Projecting the Potential Global Distribution of Carpomya vesuviana (Diptera: Tephritidae), Considering Climate Change and Irrigation Patterns
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Model and Software
2.1.1. CLIMEX Model
2.1.2. ArcGIS Software
2.2. Data Collection
2.2.1. Known Global Distribution of C. vesuviana
2.2.2. Climate Data
2.2.3. Irrigaton Data
2.2.4. Biological Data
2.3. Fitting Parameters
2.3.1. Growth Indices (GI)
Temperature Index (TI)
Moisture Index (MI)
2.3.2. Stress Indices (SI)
Cold Stress (CS)
Heat Stress (HS)
Dry Stress (DS)
Wet Stress (WS)
2.3.3. Effective Degree-Days (PDD)
2.4. Classification of EI Values
2.5. Parameter Verification
2.6. Analytical Results
3. Results
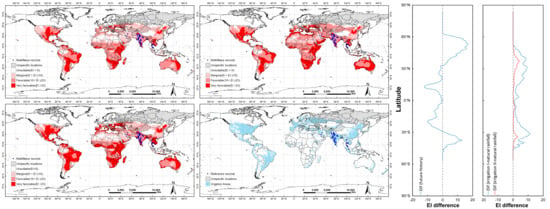
3.1. Impacts of Irrigation on the Potential Distribution of C. vesuviana
3.1.1. Potential Distribution for Two Types of Irrigation under Historical Climate Conditions
3.1.2. Comparison of the C. vesuviana Distribution for Two Types of Irrigation under Historical Climate Conditions
3.2. Potential Global Distribution of C. vesuviana under Different Climate Conditions
3.2.1. Potential Global Distribution of C. vesuviana under Historical Climate Conditions
3.2.2. Potential Global Distribution of C. vesuviana under Future Climate Conditions
3.2.3. Comparison of Distributions under Current and Future Climate Conditions
3.3. Driving Variables Limiting the Potential Distribution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yao, Y.X.; Zhao, W.X.; Huai, W.X. Discussion on exotic pests, Carpomya vesuviana and its prevention and treatment technology. In Proceedings of the National Conference on Biological Invasion, Hai Kou, China, 15–18 November 2010; p. 366. [Google Scholar]
- Lakra, R.; Singh, Z. Oviposition behaviour of ber fruitfly, Carpomyia vesuviana Costa and relationship between its incidence and ruggedness in fruits in Haryana. Indian J. Entomol. 1983, 45, 48–59. [Google Scholar]
- Azam, K.M.; Alansari, M.S.A.; Alraeesi, A.A. Fruit flies of Oman with a new record of Carpomya vasuviana Costa (Diptera: Tephritidae). Res. Crops 2004, 2, 274–277. [Google Scholar]
- Farrar, N.; Asadi, G.; Golestaneh, S. Damage and host ranges of ber fruitfly Carpomya vesuviana Costa (Diptera: Tephritidae) and its rate of parasitism. J. Agric. Sci. 2004, 1, 120–130. [Google Scholar]
- Karuppaiah, V. Biology and management of ber fruit fly, Carpomyia vesuviana Costa (Diptera: Tephritidae): A review. Afr. J. Agric. Res. 2014, 9, 1310–1317. [Google Scholar]
- Kimsanboev, K.; Murodov, B.; Yusupov, A.K. Unaby fly in Uzbekistan. Zashchita Karantin Rasteniĭ 2000, 11, 77. [Google Scholar]
- Pramanick, P.; Sharma, V.; Singh, S. Genetic behaviour of some physical and biochemical parameters of fruitfly (Carpomyia vesuviana Costa.) resistance in ber. Indian J. Hortic 2005, 62, 389–390. [Google Scholar]
- Pareek, S.; Fagera, M.; Dhaka, R. Genetic variability and association analysis for fruitfly (Carpomyia vesuviana Costa) infestation in ber. Indian J. Plant Prot. 2003, 31, 89–90. [Google Scholar]
- Gyi, M.M.; Lal, O.; Dikshit, A.; Sharma, V. Efficacy of insecticides for controlling ber fruit fly. Ann. Plant Prot. Sci. 2003, 11, 152–153. [Google Scholar]
- Zhang, R.Z.; Wang, X.J.; Satar, A. Identification and precaution of the ber fruit fly, Carpomya vesuviana, a quarantine pest insect in China. Chin. Bull. Entomol. 2007, 44, 928–930. [Google Scholar]
- Metz, B.; Davidson, O.; Bosch, P.; Dave, R.; Mayer, L. Climate Change 2007 Synthesis Report: Summary for Policymakers; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Feng, G.E. Challenges facing entomologists in a changing global climate. Chin. J. Appl. Entomol. 2011, 48, 1117–1122. [Google Scholar]
- Zhang, H.; Lin, J.T. Responses of insects to global warming. J. Environ. Entomol. 2015, 37, 1280–1286. [Google Scholar]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2010, 8, 1–16. [Google Scholar] [CrossRef]
- Singh, M. Managing menace of insect pests on ber. Indian Hortic. 2008, 53, 31. [Google Scholar]
