Differentiation and Non-Linear Responses in Temporal Phenotypic Plasticity of Seasonal Phenophases in a Common Garden of Crataegus monogyna Jacq.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Common Garden
2.2. Scoring of the Four Phenophases
2.3. Statistical Analysis of Phenological Data
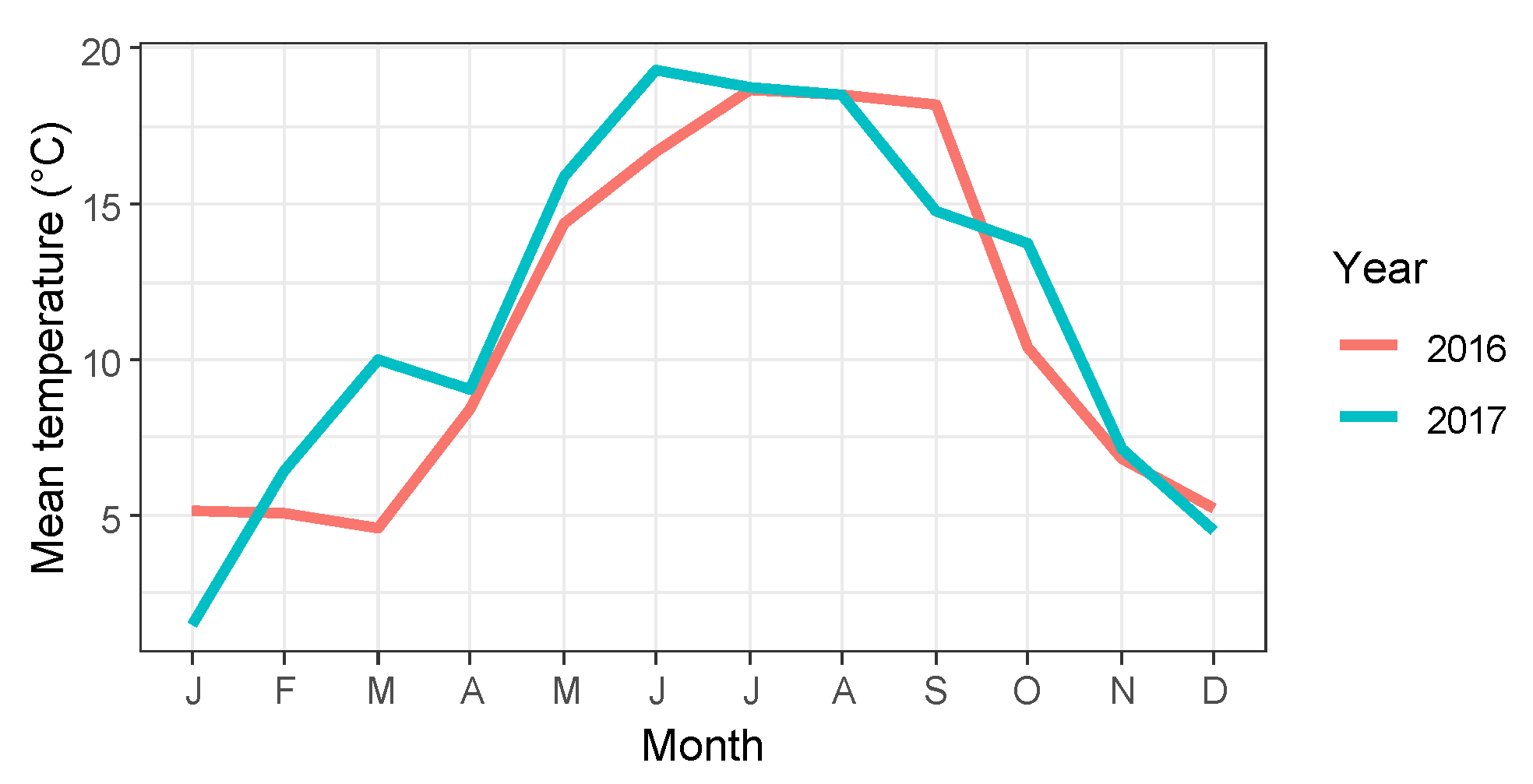
3. Results
3.1. Timing of Bud Burst
3.2. Timing of Flower Opening
3.3. Timing of Leaf Senescence and Leaf Fall
3.4. Correlation and Variance Analysis
4. Discussion
4.1. Timing of the Phenophases
4.2. Non-Linear Temporal Responses in Timing of the Phenophases
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; Wang, T.L.; Curtis-McLane, S. Adaptation, migration or extirpation: Climate change outcomes for tree populations. Evol. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef]
- Kremer, A.; Potts, B.M.; Delzon, S. Genetic divergence in forest trees: Understanding the consequences of climate change. Funct. Ecol. 2014, 28, 22–36. [Google Scholar] [CrossRef]
- Firmat, C.; Delzon, S.; Louvet, J.M.; Parmentier, J.; Kremer, A. Evolutionary dynamics of the leaf phenological cycle in an oak metapopulation along an elevation gradient. J. Evol. Biol. 2017, 30, 2116–2131. [Google Scholar] [CrossRef]
- Erwin, D.H. Climate as a driver of evolutionary change. Curr. Biol. 2009, 19, R575–R583. [Google Scholar] [CrossRef]
- Alberto, F.J.; Aitken, S.N.; Alia, R.; Gonzalez-Martinez, S.C.; Hanninen, H.; Kremer, A.; Lefevre, F.; Lenormand, T.; Yeaman, S.; Whetten, R.; et al. Potential for evolutionary responses to climate change evidence from tree populations. Glob. Chang. Biol. 2013, 19, 1645–1661. [Google Scholar] [CrossRef]
- Klisz, M.; Ukalski, K.; Ukalska, J.; Jastrzebowski, S.; Puchalka, R.; Przybylski, P.; Mionskowski, M.; Matras, J. What can we learn from an early test on the adaptation of silver fir populations to marginal environments? Forests 2018, 9, 441. [Google Scholar] [CrossRef]
- De Villemereuil, P.; Gaggiotti, O.E.; Mouterde, M.; Till-Bottraud, I. Common garden experiments in the genomic era: New perspectives and opportunities. Heredity 2016, 116, 249–254. [Google Scholar] [CrossRef]
- Gienapp, P.; Reed, T.E.; Visser, M.E. Why climate change will invariably alter selection pressures on phenology. Proc. R. Soc. B-Biol. Sci. 2014, 281, 20141611. [Google Scholar] [CrossRef]
- De Kort, H.; Vandepitte, K.; Honnay, O. A meta-analysis of the effects of plant traits and geographical scale on the magnitude of adaptive differentiation as measured by the difference between Qst and Fst. Evol. Ecol. 2013, 27, 1081–1097. [Google Scholar] [CrossRef]
- Vitasse, Y.; Lenz, A.; Korner, C. The interaction between freezing tolerance and phenology in temperate deciduous trees. Front. Plant Sci. 2014, 5, 541. [Google Scholar] [CrossRef]
