Vegetative and Edaphic Responses in a Northern Mixed Conifer Forest Three Decades after Harvest and Fire: Implications for Managing Regeneration and Carbon and Nitrogen Pools
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Location and Original Characteristics
2.2. Original Study Design
2.3. Field Sampling
2.3.1. Vegetation
Aboveground
Belowground
2.3.2. Woody Residue
2.3.3. Soil
2.4. Laboratory Analysis
2.4.1. Vegetation
2.4.2. Species Diversity
2.4.3. Soil
2.5. Statistical Analysis
3. Results
3.1. Disturbances
3.1.1. MEFE
3.1.2. XETE
3.2. Vegetation
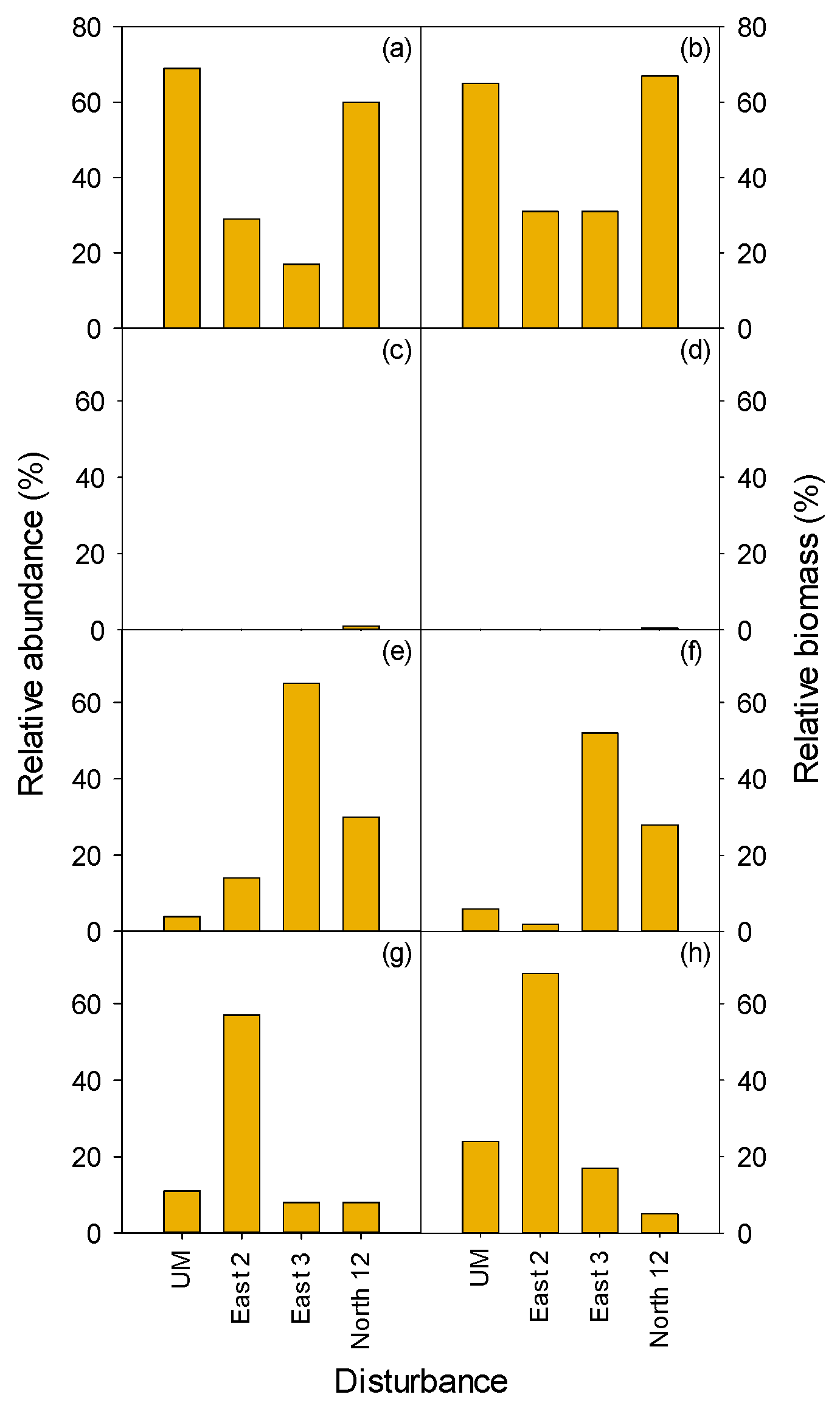
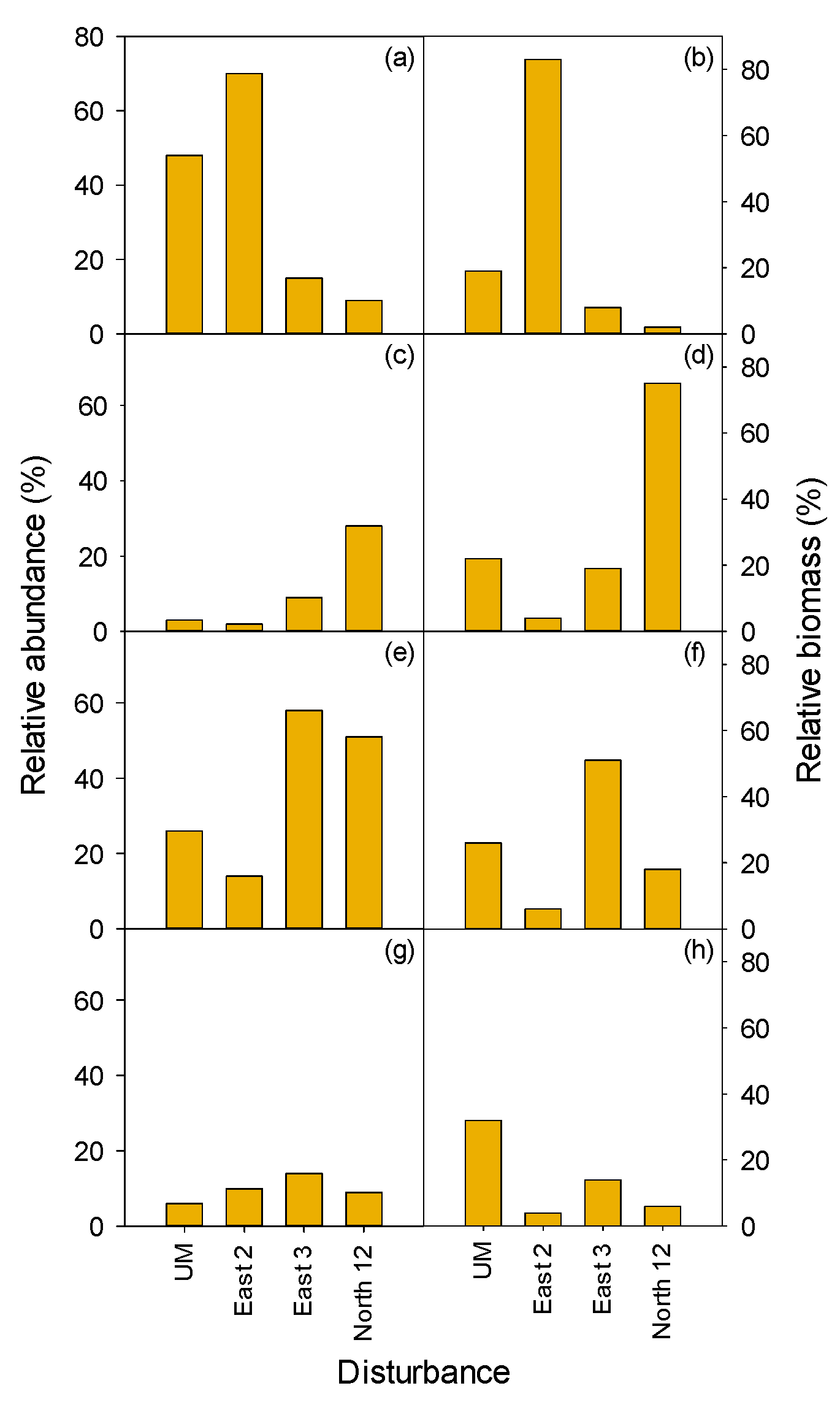
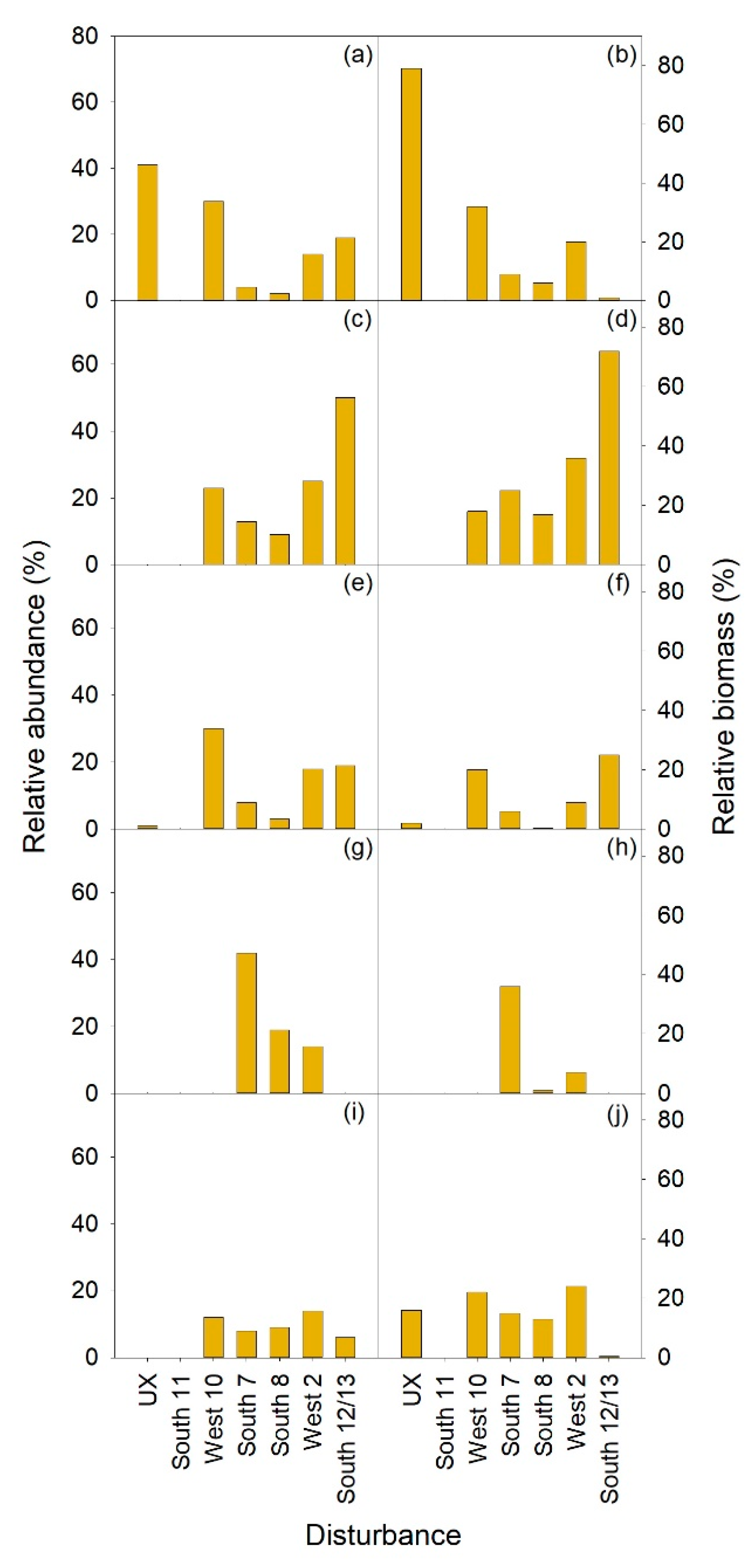
