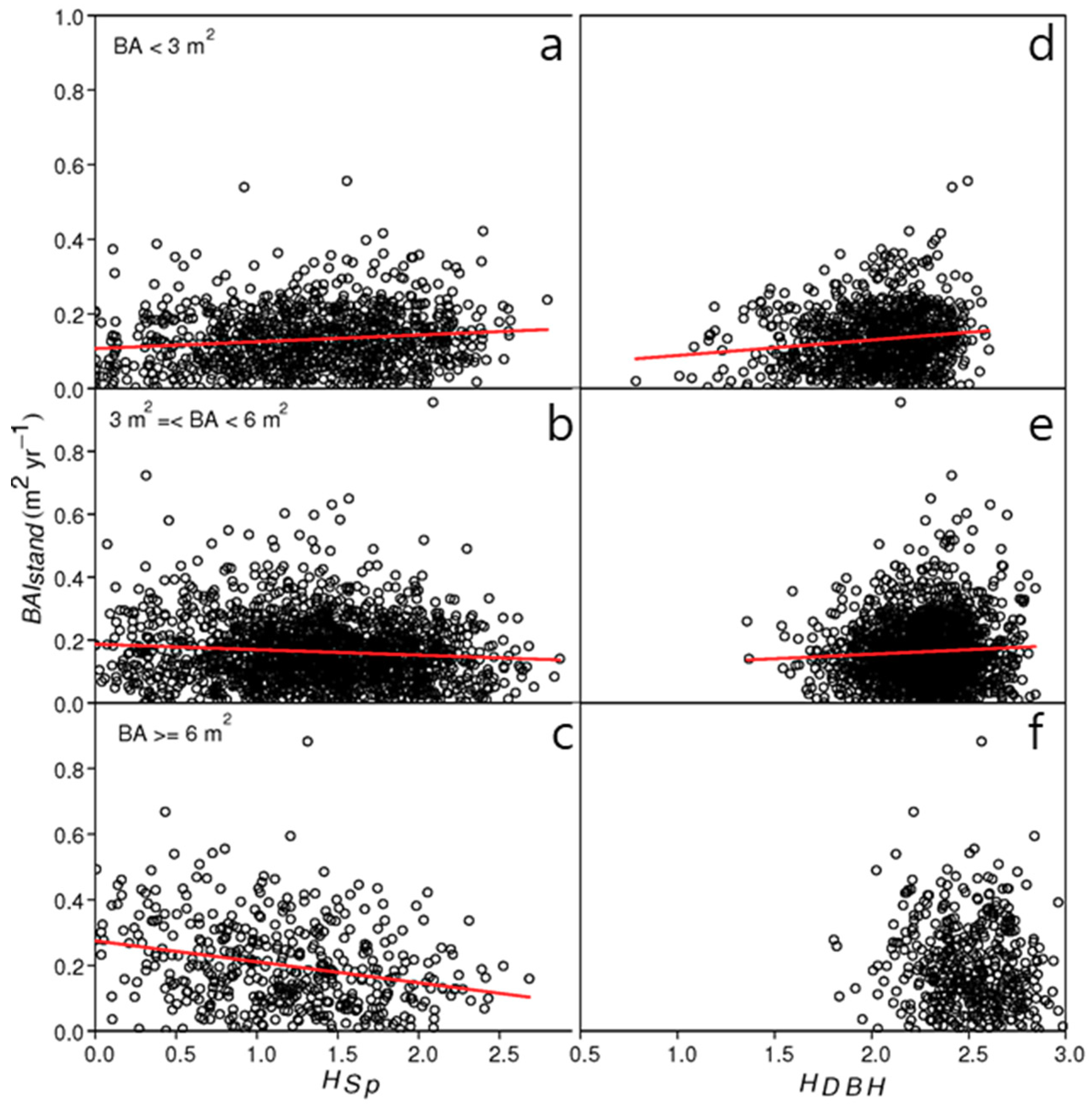
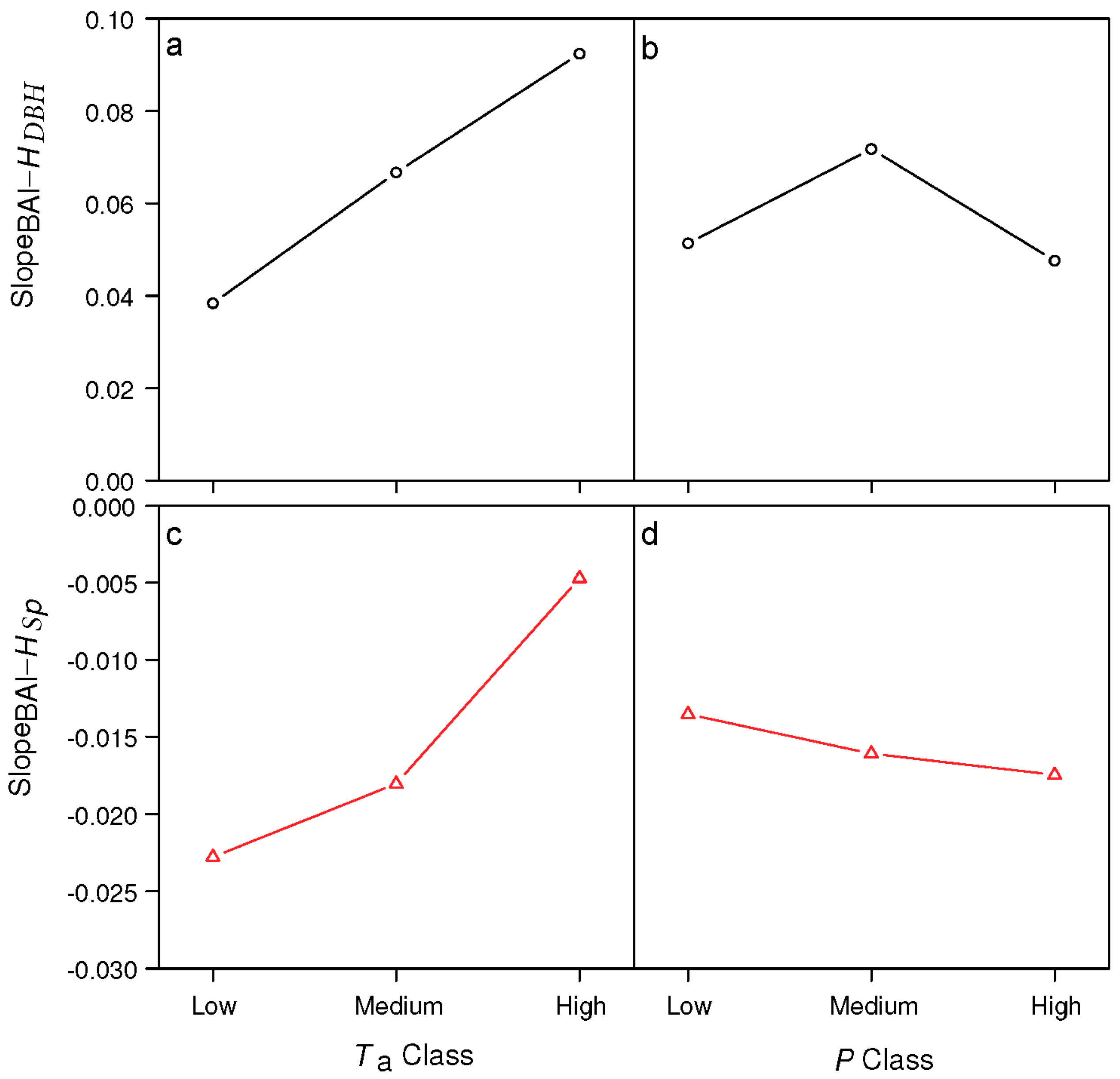
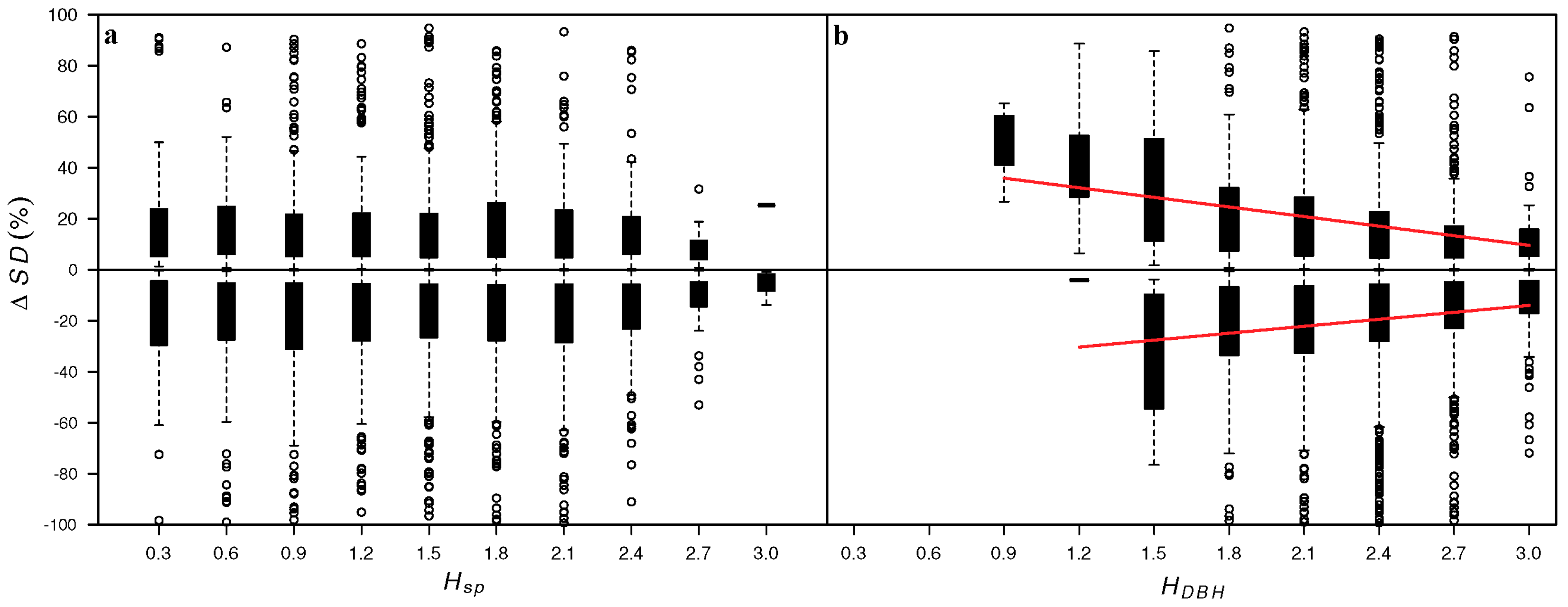
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hector, A.; Schmid, B.; Beierkuhnlein, C.; Caldeira, M.; Diemer, M.; Dimitrakopoulos, P.; Finn, J.; Freitas, H.; Giller, P.; Good, J. Plant diversity and productivity experiments in European grasslands. Science 1999, 286, 1123–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooper, D.U.; Chapin, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Reiss, J.; Bridle, J.R.; Montoya, J.M.; Woodward, G. Emerging horizons in biodiversity and ecosystem functioning research. Trends Ecol. Evol. 2009, 24, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Forrester, D.I.; Bauhus, J.; Cowie, A.L.; Vanclay, J.K. Mixed-species plantations of Eucalyptus with nitrogen-fixing trees: A review. For. Ecol. Manag. 2006, 233, 211–230. [Google Scholar] [CrossRef] [Green Version]
- Zou, C.; Barnes, P.; Archer, S.; McMurtry, C. Soil moisture redistribution as a mechanism of facilitation in savanna tree–shrub clusters. Oecologia 2005, 145, 32–40. [Google Scholar] [CrossRef]
- Riofrío, J.; del Río, M.; Pretzsch, H.; Bravo, F. Changes in structural heterogeneity and stand productivity by mixing Scots pine and Maritime pine. For. Ecol. Manag. 2017, 405, 219–228. [Google Scholar] [CrossRef]
- Tilman, D.; Reich, P.B.; Knops, J.; Wedin, D.; Mielke, T.; Lehman, C. Diversity and productivity in a long-term grassland experiment. Science 2001, 294, 843–845. [Google Scholar] [CrossRef] [Green Version]
- Edgar, C.B.; Burk, T.E. Productivity of aspen forests in northeastern Minnesota, USA, as related to stand composition and canopy structure. Can. J. For. Res. 2001, 31, 1019–1029. [Google Scholar] [CrossRef]
- Gamfeldt, L.; Snäll, T.; Bagchi, R.; Jonsson, M.; Gustafsson, L.; Kjellander, P.; Ruiz-Jaen, M.C.; Fröberg, M.; Stendahl, J.; Philipson, C.D. Higher levels of multiple ecosystem services are found in forests with more tree species. Nat. Commun. 2013, 4, 1340. [Google Scholar] [CrossRef]