- Begum, S.; Nakaba, S.; Yamagishi, Y.; Oribe, Y.; Funada, R. Regulation of cambial activity in relation to environmental conditions: Understanding the role of temperature in wood formation of trees. Physiol. Plant. 2013, 147, 46–54. [Google Scholar] [CrossRef]
- Savolainen, O.; Pyhäjärvi, T.; Knürr, T. Gene flow and local adaptation in trees. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 595–619. [Google Scholar] [CrossRef]
- Whittet, R.; Cavers, S.; Cottrell, J.; Rosique-Esplugas, C.; Ennos, R. Substantial variation in the timing of pollen production reduces reproductive synchrony between distant populations of Pinus sylvestris L. In scotland. Ecol. Evol. 2017, 7, 5754–5765. [Google Scholar] [CrossRef]
- Dragoni, D.; Schmid, H.P.; Wayson, C.A.; Potter, H.; Grimmond, C.S.B.; Randolph, J.C. Evidence of increased net ecosystem productivity associated with a longer vegetated season in a deciduous forest in south-central Indiana, USA. Glob. Chang. Biol. 2011, 17, 886–897. [Google Scholar] [CrossRef]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kubler, K.; Bissolli, P.; Braslavska, O.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Glob. Chang. Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef] [Green Version]
- Zohner, C.M.; Renner, S.S. Common garden comparison of the leaf-out phenology of woody species from different native climates, combined with herbarium records, forecasts long-term change. Ecol. Lett. 2014, 17, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Duputie, A.; Rutschmann, A.; Ronce, O.; Chuine, I. Phenological plasticity will not help all species adapt to climate change. Glob. Chang. Biol. 2015, 21, 3062–3073. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.N.; Bemmels, J.B. Time to get moving: Assisted gene flow of forest trees. Evol. Appl. 2016, 9, 271–290. [Google Scholar] [CrossRef]
- Le Corre, V.; Kremer, A. The genetic differentiation at quantitative trait loci under local adaptation. Mol. Ecol. 2012, 21, 1548–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomory, D.; Ditmarova, L.; Hrivnak, M.; Jamnicka, G.; Kmet’, J.; Krajmerova, D.; Kurjak, D. Differentiation in phenological and physiological traits in European beech (Fagus sylvatica L.). Eur. J. For. Res. 2015, 134, 1075–1085. [Google Scholar] [CrossRef]
- Vitasse, Y.; Bresson, C.C.; Kremer, A.; Michalet, R.; Delzon, S. Quantifying phenological plasticity to temperature in two temperate tree species. Funct. Ecol. 2010, 24, 1211–1218. [Google Scholar] [CrossRef] [Green Version]
