1. Introduction
Aboveground tree biomass (AGB) is an important variable used in quantifying and monitoring forest ecosystem services. Aboveground tree biomass in forest ecosystems is needed for many applications; it can be used to estimate the amount of carbon that forests can sequester or that can be emitted when forests are destroyed [
1,
2], it can be used to determine sustainable wood harvest [
3], it provides information on how many resources are available for use [
4], and it is important for assessing forest productivity [
5,
6]. For these and other applications, reliable and accurate biomass estimates are required.
Aboveground tree biomass can be determined directly, by harvesting all trees in a known area and weighing their biomass, or indirectly, by using an allometric model that relates forest biomass to a single or a combination of dendrometrical variables that are easy to measure such as diameter or height [
7,
8]. In addition to diameter or height, the importance of wood basic density as a predictor variable in AGB models has been confirmed in several studies (e.g., [
9,
10,
11]). Furthermore, for shrubs or trees with multi-stem patterns, crown dimension could be a useful variable to explain the variability in biomass [
12]. Although the direct method is the most accurate, it is impractical for large areas because it is time consuming and destructive [
13]. Moreover, applying the direct method in protected or threatened forests is not always possible. Hence, use of allometric models is a commonly used method [
14,
15].
The accuracy of AGB estimation using allometric models is highly dependent on the use of appropriate models [
11]. There are different arguments when it comes to choosing between the use of existing general (data from multiple sites) allometric models or to develop local (data from one site) allometric models for estimating biomass of a particular forest. Furthermore, general models can be developed for multiple species (general multispecies models) or for single species (general species-specific models). Similarly, local models can be developed for multiple species (local multispecies models) or for single species (local species-specific models). Because developing biomass models is costly, very often people prefer to adopt existing general (multispecies or species-specific) models from the literature rather than develop local models [
16,
17]. However, use of general models outside the locations reflecting the conditions on which they were developed can lead to significant biases in AGB estimations [
15]. For instance, the general multispecies pan-tropical models developed by Chave et al. [
10,
11] have been used for estimating AGB of different forest types in many parts of the world, including Ethiopia. Nevertheless, there are many studies (e.g., [
15,
18,
19]) that showed a significant bias in AGB for different forest types when these models were applied.
Local models may have limited use beyond the site for which they are developed. However, they generally provide less bias than general models [
20] because tree growth characteristics are affected by local geographical conditions such as climate, soil properties, altitude, and land-use history [
21]. As it is indicated by Mokria et al. [
2], lack of local AGB estimation models is the main reason for persistent inaccuracy in biomass estimation, particularly in sub-Saharan Africa.
There have been some attempts to develop allometric models (mostly local species-specific) for estimating biomass of trees and shrubs in various parts of Ethiopia. In a review made by Henry et al. [
22] on available AGB models in sub-Saharan Africa, 63 models addressing six species were registered from Ethiopia, of which 70% of the models were developed for eucalypt species. None of the models included multiple species. Even though there are developments after the review by Henry et al. [
22], still very few models (e.g., [
2,
19]) are multispecies. Species-specific models can result in accurate biomass estimates. However, developing models for every species is not feasible in areas where there is high number of species because destructive sampling is limited by high costs and legal restrictions on tree harvesting. As it is suggested by Chave et al. [
10], use of multispecies AGB models is a more feasible solution for tropical forests, which are characterized by high species diversity.
Dry Afromontane forests form the largest part of the existing natural vegetation in Ethiopia [
23]. Nevertheless, AGB models developed particularly for these forests are rare. In the literature, we found only four published studies regarding AGB models developed for the Ethiopian Afromontane forests: (1) local species-specific models developed by Worku and Soromessa [
24] for
Juniperus procera Hochst. ex Endl. and
Afrocarpus falcatus (Thunb.) R. Br. Ex Mirb. in Wof-Washa dry Afromontane forest using a non-destructive method; (2) local species-specific models developed by Tesfaye et al. [
18] for
Allophyllus abyssinicus (Hochst.) Radlk.,
Olea europaea L. subsp.