- Yonow, T.; Kriticos, D.J.; Ota, N.; Berg, J.V.D.; Hutchison, W.D. The potential global distribution of Chilo partellus, including consideration of irrigation and cropping patterns. J. Pest Sci. 2017, 90, 459–477. [Google Scholar] [CrossRef]
- De Villiers, M.; Hattingh, V.; Kriticos, D.J.; Brunel, S.; Vayssières, J.-F.; Sinzogan, A.; Billah, M.; Mohamed, S.; Mwatawala, M.; Abdelgader, H. The potential distribution of Bactrocera dorsalis: Considering phenology and irrigation patterns. Bull. Entomol. Res. 2016, 106, 19–33. [Google Scholar] [CrossRef]
- Annam, M.; Jonathana, N. Climate change scenarios and models yield conflicting predictions about the future risk of an invasive species in North America. Agric. For. Entomol. 2010, 12, 213–221. [Google Scholar]
- Li, Z.H.; Jiang, F.; Ma, X.L.; Fang, Y.; Sun, Z.Z.; Qin, Y.J.; Wang, Q.L. Review on prevention and control techniques of Tephritidae invasion. Plant Quar. 2013, 27, 1–10. [Google Scholar]
- Lv, W.G.; Lin, W.; Li, Z.H.; Geng, J.; Wan, F.H.; Wang, Z.L. Potential geographic distribution of Ber fruit fly, Carpomya vesuviana Costa, in China. Plant Quar. 2008, 22, 343–347. [Google Scholar]
- He, S.Y. Study on the Bioecology of Carpomya vesuviana Costa and the Forecast of Its Potential Geographic Distribution in China. Master’s Thesis, Beijing Forestry University, Beijing, China, 2009. [Google Scholar]
- He, S.Y.; Zhu, Y.F.; Satar, A.; Wen, J.B.; Chen, M.; Tian, C.M. Pest Risk Assessment of Carpomya veusuviana in China. Sci. Silvae Sin. 2011, 47, 107–116. [Google Scholar]
- Chen, Y.; Ma, C.S. Effect of global warming on insect: A literature review. Acta Ecol. Sin. 2010, 30, 2159–2172. [Google Scholar]
- Kriticos, D.; Maywald, G.; Yonow, T.; Zurcher, E.; Herrmann, N.; Sutherst, R. CLIMEX Version 4: Exploring the Effects of Climate on Plants, Animals and Diseases; CSIRO: Canberra, Australia, 2015. [Google Scholar]
- Ge, X.; He, S.; Zhu, C.; Wang, T.; Xu, Z.; Shixiang, Z. Projecting the current and future potential global distribution of Hyphantria cunea (Lepidoptera: Arctiidae) using CLIMEX. Pest Manag. Sci. 2018, 75, 160–169. [Google Scholar] [CrossRef]
- Harris, I.C.; Jones, P.D. CRU TS4.01: Climatic Research Unit (CRU) Time-Series (TS) Version 4.01 of High-Resolution Gridded Data of Month-By-Month Variation in Climate (Jan. 1901–Dec. 2016). Centre for Environmental Data Analysis, 4 December 2017. Available online: http://dx.doi.org/10.5285/58a8802721c94c66ae45c3baa4d814d0 (accessed on 3 July 2018).
- Da Silva Silveira, C.; de Souza Filho, F.d.A.; das Chagas Vasconcelos, F. Projections of the Affluent Natural Energy (ANE) for the Brazilian electricity sector based on RCP 4.5 and RCP 8.5 2 scenarios of IPCC-AR5 3. Hydrol. Earth Syst. Sci. 2016. [Google Scholar] [CrossRef]
- Siebert, S.; Doll, P.; Hoogeveen, J.; Faures, J.M.; Frenken, K.; Feick, S. Development and validation of the global map of irrigation areas. Hydrol Earth Syst. Sci. 2005, 9, 535–547. [Google Scholar] [CrossRef]
- Hu, L.; Tian, C.; Zhu, Y.; Zhou, Z.; Ren, L.; Qi, C. Biological characteristics of the ber fruit fly, Carpomya vesuviana (Diptera: Tephritidae). Acta Entomol. Sin. 2013, 56, 69–78. [Google Scholar]
- Aljaryian, R.; Kumar, L. Changing global risk of invading greenbug Schizaphis graminum under climate change. Crop Prot. 2016, 88, 137–148. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Sutherst, R.W.; Brown, J.R.; Adkins, S.W.; Maywald, G.F. Climate change and the potential distribution of an invasive alien plant: Acacia nilotica ssp. indica in Australia. J. Appl. Ecol. 2003, 40, 111–124. [Google Scholar] [CrossRef]
- Sattar, A.; He, S.Y.; Tian, C.M.; Luo, Y.Q.; Yu, F.; Feng, X.F. The occurrence of Carpomya vesuviana in the Turpan area and the distribution of pupae. Plant Quar. 2008, 22, 295–297. [Google Scholar]
- Ding, J.T. Flight Capacity and Environmental Factors Adaptation of Carpomya vesuviana. Master’s Thesis, Xinjiang Agricultural University, Ürümqi, China, 2015. [Google Scholar]