- Pluess, A.R.; Frank, A.; Heiri, C.; Lalaguee, H.; Vendramin, G.G.; Oddou-Muratorio, S. Genome-environment association study suggests local adaptation to climate at the regional scale in Fagus sylvatica. New Phytol. 2016, 210, 589–601. [Google Scholar] [CrossRef]
- Puchalka, R.; Koprowski, M.; Gricar, J.; Przybylak, R. Does tree-ring formation follow leaf phenology in pedunculate oak (Quercus robur L.)? Eur. J. For. Res. 2017, 136, 259–268. [Google Scholar] [CrossRef]
- Kraj, W.; Sztorc, A. Genetic structure and variability of phenological forms in the european beech (Fagus sylvatica L.). Ann. For. Sci. 2009, 66, 1–7. [Google Scholar] [CrossRef]
- Jordan, C.Y.; Ally, D.; Hodgins, K.A. When can stress facilitate divergence by altering time to flowering? Ecol. Evol. 2015, 5, S962–S973. [Google Scholar] [CrossRef]
- Soularue, J.P.; Kremer, A. Assortative mating and gene flow generate clinal phenological variation in trees. BMC Evol. Biol. 2012, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Soularue, J.P.; Kremer, A. Evolutionary responses of tree phenology to the combined effects of assortative mating, gene flow and divergent selection. Heredity 2014, 113, 485–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.S.; Campioli, M.; Vitasse, Y.; De Boeck, H.J.; Van den Berge, J.; AbdElgawad, H.; Asard, H.; Piao, S.; Deckmyn, G.; Janssens, I.A. Variation in leaf flushing date influences autumnal senescence and next year’s flushing date in two temperate tree species. Proc. Natl. Acad. Sci. USA 2014, 111, 7355–7360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delpierre, N.; Dufrene, E.; Soudani, K.; Ulrich, E.; Cecchini, S.; Boe, J.; Francois, C. Modelling interannual and spatial variability of leaf senescence for three deciduous tree species in France. Agric. For. Meteorol. 2009, 149, 938–948. [Google Scholar] [CrossRef]
- Estiarte, M.; Penuelas, J. Alteration of the phenology of leaf senescence and fall in winter deciduous species by climate change: Effects on nutrient proficiency. Glob. Chang. Biol. 2015, 21, 1005–1017. [Google Scholar] [CrossRef]
- Rohde, A.; Bastien, C.; Boerjan, W. Temperature signals contribute to the timing of photoperiodic growth cessation and bud set in poplar. Tree Physiol. 2011, 31, 472–482. [Google Scholar] [CrossRef] [Green Version]
- Rohde, A.; Storme, V.; Jorge, V.; Gaudet, M.; Vitacolonna, N.; Fabbrini, F.; Ruttink, T.; Zaina, G.; Marron, N.; Dillen, S.; et al. Bud set in poplar—Genetic dissection of a complex trait in natural and hybrid populations. New Phytol. 2011, 189, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Polgar, C.A.; Primack, R.B. Leaf-out phenology of temperate woody plants: From trees to ecosystems. New Phytol. 2011, 191, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Vitasse, Y.; Delzon, S.; Bresson, C.C.; Michalet, R.; Kremer, A. Altitudinal differentiation in growth and phenology among populations of temperate-zone tree species growing in a common garden. Can. J. For. Res. 2009, 39, 1259–1269. [Google Scholar] [CrossRef]