cuspidata (Wall. ex DC.) Ciffieri.,
Olinia rochetiana A. Juss,
Rhus glutinosa Hochst. Ex A. Rich., and
Scolopia theifolia Gilg in Chilimo dry Afromontane forest; (3) local species-specific model developed by Kebede and Soromessa [
25] for
Olea europaea L. subsp.
cuspidata (Wall. ex DC.) Ciffieri. in Mana Angetu moist Afromontane forest using a semi-destructive method; and (4) local species-specific models developed by Solomon et al. [
26] for
Juniperus procera Hochst. ex Endl. and
Acacia abyssinica Hochst. ex Benth. in Gergera watershed.
The main objective of this study was to develop local AGB models for dry Afromontane forests in northern Ethiopia. More specifically, we aimed (1) to develop local multispecies models and local species-specific models for dominant tree species, (2) to develop models with different combinations of predictor variables to secure flexibility in application, and (3) to test the accuracy of some potentially relevant, previously developed AGB models on data from our study site.
3. Results
The parameter estimates and statistical summary for the multispecies AGB models under Model System I are presented in
Table 3. All models that included H as a predictor variable had nonsignificant model parameters estimate (
p > 0.05) and were, therefore, not considered as valid. On the other hand, M1, M3, M4, and M7 (from the first option with DBH as predictor) and M9, M11, M12, and M15 (from the second option with DSH as predictor) all had significant model parameter estimates (
p < 0.05). Generally, there were only small differences between the models regarding adjusted
