Drought and Moisture Availability and Recent Western Spruce Budworm Outbreaks in the Western United States
Abstract
:1. Introduction
1.1. Ecology of Western Spruce Budworm
1.2. Climate and WSBW Relationships
2. Materials and Methods
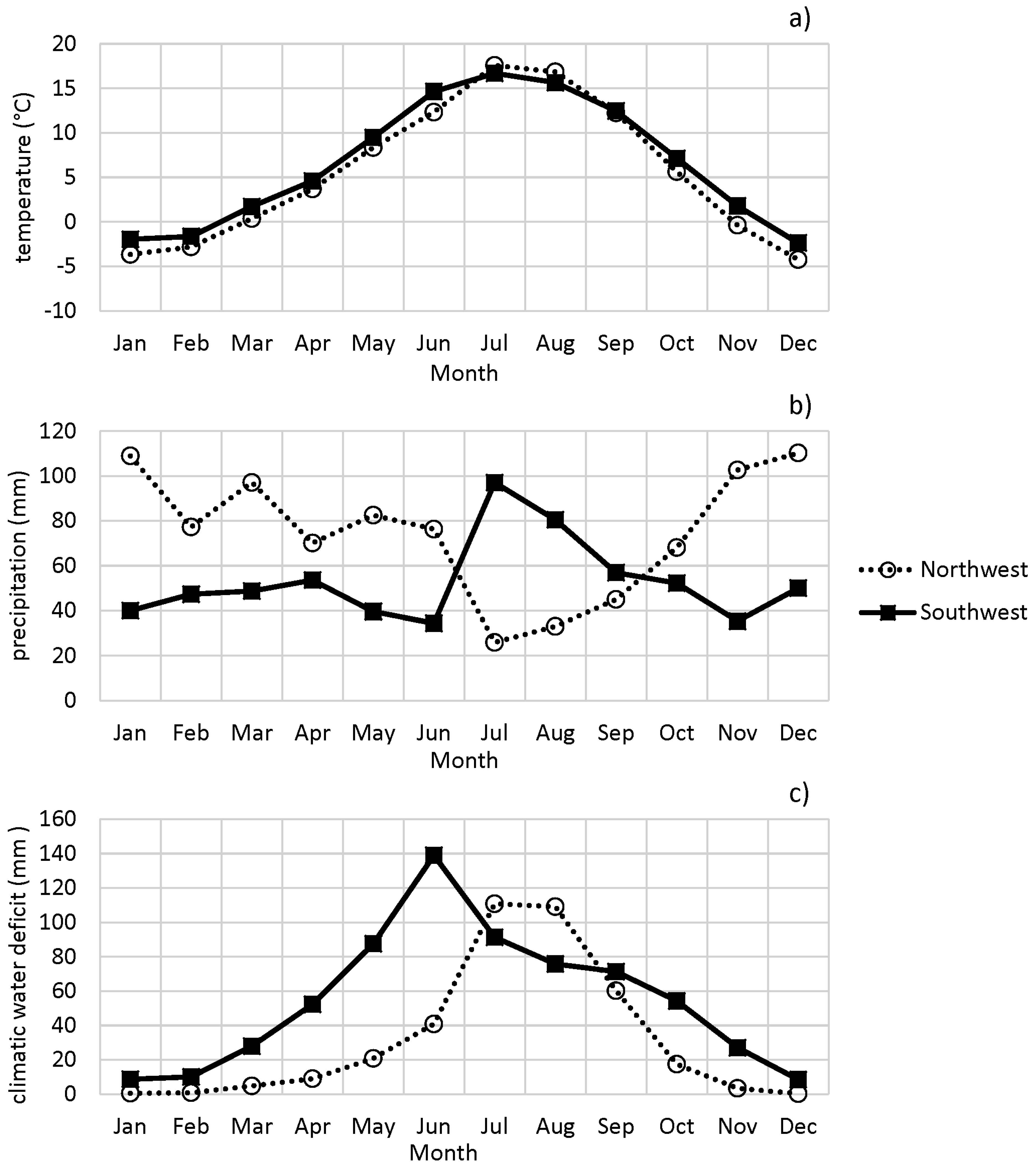
2.1. Study Regions
2.2. Data Sources
2.3. Data Processing and Analysis