- Cavard, X.; Bergeron, Y.; Chen, H.Y.; Pare, D. Mixed-species effect on tree aboveground carbon pools in the east-central boreal forests. Can. J. For. Res. 2010, 40, 37–47. [Google Scholar] [CrossRef]
- Chen, H.Y.H.; Klinka, K. Aboveground productivity of western hemlock and western redcedar mixed-species stands in southern coastal British Columbia. For. Ecol. Manag. 2003, 184, 55–64. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.Y.H.; Reich, P.B. Forest productivity increases with evenness, species richness and trait variation: A global meta-analysis. J. Ecol. 2012, 100, 742–749. [Google Scholar] [CrossRef]
- Liang, J.; Crowther, T.W.; Picard, N.; Wiser, S.; Zhou, M.; Alberti, G.; Schulze, E.D.; McGuire, A.D.; Bozzato, F.; Pretzsch, H.; et al. Positive biodiversity-productivity relationship predominant in global forests. Science 2016, 354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.; Hector, A.; Hooper, D.; Huston, M.; Raffaelli, D.; Schmid, B. Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef] [Green Version]
- Fotis, A.T.; Murphy, S.J.; Ricart, R.D.; Krishnadas, M.; Whitacre, J.; Wenzel, J.W.; Queenborough, S.A.; Comita, L.S. Aboveground biomass is driven by mass-ratio effects and stand structural attributes in a temperate deciduous forest. J. Ecol. 2017, 106, 561–570. [Google Scholar] [CrossRef]
- Danescu, A.; Albrecht, A.T.; Bauhus, J. Structural diversity promotes productivity of mixed, uneven-aged forests in southwestern Germany. Oecologia 2016, 182, 319–333. [Google Scholar] [CrossRef]
- Morin, X.; Fahse, L.; Scherer-Lorenzen, M.; Bugmann, H. Tree species richness promotes productivity in temperate forests through strong complementarity between species. Ecol. Lett. 2011, 14, 1211–1219. [Google Scholar] [CrossRef]
- Cordonnier, T.; Kunstler, G. The Gini index brings asymmetric competition to light. Perspect. Plant Ecol. Evol. Syst. 2015, 17, 107–115. [Google Scholar] [CrossRef]