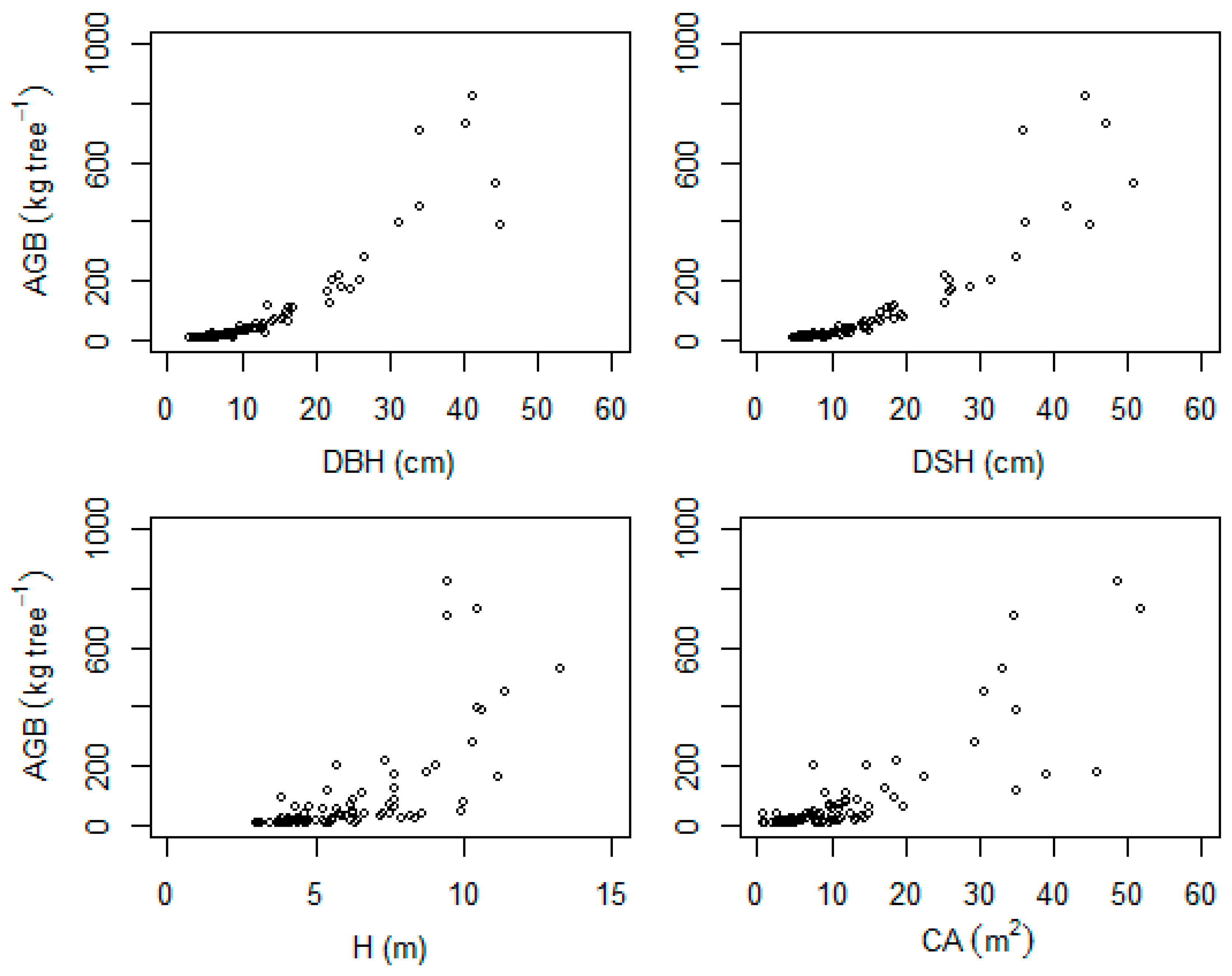
R2. Among the valid models, M7 and M15, respectively for the two options, were the best ones based on the statistical performance criteria (rRMSE, rBias, and AIC). The simple models using DBH and DSH solely as predictor variables (M1 and M9, respectively) both explained about 94% of the AGB variation but had higher rRMSE and rBias compared to the other valid models.
Among all the tested AGB models for two dominant species and for other species under Model System II, only those with DBH and DSH as sole predictor variables had significant parameter estimates (
p < 0.05) (
Table 4). Generally, the rBias values of these models were higher than those of the multispecies AGB models.
We predicted AGB of the 86 sample trees using the two systems and compared their biases (
Table 5). In Model System I, we used the best multispecies models (M7 and M15). In Model System II, we used independently the best species-specific models for
Juniperus procera and
Olea europaea (M17 and M18 for
Juniperus procera, and M19 and M20 for
Olea europaea) and the best multispecies models for others (M21 and M22). Finally, an average bias was computed. Although the differences between observed and predicted values were not significantly different from zero, Model System I slightly underpredicted while Model System II overpredicted AGB.
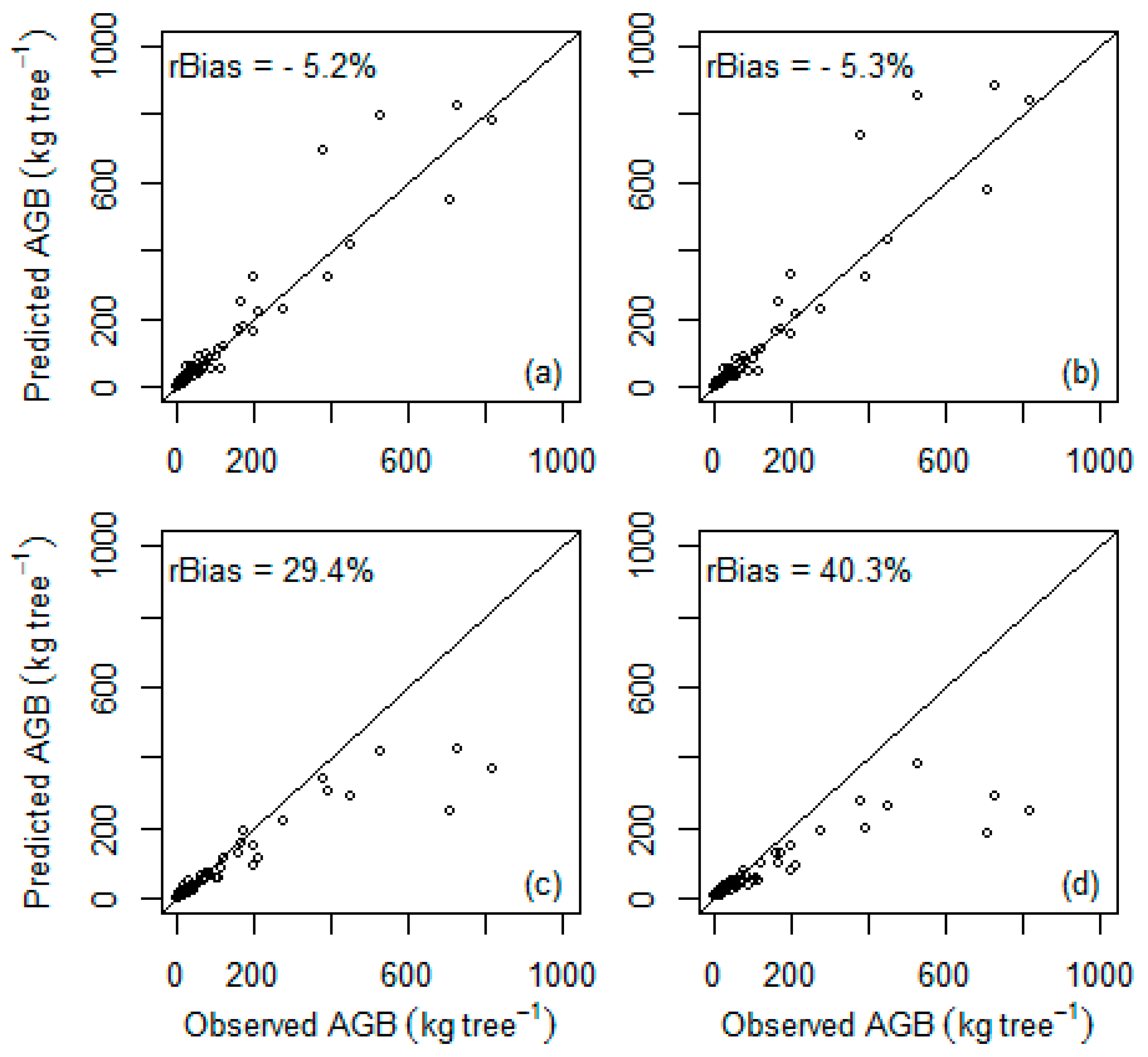
When the previously published general multispecies models were tested in our data set (
Table 6,
Figure 4), the models developed by Chave et al. [
10] and Chave et al. [