- Chen, Y.J.; Liu, S.L. Physiology and drought resistance application of jujube dry elephant. Forest Sci. Technol. 1994, 25. [Google Scholar] [CrossRef]
- Ding, J.T.; Sattar, A.; Cheng, X.T.; Huang, X.Y. Amina. Supercooling points and freezing points of Carpomya vesuviana Costa. Acta Agric. Boreali Occident. Sin. 2014, 23, 163–167. [Google Scholar]
- He, S.Y.; Zhu, Y.F.; Satar, A.; Yu, F.; Wen, J.B.; Tian, C.M. Occurrence of Carpomya vesuviana in Turpan region. Chin. Bull. Entomol. 2009, 46, 930–934. [Google Scholar]
- Aljaryian, R.; Kumar, L.; Taylor, S. Modelling the current and potential future distributions of the sunn pest Eurygaster integriceps (Hemiptera: Scutelleridae) using CLIMEX. Pest Manag. Sci. 2016, 72, 1989–2000. [Google Scholar] [CrossRef]
- Sutherst, R.W. Prediction of species geographical ranges. J. Biogeogr. 2010, 30, 805–816. [Google Scholar] [CrossRef]
- Geng, X.; Zhao, X.Z.; Hou, J.M.; Han, H.Z. Biological characteristics and control strategies of Carpomya vesuviana. J. Hebei For. Sci. Technol. 2010, 73–75. [Google Scholar] [CrossRef]
- Baker, R.; Sansford, C.; Jarvis, C.; Cannon, R.; MacLeod, A.; Walters, K. The role of climatic mapping in predicting the potential geographical distribution of non-indigenous pests under current and future climates. Agric. Ecosyst. Environ. 2000, 82, 57–71. [Google Scholar] [CrossRef]
- Shabani, F.; Kumar, L.; Taylor, S. Climate change impacts on the future distribution of date palms: A modeling exercise using CLIMEX. PLoS ONE 2012, 7, e48021. [Google Scholar] [CrossRef]









| Parameters | Descriptions | Values | ||
|---|---|---|---|---|
| Lv et al. (2008) | He et al. (2011) | Current Model | ||
| Moisture | ||||
| SM0 | Lower soil moisture threshold | 0.1 | 0 | 0.028 |
| SM1 | Lower optimum soil moisture | 0.2 | 0.2 | 0.2 |
| SM2 | Upper optimum soil moisture | 0.85 | 0.4 | 0.4 |
| SM3 | Upper soil moisture threshold | 1.2 | 1.1 | 1.1 |
| Temperature | ||||
| DV0 | Lower threshold | 7.7 | 13 | 13 |
| DV1 | Lower optimum temperature | 17 | 21 | 21 |
| DV2 | Upper optimum temperature | 30 | 36 | 35 |
| DV3 | Upper threshold | 39 | 40 | 41 |
| Cold stress | ||||
| TTCS | Cold stress temperature threshold | 1.7 | −10 | −16 |
| THCS | Temperature threshold stress accumulation rate | −0.00008 | −0.00008 | −0.00008 |
| Heat stress | ||||
| TTHS | Heat stress temperature threshold | 39 | 40 | 41 |
| THHS | Temperature threshold stress accumulation rate | 0.0005 | 0.005 | 0.005 |
| Dry stress | ||||
| SMDS | Soil moisture dry stress threshold | 0.08 | 0 | 0.028 |
| HDS | Stress accumulation rate | −0.0007 | −0.00007 | −0.00007 |
| Wet stress | ||||
| SMWS | Soil moisture wet stress threshold | 1.2 | 1.1 | 1.1 |
| HWS | Stress accumulation rate | 0.007 | 0.00003 | 0.00003 |
| Threshold heat sum | ||||
| PDD | Number of degree-days above DV0 needed to complete one generation | 890 | 1100 | 800 |
| Irrigation scenario | 3.5 mm day−1 in winter | 0.3 mm day−1 in April, 1.4 mm day−1 in May and August, 2.8 mm day−1 in June and July, 0.2 mm day−1 in September | 1.5 mm day−1 in summer as top-up irrigation |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Ge, X.; Zou, Y.; Zhou, Y.; Wang, T.; Zong, S. Projecting the Potential Global Distribution of Carpomya vesuviana (Diptera: Tephritidae), Considering Climate Change and Irrigation Patterns. Forests 2019, 10, 355. https://doi.org/10.3390/f10040355
Guo S, Ge X, Zou Y, Zhou Y, Wang T, Zong S. Projecting the Potential Global Distribution of Carpomya vesuviana (Diptera: Tephritidae), Considering Climate Change and Irrigation Patterns. Forests. 2019; 10(4):355. https://doi.org/10.3390/f10040355
Chicago/Turabian StyleGuo, Siwei, Xuezhen Ge, Ya Zou, Yuting Zhou, Tao Wang, and Shixiang Zong. 2019. "Projecting the Potential Global Distribution of Carpomya vesuviana (Diptera: Tephritidae), Considering Climate Change and Irrigation Patterns" Forests 10, no. 4: 355. https://doi.org/10.3390/f10040355