- Ryan, M.G.; Stape, J.L.; Binkley, D.; Fonseca, S.; Loos, R.A.; Takahashi, E.N.; Silva, C.R.; Silva, S.R.; Hakamada, R.E.; Ferreira, J.M. Factors controlling Eucalyptus productivity: How water availability and stand structure alter production and carbon allocation. For. Ecol. Manag. 2010, 259, 1695–1703. [Google Scholar] [CrossRef]
- Lei, X.; Wang, W.; Peng, C. Relationships between stand growth and structural diversity in spruce-dominated forests in New Brunswick, Canada. Can. J. For. Res. 2009, 39, 1835–1847. [Google Scholar] [CrossRef]
- Holling, C.S. Resilience and stability of ecological systems. Annu. Rev. Ecol. Syst. 1973, 4, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Grimm, V.; Wissel, C. Babel, or the ecological stability discussions: An inventory and analysis of terminology and a guide for avoiding confusion. Oecologia 1997, 109, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Hector, A.; Hautier, Y.; Saner, P.; Wacker, L.; Bagchi, R.; Joshi, J.; Scherer-Lorenzen, M.; Spehn, E.M.; Bazeley-White, E.; Weilenmann, M. General stabilizing effects of plant diversity on grassland productivity through population asynchrony and overyielding. Ecology 2010, 91, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Jucker, T.; Bouriaud, O.; Avacaritei, D.; Coomes, D.A. Stabilizing effects of diversity on aboveground wood production in forest ecosystems: Linking patterns and processes. Ecol. Lett. 2014, 17, 1560–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, C. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Yun, J.I. Visualization of Local Climates Based on Geospatial Climatology. Korean J. Agric. For. Meteorol. 2004, 6, 272–289. [Google Scholar]
- Yun, J.I. Agroclimatic Maps Augmented by a GIS Technology. Korean J. Agric. For. Meteorol. 2010, 12, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Rosseel, Y. Lavaan: An R package for structural equation modeling and more. Version 0.5–12 (BETA). J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Chapman, R.A.; Heitzman, E.; Shelton, M.G. Long-term changes in forest structure and species composition of an upland oak forest in Arkansas. For. Ecol. Manag. 2006, 236, 85–92. [Google Scholar] [CrossRef]