3. Results
3.1. Characteristics of the Study Regions
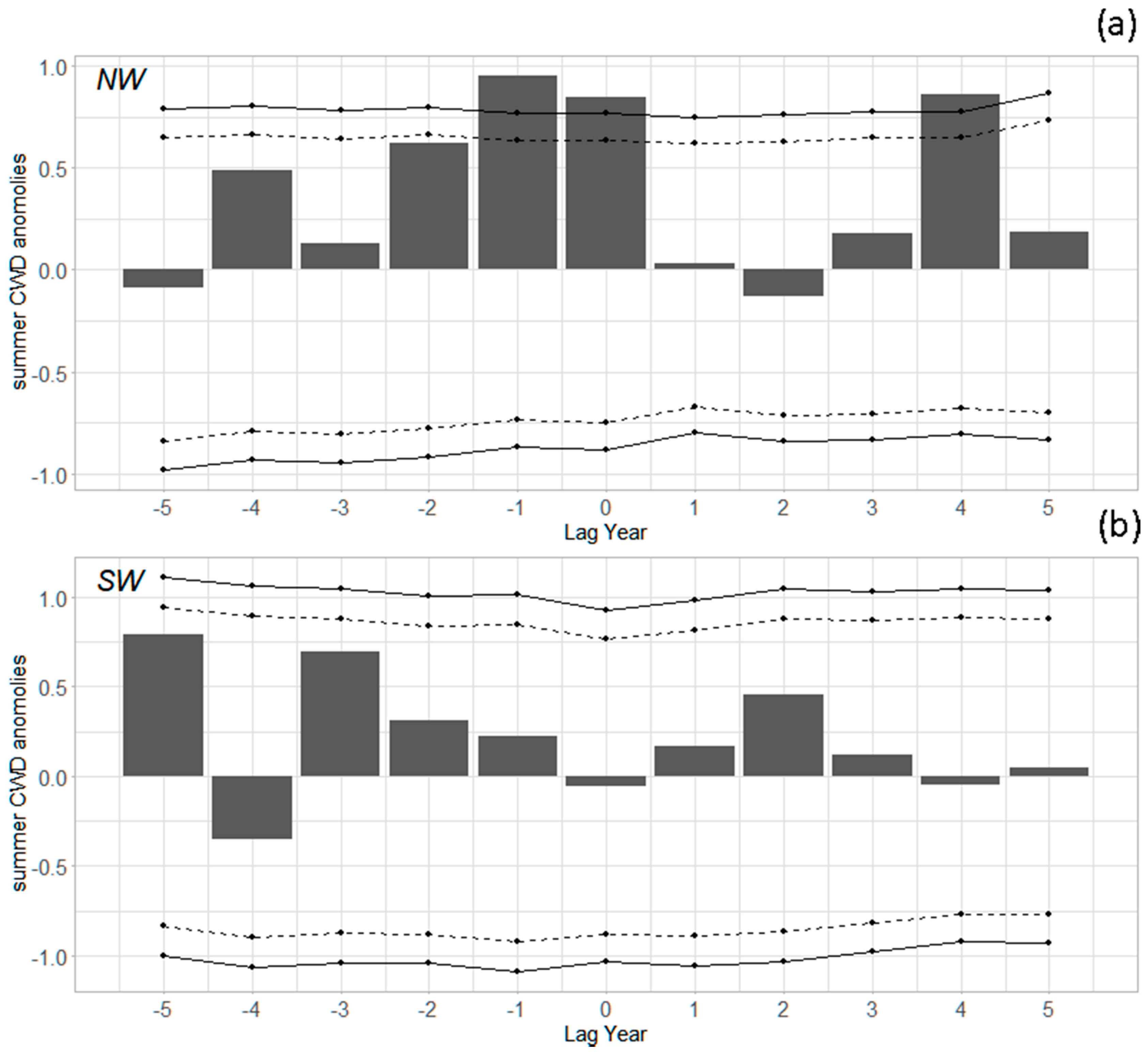
3.2. Outbreak Initiation, Climate, and Superposed Epoch Analysis
3.3. Growth Rates and Climate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fellin, D.G.; Dewey, J.E. Western Spruce Budworm; Forest Service: Washington, DC, USA, 1982.