- Panchen, Z.A.; Primack, R.B.; Gallinat, A.S.; Nordt, B.; Stevens, A.D.; Du, Y.J.; Fahey, R. Substantial variation in leaf senescence times among 1360 temperate woody plant species: Implications for phenology and ecosystem processes. Ann. Bot.-Lond. 2015, 116, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Zohner, C.M.; Rockinger, A.; Renner, S.S. Increased autumn productivity permits temperate trees to compensate for spring frost damage. New Phytol. 2019, 221, 789–795. [Google Scholar] [CrossRef]
- Nicotra, a.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleland, E.E.; Allen, J.M.; Crimmins, T.M.; Dunne, J.A.; Pau, S.; Travers, S.E.; Zavaleta, E.S.; Wolkovich, E.M. Phenological tracking enables positive species responses to climate change. Ecology 2012, 93, 1765–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, H.F.; Grady, K.C.; Cowan, J.A.; Best, R.J.; Allan, G.J.; Whitham, T.G. Genotypic variation in phenological plasticity: Reciprocal common gardens reveal adaptive responses to warmer springs but not to fall frost. Glob. Chang. Biol. 2019, 25, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.M.; Kaluthota, S.; Pearce, D.W.; Allan, G.J.; Floate, K.; Rood, S.B.; Whitham, T.G. Bud phenology and growth are subject to divergent selection across a latitudinal gradient in Populus angustifolia and impact adaptation across the distributional range and associated arthropods. Ecol. Evol. 2016, 6, 4565–4581. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Beatty, G.E.; Finlay, C.M.V.; Montgomery, W.I.; Tosh, D.G.; Provan, J. Genetic analyses reveal high levels of seed and pollen flow in hawthorn (Crataegus monogyna Jacq.), a key component of hedgerows. Tree Genet. Genomes 2016, 12, 58. [Google Scholar] [CrossRef]
- Vander Mijnsbrugge, K.; Onkelinx, T.; De Cuyper, B. Variation in bud burst and flower opening responses of local versus non-local provenances of hawthorn (Crataegus monogyna Jacq.) in Belgium. Plant Syst. Evol. 2015, 301, 1171–1179. [Google Scholar] [CrossRef]
- Council of the European Union. Council directive 1999/105/ec of 22 december 1999 on the marketing of forest reproductive material. Off. J. Eur. Communion 2000, L11, 17–40. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: http://www.R-project.org/ (accessed on 5 January 2018).
- Christensen, R.H.B. Ordinal: Regression Models for Ordinal Data. R Package Version 2015.6-28. 2015. Available online: http://www.Cran.R-project.Org/package=ordinal/ (accessed on 5 January 2015).