11] resulted in relatively low differences between observed and predicted values, which were not significantly different from zero (
p > 0.05). On the other hand, the two general multispecies models developed by Ubuy et al. [
19] and Mokria et al. [
2] were significantly biased (
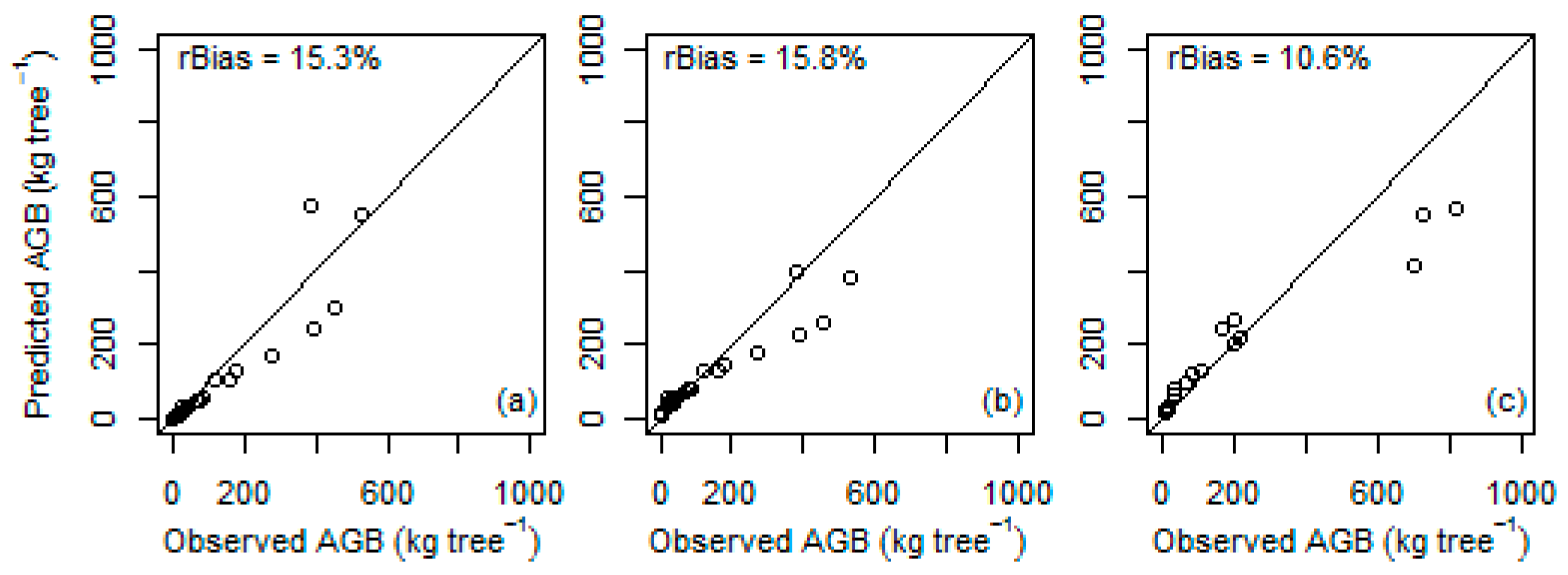
p < 0.05) and underpredicted AGB by 29.4% and 40.3%, respectively. Similarly, when the previously developed species-specific models were tested, the differences between observed and predicted values were not significantly different from zero (
Table 6,
Figure 5).
4. Discussion
Use of a small sample size in the development of biomass models is a potential source of error in biomass estimation [
10,
41,
42]. Particularly, this is mentioned as a limitation of local biomass models [
15]. In the present study, we used 86 trees (consisting of ten dominant species, representing 76% of the total basal area of the forest) destructively sampled from a single site to develop local multispecies and species-specific biomass models. Considering the restrictions on destructive sampling in Ethiopia and costs associated with biomass measurement in general, the number of sample trees used in this study was much higher than in most previously published models based on data from the country.
We have used four commonly suggested predictor variables (i.e., DBH/DSH, H, CA, and ρ) to check their ability in explaining the variability in AGB. As confirmed in many studies, we found diameter (DBH/DSH) to be an important predictor variable explaining most of the variability (from about 94% and above). Several authors found stem diameter alone to be an effective predictor for AGB (e.g., [
26,
43]). In the review by Henry et al. [
22] on available allometric models in sub-Saharan African forests, 63% of the biomass models used stem diameter (DBH) as a predictor variable along with other variables, which shows how important diameter is in explaining the variability in tree biomass.
Including tree height as a predictor variable in AGB models is usually recommended as it can improve model fit [
44,
45]. In our case, however, the inclusion of tree height did not improve the models. All models that included tree height as a predictor variable had nonsignificant model parameters for tree height. This could be due to frequent cutting and grazing activities in the forest [
46] leading to more open-stand conditions where competition for light is low. Under such conditions, trees tend to invest more on radial growth than apical growth [
47]. As a result of this, there will be limited increase in tree height while there is an increase in tree biomass, particularly as the tree gets older.
Crown dimension is known to vary greatly between species, reflecting different strategies of carbon allocation [
48]. In our case, integrating crown area as a predictor variable for the multispecies models resulted in significant improvements regarding the goodness-of-fit criteria (rRMSE, rBias, and AIC). In support of our result, significant reduction in biases have been reported in many studies when crown dimension was taken into account in biomass models of different tropical forests [
19,
48,
49].