- Williams, D.W.; Birdsey, R.A. Historical Patterns of Spruce Budworm Defoliation and Bark Beetle Outbreaks in North American Conifer Forests: An Atlas and Description of Digital Maps; General Technical Report NE-308; Forest Service: Newtown Square, PA, USA, 2003; p. 33. [CrossRef]
- Hicke, J.A.; Allen, C.D.; Desai, A.R.; Dietze, M.C.; Hall, R.J.; Hogg, E.H.T.; Kashian, D.M.; Moore, D.; Raffa, K.F.; Sturrock, R.N.; et al. Effects of biotic disturbances on forest carbon cycling in the United States and Canada. Glob. Chang. Biol. 2012, 18, 7–34. [Google Scholar] [CrossRef]
- Campbell, R.W. Population Dynamics of the Major North American Needle-Eating Budworms; Research Paper PNW-RP-463; Forest Service: Portland, OR, USA, 1993; p. 242. [CrossRef]
- Flower, A. Three Centuries of synchronous forest defoliator outbreaks in western North America. PLoS ONE 2016, 11, e0164737. [Google Scholar] [CrossRef]
- Ryerson, D.E.; Swetnam, T.W.; Lynch, A.M. A tree-ring reconstruction of western spruce budworm outbreaks in the San Juan Mountains, Colorado, USA. Can. J. For. Res. 2003, 33, 1010–1028. [Google Scholar] [CrossRef]
- Swetnam, T.W.; Lynch, A.M. Multicentury, Regional-Scale Patterns of Western Spruce Budworm Outbreaks. Ecol. Monogr. 1993, 63, 399–424. [Google Scholar] [CrossRef]
- Swetnam, T.W.; Lynch, A.M. A tree-ring reconstruction of western spruce budworm history in the southern Rocky Mountains. For. Sci. 1989, 35, 962–986. [Google Scholar]
- Kolb, T.E.; Fettig, C.J.; Ayres, M.P.; Bentz, B.J.; Hicke, J.A.; Mathiasen, R.; Stewart, J.E.; Weed, A.S. Observed and anticipated impacts of drought on forest insects and diseases in the United States. For. Ecol. Manag. 2016, 380, 321–334. [Google Scholar] [CrossRef]
- Huberty, A.F.; Denno, R.F. Plant water stress and its consequences for herbivorous insects: A new synthesis. Ecology 2004, 85, 1383–1398. [Google Scholar] [CrossRef]
- Mattson, W.J.; Haack, R.A. The role of drought in outbreaks of plant-eating insects. Bioscience 1987, 37, 110–118. [Google Scholar] [CrossRef]
- White, T.T. The abundance of invertebrate herbivores in relation to the availability of nitrogen in stressed food plants. Oecologia 1984, 63, 90–105. [Google Scholar] [CrossRef]
- Parks, C.G. The Influence of Induced Host Moisture Stress on the Growth and Development of Western Spruce Budworm and Armillaria Ostoyae on Grand Fir Seedlings. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, April 1993. [Google Scholar]
- Gower, S.T.; Vogt, K.A.; Grier, C.C. Carbon dynamics of Rocky Mountain Douglas-fir: influence of water and nutrient availability. Ecol. Monogr. 1992, 62, 43–65. [Google Scholar] [CrossRef]
- McMillin, J.D.; Wagner, M.R. Season and intensity of water stress effects on needle toughness of ponderosa pine. Can. J. For. Res. 1996, 26, 1166–1173. [Google Scholar] [CrossRef]
- Brewer, J.W.; Capinera, J.L.; Deshon, R.E.; Walmsley, M.L. Influence of Foliar Nitrogen Levels on Survival, Development, and Reproduction of Western Spruce Budworm, Choristoneura Occidentals (Lepidoptera: Tortricidae). Can. Entomol. 1985, 117, 23–32. [Google Scholar] [CrossRef]
- Hard, J.S.; Tunnock, S.; Eder, R.G. Western Spruce Budworm Defoliation Trend Relative to Weather in the Northern Region, 1969–1979. Available online: https://ir.library.oregonstate.edu/concern/defaults/1v53jz24t (accessed on 5 September 2017).
- Thomson, A.; Shepherd, R.; Harris, J.; Silversides, R. Relating weather to outbreaks of western spruce budworm, Choristoneura occidentalis (Lepidoptera: Tortricidae), in British Columbia. Can. Entomol. 1984, 116, 375–381. [Google Scholar] [CrossRef]
- Senf, C.; Wulder, M.A.; Campbell, E.M.; Hostert, P. Using Landsat to assess the relationship between spatiotemporal patterns of western spruce budworm outbreaks and regional-scale weather variability. Can. J. Remote Sens. 2016, 42, 706–718. [Google Scholar] [CrossRef]
- Flower, A.; Gavin, D.; Heyerdahl, E.; Parsons, R.; Cohn, G. Drought-triggered western spruce budworm outbreaks in the interior Pacific Northwest: A multi-century dendrochronological record. For. Ecol. Manag. 2014, 324, 16–27. [Google Scholar] [CrossRef]
- Thomson, A.J.; Benton, R. A 90-year sea warming trend explains outbreak patterns of western spruce budworm on Vancouver Island. For. Chron. 2007, 83, 867–869. [Google Scholar] [CrossRef] [Green Version]
- Mopper, S.; Whitham, T.G. The plant stress paradox: effects on pinyon sawfly sex ratios and fecundity. Ecology 1992, 73, 515–525. [Google Scholar] [CrossRef]
- Wuebbles, D.; Fahey, D.; Hibbard, K.; Dokken, B.; Stewart, B.; Maycock, T. Climate Science Special Report: Fourth National Climate Assessment, Volume I. Available online: https://repository.library.noaa.gov/view/noaa/19486/noaa_19486_DS1.pdf (accessed on 8 January 2018).