- Alberto, F.J.; Derory, J.; Boury, C.; Frigerio, J.M.; Zimmermann, N.E.; Kremer, A. Imprints of natural selection along environmental gradients in phenology-related genes of Quercus petraea. Genetics 2013, 195, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Salmela, M.J.; Cavers, S.; Cottrell, J.E.; Iason, G.R.; Ennos, R.A. Spring phenology shows genetic variation among and within populations in seedlings of scots pine (Pinus sylvestris L.) in the Scottish highlands. Plant Ecol. Divers 2013, 6, 523–536. [Google Scholar] [CrossRef]
- Lobo, A.; Hansen, J.K.; Hansen, L.N.; Kjaer, E.D. Differences among six woody perennials native to northern Europe in their level of genetic differentiation and adaptive potential at fine local scale. Ecol. Evol. 2018, 8, 2231–2239. [Google Scholar] [CrossRef]
- Chmielewski, F.M.; Rotzer, T. Response of tree phenology to climate change across Europe. Agric. For. Meteorol. 2001, 108, 101–112. [Google Scholar] [CrossRef]
- Franks, S.J.; Weber, J.J.; Aitken, S.N. Evolutionary and plastic responses to climate change in terrestrial plant populations. Evol. Appl. 2014, 7, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Hampe, A.; Petit, R.J. Conserving biodiversity under climate change: The rear edge matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef] [PubMed]





| Region | Village | Provenance abb. | Latitude | Longitude | Altitude (m) | n |
|---|---|---|---|---|---|---|
| Flanders | Landegem | FL1 | 51.0546 | 3.5888 | 10 | 53 |
| Flanders | Riemst | FL2 | 50.8116 | 5.5978 | 112 | 52 |
| Flanders | Hansbeke | FL3 | 51.0747 | 3.5345 | 10 | 31 |
| Flanders | Zomergem | FL4 | 51.1195 | 3.5642 | 7.5 | 46 |
| Flanders | Melle | FL5 | 51.0030 | 3.7988 | 10 | 55 |
| Flanders | Muizen | FL6 | 51.0109 | 4.5143 | 12.5 | 52 |
| Wallonia | Smuid | WA1 | 50.0188 | 5.2662 | 370 | 53 |
| Wallonia | Viroin | WA2 | 50.0727 | 4.6072 | 305 | 49 |
| Hungary | - | HU | - | - | - | 46 |
| Italy | - | IT | - | - | - | 52 |
| United Kingdom | - | UK | - | - | - | 48 |
| Phenophase | Score Level | Description |
|---|---|---|
| bud burst | 1 | winter buds |
| 2 | buds swelling | |
| 3 | buds opening and first green tips of the leaves visible | |
| 4 | leaves emerging from the bud but not yet unfolding | |
| 5 | leaves emerged and unfolding | |
| 6 | leaves fully unfolded and expanding | |
| flower opening | 1 | flower buds closed and green |
| 2 | flower buds closed and white | |
| 3 | less than half of the flowers in an inflorescence opened | |
| 4 | half to more than half of the flowers in an inflorescence opened | |
| 5 | most but not all flowers in an inflorescence opened | |
| 6 | all flowers opened | |
| leaf senescence | 1 | (mainly) green leaves |
| 2 | mainly light green to yellow leaves | |
| 3 | mainly yellow leaves | |
| 4 | yellow and brown leaves | |
| 5 | (mainly) brown leaves | |
| leaf fall | 1 | (nearly) no leaves fallen |
| 2 | About 1/4of the leaves are fallen | |
| 3 | About 1/2 of the leaves are fallen | |