- Wang, W.; Lei, X.; Ma, Z.; Kneeshaw, D.D.; Peng, C. Positive relationship between aboveground carbon stocks and structural diversity in spruce-dominated forest stands in New Brunswick, Canada. For. Sci. 2011, 57, 506–515. [Google Scholar]
- Liang, J.; Buongiorno, J.; Monserud, R.A.; Kruger, E.L.; Zhou, M. Effects of diversity of tree species and size on forest basal area growth, recruitment, and mortality. For. Ecol. Manag. 2007, 243, 116–127. [Google Scholar] [CrossRef]
- Binkley, D.; Stape, J.L.; Bauerle, W.L.; Ryan, M.G. Explaining growth of individual trees: Light interception and efficiency of light use by Eucalyptus at four sites in Brazil. For. Ecol. Manag. 2010, 259, 1704–1713. [Google Scholar] [CrossRef]
- Long, J.N.; Shaw, J.D. The influence of compositional and structural diversity on forest productivity. For. Int. J. For. Res. 2010, 83, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Stape, J.L.; Binkley, D.; Ryan, M.G.; Fonseca, S.; Loos, R.A.; Takahashi, E.N.; Silva, C.R.; Silva, S.R.; Hakamada, R.E.; Ferreira, J.M.d.A.; et al. The Brazil Eucalyptus Potential Productivity Project: Influence of water, nutrients and stand uniformity on wood production. For. Ecol. Manag. 2010, 259, 1684–1694. [Google Scholar] [CrossRef]
- Fahey, R.; Fotis, A.; Woods, K. Quantifying canopy complexity and effects on productivity and resilience in late-successional hemlock–hardwood forests. Ecol. Appl. 2015, 25, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Fotis, A.T.; Curtis, P.S. Effects of structural complexity on within-canopy light environments and leaf traits in a northern mixed deciduous forest. Tree Physiol. 2017, 37, 1426–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardiman, B.S.; Gough, C.M.; Halperin, A.; Hofmeister, K.L.; Nave, L.E.; Bohrer, G.; Curtis, P.S. Maintaining high rates of carbon storage in old forests: A mechanism linking canopy structure to forest function. For. Ecol. Manag. 2013, 298, 111–119. [Google Scholar] [CrossRef]