Wood basic density variation in tropical tree species is high [
50], particularly in dry forests [
34,
51]. Hence, including wood basic density as a predictor variable in multispecies AGB models is highly recommended by many authors [
9,
10,
11,
41]. As expected, the inclusion of wood basic density as a predictor variable improved the model fit in the case of multispecies models. But, wood basic density was not a significant predictor of AGB in the species-specific models, which implies that wood basic density variation within a species is narrow.
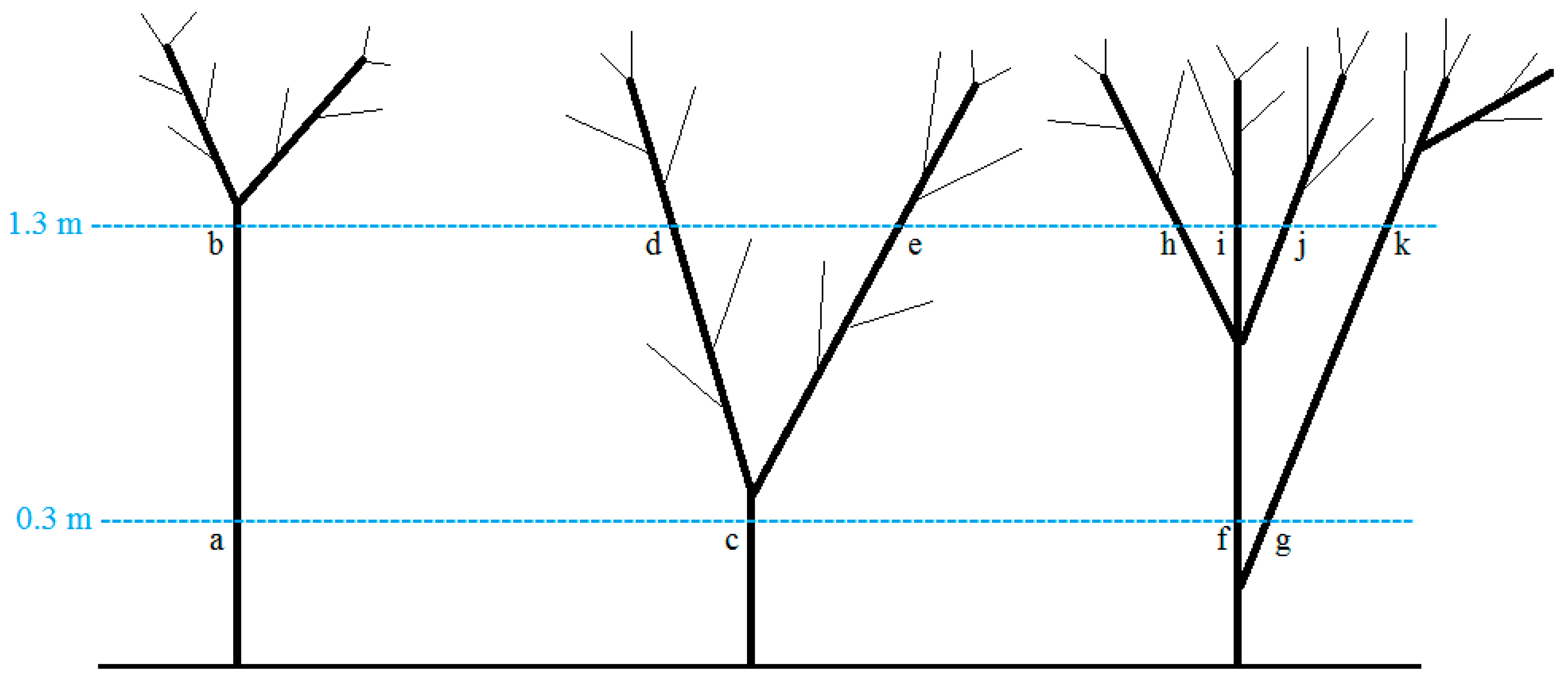
It can be difficult to measure DBH in woodlands and open forests where trees are short, with high basal ramification, resulting in large crown areas. The same applies to forests where trees are repeatedly cut and are therefore coppice. In such cases, the use of DSH measurements for predicting tree AGB would be a better option. Since Desa’a forest, and similar forests in Ethiopia, are largely dominated by shrub species and often cut [
46], providing models with both options gives an advantage to use either DBH- or DSH-based models for estimating AGB depending on the availability of data. When there is a need to quantify the biomass removed in illegal cutting, the DSH-based models gives the opportunity to estimate AGB of harvested trees directly from measurements of stump diameter [
43,
52].