| 4 | About 3/4 of the leaves are fallen | |
| 5 | (nearly) all leaves fallen |
| Variable | Bud Burst | Flower Opening | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | Std. Error | z-Value | p-Value | Estimate | Std. Error | z-Value | p-Value | |
| DOY | −0.64 | 0.01 | −44.79 | <0.001 *** | −0.46 | 0.01 | −38.90 | <0.001 *** |
| 2017 | −8.29 | 0.30 | −27.26 | <0.001 *** | −2.84 | 0.24 | −12.07 | <0.001 *** |
| FL2 | −0.28 | 0.48 | −0.59 | 0.558 | −0.28 | 0.31 | −0.90 | 0.367 |
| FL3 | −0.08 | 0.55 | −0.15 | 0.878 | −0.47 | 0.35 | −1.32 | 0.188 |
| FL4 | 1.30 | 0.49 | 2.63 | 0.009 ** | 0.42 | 0.32 | 1.32 | 0.188 |
| FL5 | 0.31 | 0.47 | 0.66 | 0.513 | −0.04 | 0.30 | −0.12 | 0.905 |
| FL6 | 0.50 | 0.48 | 1.06 | 0.290 | −0.10 | 0.31 | −0.31 | 0.753 |
| HO | −6.19 | 0.51 | −12.06 | <0.001 *** | −3.02 | 0.35 | −8.65 | <0.001 *** |
| IT | −7.14 | 0.51 | −14.14 | <0.001 *** | −3.19 | 0.32 | −10.04 | <0.001 *** |
| WA1 | 3.01 | 0.48 | 6.26 | <0.001 *** | 1.46 | 0.32 | 4.55 | <0.001 *** |
| WA2 | 3.62 | 0.49 | 7.33 | <0.001 *** | 0.69 | 0.34 | 2.04 | 0.041* |
| UK | 0.43 | 0.49 | 0.89 | 0.375 | −0.65 | 0.36 | −1.82 | 0.069 |
| 2017:FL2 | 0.56 | 0.34 | 1.64 | 0.101 | 0.39 | 0.33 | 1.19 | 0.233 |
| 2017:FL3 | 1.14 | 0.39 | 2.94 | 0.003 ** | 0.77 | 0.39 | 1.97 | 0.049 * |
| 2017:FL4 | −0.07 | 0.35 | −0.21 | 0.837 | 0.44 | 0.34 | 1.32 | 0.188 |
| 2017:FL5 | 0.10 | 0.34 | 0.31 | 0.760 | 0.50 | 0.32 | 1.56 | 0.119 |
| 2017:FL6 | 0.51 | 0.34 | 1.51 | 0.130 | 0.40 | 0.33 | 1.19 | 0.236 |
| 2017:HO | 2.44 | 0.37 | 6.54 | <0.001 *** | 1.91 | 0.36 | 5.30 | <0.001 *** |
| 2017:IT | 3.15 | 0.36 | 8.73 | <0.001 *** | 0.99 | 0.33 | 3.05 | 0.002 ** |
| 2017:WA1 | 1.01 | 0.35 | 2.91 | 0.004 ** | 0.90 | 0.38 | 2.40 | 0.016 * |
| 2017:WA2 | 0.62 | 0.36 | 1.75 | 0.080 | 1.59 | 0.39 | 4.04 | <0.001 *** |
| 2017:UK | 0.94 | 0.35 | 2.73 | 0.006 ** | 1.21 | 0.39 | 3.11 | 0.002 * |
| Phenophase | Provenance | Difference in Timing with FL1 (days) | |
|---|---|---|---|
| 2016 | 2017 | ||
| bud burst | FL3 | 1.7 | |
| FL4 | 2 | 1.9 | |
| FL6 | 1.6 | ||
| HO | −9.7 | −5.9 | |
| IT | −11.2 | −6.3 | |
| WA1 | 4.7 | 6.3 | |
| WA2 | 5.7 | 6.7 | |
| UK | 2.2 | ||
| flower opening | FL4 | 1.9 | |
| HO | −6.6 | −2.4 | |
| IT | −7 | −4.8 | |
| WA1 | 3.2 | 5.2 | |
| WA2 | 1.5 | 5 | |
| UK | 1.2 | ||
| leaf senescence | HO | 15.7 | 11.9 |
| IT | 9 | 12.3 | |
| UK | −7.5 | ||
| leaf fall | FL5 | 4.4 | |
| HO | 6.8 | 8 | |
| IT | 8.7 | 6.3 | |
| Variable | Leaf Senescence | Leaf Fall | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | Std. Error | z-Value | p-Value | Estimate | Std. Error | z-Value | p-Value | |
| DOY | 0.10 | 0.01 | 14.97 | <0.001 *** | 0.27 | 0.01 | 31.11 | <0.001 *** |
| 2017 | −1.32 | 0.33 | −4.06 | <0.001 *** | 1.89 | 0.29 | 6.45 | <0.001 *** |
| FL2 | −0.07 | 0.35 | −0.19 | 0.851 | −0.13 | 0.46 | −0.28 | 0.782 |
| FL3 | 0.13 | 0.41 | 0.32 | 0.747 | 0.49 | 0.54 | 0.91 | 0.366 |
| FL4 | −0.10 | 0.35 | −0.29 | 0.771 | −0.81 | 0.48 | −1.70 | 0.090 |