- Purves, D.W.; Lichstein, J.W.; Pacala, S.W. Crown Plasticity and Competition for Canopy Space: A New Spatially Implicit Model Parameterized for 250 North American Tree Species. PLoS ONE 2007, 2, e870. [Google Scholar] [CrossRef]
- Jucker, T.; Bouriaud, O.; Coomes, D.A.; Baltzer, J. Crown plasticity enables trees to optimize canopy packing in mixed-species forests. Funct. Ecol. 2015, 29, 1078–1086. [Google Scholar] [CrossRef] [Green Version]
- Garber, S.M.; Maguire, D.A. Stand Productivity and Development in Two Mixed-Species Spacing Trials in the Central Oregon Cascades. For. Sci. 2004, 50, 92–105. [Google Scholar]
- Forrester, D.I.; Bauhus, J. A review of processes behind diversity—Productivity relationships in forests. Curr. For. Rep. 2016, 2, 45–61. [Google Scholar] [CrossRef] [Green Version]
- Bouriaud, O.; Marin, G.; Bouriaud, L.; Hessenmöller, D.; Schulze, E.-D. Romanian legal management rules limit wood production in Norway spruce and beech forests. For. Ecosyst. 2016, 3, 20. [Google Scholar] [CrossRef] [Green Version]
- Schulze, E.D.; Bouriaud, O.; Weber, U.; Roscher, C.; Hessenmoeller, D.; Kroiher, F.; Schall, P. Management breaks the natural productivity-biodiversity relationship in forests and grassland: An opinion. For. Ecosyst. 2018, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Forrester, D.I.; Kohnle, U.; Albrecht, A.T.; Bauhus, J. Complementarity in mixed-species stands of Abies alba and Picea abies varies with climate, site quality and stand density. For. Ecol. Manag. 2013, 304, 233–242. [Google Scholar] [CrossRef]
- Hunt, M.A.; Unwin, G.L.; Beadle, C.L. Effects of naturally regenerated Acacia dealbata on the productivity of a Eucalyptus nitens plantation in Tasmania, Australia. For. Ecol. Manag. 1999, 117, 75–85. [Google Scholar] [CrossRef]