- Abatzoglou, J.T.; Dobrowski, S.Z.; Parks, S.A.; Hegewisch, K.C. TerraClimate, a high-resolution global dataset of monthly climate and climatic water balance from 1958–2015. Sci. Data 2018, 5, 170191. [Google Scholar] [CrossRef] [PubMed]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef] [Green Version]
- Harris, I.; Jones, P.D.; Osborn, T.J.; Lister, D.H. Updated high-resolution grids of monthly climatic observations–the CRU TS3. 10 Dataset. Int. J. Climatol. 2014, 34, 623–642. [Google Scholar] [CrossRef]
- Kobayashi, S.; Ota, Y.; Harada, Y.; Ebita, A.; Moriya, M.; Onoda, H.; Onogi, K.; Kamahori, H.; Kobayashi, C.; Endo, H.; et al. The JRA-55 reanalysis: General specifications and basic characteristics. J. Meteorol. Soc. Jpn. Ser. II 2015, 93, 5–48. [Google Scholar] [CrossRef]
- Palmer, C.W. Meteorological drought. In US Weather Bureau Research Paper; US Department of Commerce: Washington, DC, USA, 1965. [Google Scholar]
- Oyler, J.W.; Ballantyne, A.; Jencso, K.; Sweet, M.; Running, S.W. Creating a topoclimatic daily air temperature dataset for the conterminous United States using homogenized station data and remotely sensed land skin temperature. Int. J. Climatol. 2015, 35, 2258–2279. [Google Scholar] [CrossRef]
- Daly, C.; Halbleib, M.; Smith, J.I.; Gibson, W.P.; Doggett, M.K.; Taylor, G.H.; Curtis, J.; Pasteris, P.P. Physiographically sensitive mapping of climatological temperature and precipitation across the conterminous United States. Int. J. Climatol. 2008, 28, 2031–2064. [Google Scholar] [CrossRef] [Green Version]
- Turchin, P. Complex Population Dynamics: A Theoretical/Empirical Synthesis; Princeton University Press: Princeton, NJ, USA, 2003; Volume 35. [Google Scholar]
- Nealis, V.G.; Régnière, J. Risk of dispersal in western spruce budworm. Agric. For. Entomol. 2009, 11, 213–223. [Google Scholar] [CrossRef]
- Gergel, D.R.; Nijssen, B.; Abatzoglou, J.T.; Lettenmaier, D.P.; Stumbaugh, M.R. Effects of climate change on snowpack and fire potential in the western USA. Clim. Chang. 2017, 141, 287–299. [Google Scholar] [CrossRef]
- Seager, R.; Ting, M.; Li, C.; Naik, N.; Cook, B.; Nakamura, J.; Liu, H. Projections of declining surface-water availability for the southwestern United States. Nat. Clim. Chang. 2013, 3, 482. [Google Scholar] [CrossRef]
- Rupp, D.E.; Abatzoglou, J.T.; Mote, P.W. Projections of 21st century climate of the Columbia River Basin. Clim. Dyn. 2017, 49, 1783–1799. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Hicke, J.A.; Abatzoglou, J.T. Drought and Moisture Availability and Recent Western Spruce Budworm Outbreaks in the Western United States. Forests 2019, 10, 354. https://doi.org/10.3390/f10040354
Xu B, Hicke JA, Abatzoglou JT. Drought and Moisture Availability and Recent Western Spruce Budworm Outbreaks in the Western United States. Forests. 2019; 10(4):354. https://doi.org/10.3390/f10040354
Chicago/Turabian StyleXu, Bingbing, Jeffrey A. Hicke, and John T. Abatzoglou. 2019. "Drought and Moisture Availability and Recent Western Spruce Budworm Outbreaks in the Western United States" Forests 10, no. 4: 354. https://doi.org/10.3390/f10040354
APA StyleXu, B., Hicke, J. A., & Abatzoglou, J. T. (2019). Drought and Moisture Availability and Recent Western Spruce Budworm Outbreaks in the Western United States. Forests, 10(4), 354. https://doi.org/10.3390/f10040354