| FL5 | −0.16 | 0.34 | −0.48 | 0.635 | −1.17 | 0.46 | −2.56 | 0.010* |
| FL6 | −0.06 | 0.35 | −0.16 | 0.874 | 0.17 | 0.47 | 0.36 | 0.721 |
| HO | −1.60 | 0.35 | −4.61 | <0.001 *** | −1.82 | 0.47 | −3.83 | <0.001 *** |
| IT | −0.92 | 0.33 | −2.76 | 0.006 ** | −2.34 | 0.47 | −5.03 | <0.001 *** |
| WA1 | 0.00 | 0.34 | −0.01 | 0.996 | −0.84 | 0.46 | −1.83 | 0.068 |
| WA2 | 0.10 | 0.35 | 0.29 | 0.768 | −0.23 | 0.47 | −0.49 | 0.624 |
| UK | −0.05 | 0.35 | −0.15 | 0.884 | −0.47 | 0.47 | −1.00 | 0.317 |
| 2017:FL2 | 0.73 | 0.45 | 1.62 | 0.106 | 0.79 | 0.41 | 1.94 | 0.052 |
| 2017:FL3 | 0.16 | 0.53 | 0.31 | 0.757 | 0.01 | 0.47 | 0.02 | 0.984 |
| 2017:FL4 | 0.20 | 0.45 | 0.44 | 0.658 | 0.43 | 0.41 | 1.04 | 0.299 |
| 2017:FL5 | 0.09 | 0.44 | 0.21 | 0.834 | 0.40 | 0.39 | 1.02 | 0.308 |
| 2017:FL6 | −0.06 | 0.46 | −0.13 | 0.898 | 0.22 | 0.41 | 0.53 | 0.598 |
| 2017:HO | 0.39 | 0.46 | 0.86 | 0.391 | −0.34 | 0.41 | −0.84 | 0.402 |
| 2017:IT | −0.33 | 0.44 | −0.76 | 0.450 | 0.66 | 0.40 | 1.65 | 0.100 |
| 2017:WA1 | 0.47 | 0.45 | 1.05 | 0.294 | 0.72 | 0.40 | 1.80 | 0.072 |
| 2017:WA2 | 0.19 | 0.46 | 0.41 | 0.683 | 0.99 | 0.41 | 2.42 | 0.016 * |
| 2017:UK | 0.82 | 0.45 | 1.81 | 0.070 | 1.13 | 0.41 | 2.76 | 0.006 ** |
| Bb2016 | Fo2016 | Se2016 | Fa216 | Bb2017 | Fo2017 | Se2017 | Fa2017 | |
|---|---|---|---|---|---|---|---|---|
| Bb2016 | 0.98 | −0.87 | −0.7 | 0.97 | 0.95 | −0.85 | −0.8 | |
| Fo2016 | < 0.001 | −0.86 | −0.65 | 0.94 | 0.92 | −0.81 | −0.73 | |
| Se2016 | < 0.001 | < 0.001 | 0.79 | −0.83 | −0.7 | 0.86 | 0.9 | |
| Fa2016 | 0.017 | 0.030 | 0.003 | −0.64 | −0.53 | 0.75 | 0.89 | |
| Bb2017 | < 0.001 | < 0.001 | 0.002 | 0.036 | 0.97 | −0.82 | −0.78 | |
| Fo2017 | < 0.001 | < 0.001 | 0.019 | 0.097 | < 0.001 | −0.75 | −0.65 | |
| Se2017 | < 0.001 | 0.002 | < 0.001 | 0.008 | 0.002 | 0.008 | 0.9 | |
| Fa2017 | 0.003 | 0.010 | < 0.001 | < 0.001 | 0.004 | 0.030 | < 0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vander Mijnsbrugge, K.; Janssens, A. Differentiation and Non-Linear Responses in Temporal Phenotypic Plasticity of Seasonal Phenophases in a Common Garden of Crataegus monogyna Jacq. Forests 2019, 10, 293. https://doi.org/10.3390/f10040293
Vander Mijnsbrugge K, Janssens A. Differentiation and Non-Linear Responses in Temporal Phenotypic Plasticity of Seasonal Phenophases in a Common Garden of Crataegus monogyna Jacq. Forests. 2019; 10(4):293. https://doi.org/10.3390/f10040293
Chicago/Turabian StyleVander Mijnsbrugge, Kristine, and Astrid Janssens. 2019. "Differentiation and Non-Linear Responses in Temporal Phenotypic Plasticity of Seasonal Phenophases in a Common Garden of Crataegus monogyna Jacq." Forests 10, no. 4: 293. https://doi.org/10.3390/f10040293
APA StyleVander Mijnsbrugge, K., & Janssens, A. (2019). Differentiation and Non-Linear Responses in Temporal Phenotypic Plasticity of Seasonal Phenophases in a Common Garden of Crataegus monogyna Jacq. Forests, 10(4), 293. https://doi.org/10.3390/f10040293