- Del Río, M.; Sterba, H. Comparing volume growth in pure and mixed stands of Pinus sylvestris and Quercus pyrenaica. Ann. For. Sci. 2009, 66, 1–11. [Google Scholar]
- Tilman, D. Biodiversity: Population versus ecosystem stability. Ecology 1996, 77, 350–363. [Google Scholar] [CrossRef]
- de Mazancourt, C.; Isbell, F.; Larocque, A.; Berendse, F.; De Luca, E.; Grace, J.B.; Haegeman, B.; Wayne Polley, H.; Roscher, C.; Schmid, B.; et al. Predicting ecosystem stability from community composition and biodiversity. Ecol. Lett. 2013, 16, 617–625. [Google Scholar] [CrossRef] [Green Version]
- Tilman, D.; Isbell, F.; Cowles, J.M. Biodiversity and Ecosystem Functioning. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 471–493. [Google Scholar] [CrossRef]
- Cleve, K.V.; Oliver, L.; Schlentner, R.; Viereck, L.A.; Dyrness, C. Productivity and nutrient cycling in taiga forest ecosystems. Can. J. For. Res. 1983, 13, 747–766. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Sanford, R.L., Jr. Nutrient cycling in moist tropical forest. Annu. Rev. Ecol. Syst. 1986, 17, 137–167. [Google Scholar] [CrossRef]
- Ulrich, B. A concept of forest ecosystem stability and of acid deposition as driving force for destabilization. In Effects of Accumulation of Air Pollutants in Forest Ecosystems; Springer: Dordrecht, The Netherlands, 1983; Volume 29. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Kim, H.S.; Jo, H.K.; Jung, I.B. The Influence of Tree Structural and Species Diversity on Temperate Forest Productivity and Stability in Korea. Forests 2019, 10, 1113. https://doi.org/10.3390/f10121113
Park J, Kim HS, Jo HK, Jung IB. The Influence of Tree Structural and Species Diversity on Temperate Forest Productivity and Stability in Korea. Forests. 2019; 10(12):1113. https://doi.org/10.3390/f10121113
Chicago/Turabian StylePark, Juhan, Hyun Seok Kim, Hyun Kook Jo, and II Bin Jung. 2019. "The Influence of Tree Structural and Species Diversity on Temperate Forest Productivity and Stability in Korea" Forests 10, no. 12: 1113. https://doi.org/10.3390/f10121113