All models with significant model parameter estimates (
Table 3) can potentially be applied for estimating AGB depending on the availability of data from a forest inventory. All models explained more than 94% of the variability in AGB with less than 5% bias. If data on tree DSH, CA, and ρ are available, the best model (M15) should be applied. Although it is important to incorporate all appropriate structural tree variables that affect variation in AGB [
49], the use of a single and easy measurable variable is beneficial [
47], especially when it is able to explain much of the variability. In our case, the two simple models, M1 and M9, which uses only DBH or DSH as a predictor variable, provided more or less the same predictive power (adjusted
R2 = 0.94) as those including several predictor variables. Since many forest inventories are restricted to stem diameter measurements [
45], it would be more convenient and cost effective to apply the simple models (M1 or M9) for estimating AGB.
It is not surprising that the best local multispecies model from this study performed better than the previously published general models (
Table 6). Such a result also emphasizes the importance to develop local models to get more accurate biomass estimations when local estimates are required [
9,
18]. The two pan-tropical general multispecies models developed for dry tropical forests [
10] and for all tropical forests [
11] gave relatively good predictions with nonsignificant differences for our site (5.2% and 5.3% respectively). In agreement with our findings, Vieilledent et al. [
51] found that the model by Chave et al. [
10] performed well for dry forests in Madagascar. Contrary to our findings, significant biases were reported when the models by Chave et al. [
10,
11] were applied to data sets from other dry tropical forests [
18,
19,
53].
The two general multispecies models by Ubuy et al. [
19] and Mokria et al. [
2] developed for exclosures resulted in significant biases (29.4% and 40.3%, respectively) when tested on our data. Our study site is found in the same geographical region (Tigray region) where the study by Ubuy et al. [
19] was conducted. Similarly, the model by Mokria et al. [
2] is based on sample trees from Ethiopia (Amhara region). But, trees in exclosures are relatively younger compared to the trees growing in Desa’a forest (i.e., climax forest) because the exclosures are at the early stage of succession. Hence, applying biomass models developed for exclosures to a climax forest can lead to considerable bias in biomass estimates, especially for larger trees (
Figure 4). This suggests that different models should be developed for different forest types even if they are found in the same geographical regions.
Also, the local species-specific models by Worku and Soromessa [
24] and by Solomon et al. [
26] for
Juniperus procera and by Kebede and Soromessa [
25] for
Olea europaea resulted in relatively large differences between observed and predicted values, although not significantly different from zero, when tested on our data. These results as well confirmed the need to develop local models also for individual species.
Although the models developed in this study are local, they can potentially be applied in dry Afromontane forests elsewhere in Ethiopia since no general models previously have been developed for this particular forest type. However, it is important at least to evaluate species composition and growing conditions related to rainfall and temperature before an application is done. For instance, applying the models developed in this study for moist Afromontane forests may not be appropriate, as the species composition and growing conditions in dry and moist areas are different. Information on rainfall and temperature may easily be derived from nearby weather stations. It is also likely that information on species distribution from a sample plot inventory is available in a situation where application of the models is under consideration, but if not, at least the presence of Juniperus procera and Olea europaea should be assessed because these two species represent almost half of the basal area observed in the study site where the data used for model development were collected. In the future, more data from other Afromontane forests in Ethiopia should be added to the present data in order to develop general models that cover a larger portion of this forest type in Ethiopia.