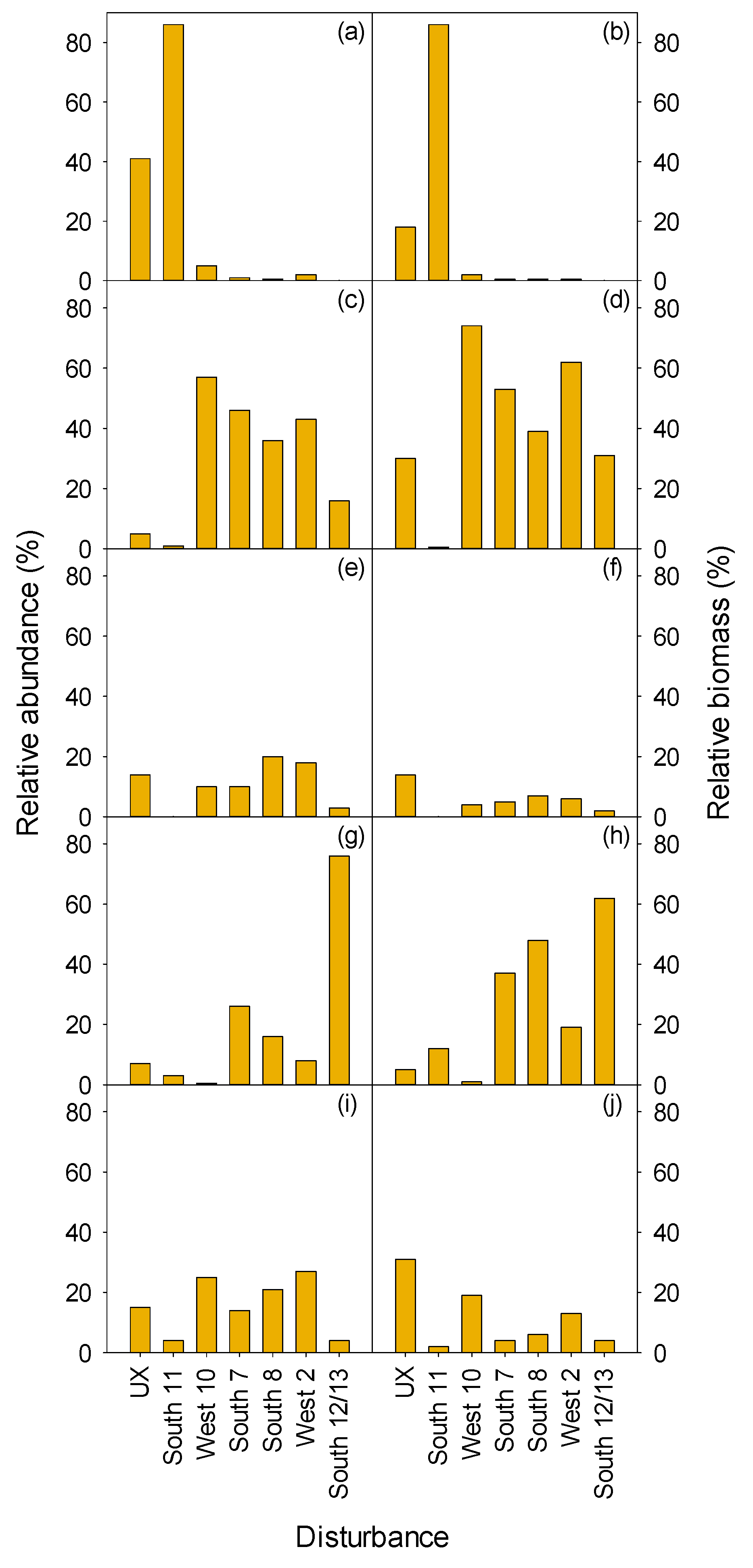
3.2.1. Herbaceous and Shrubs
MEFE
XETE
3.2.2. Seedlings and Trees
MEFE
XETE
3.2.3. Total Vegetation
3.3. Woody Residue and Soil
3.3.1. MEFE
3.3.2. XETE
3.4. C and N Pools
3.4.1. MEFE
3.4.2. XETE
4. Discussion
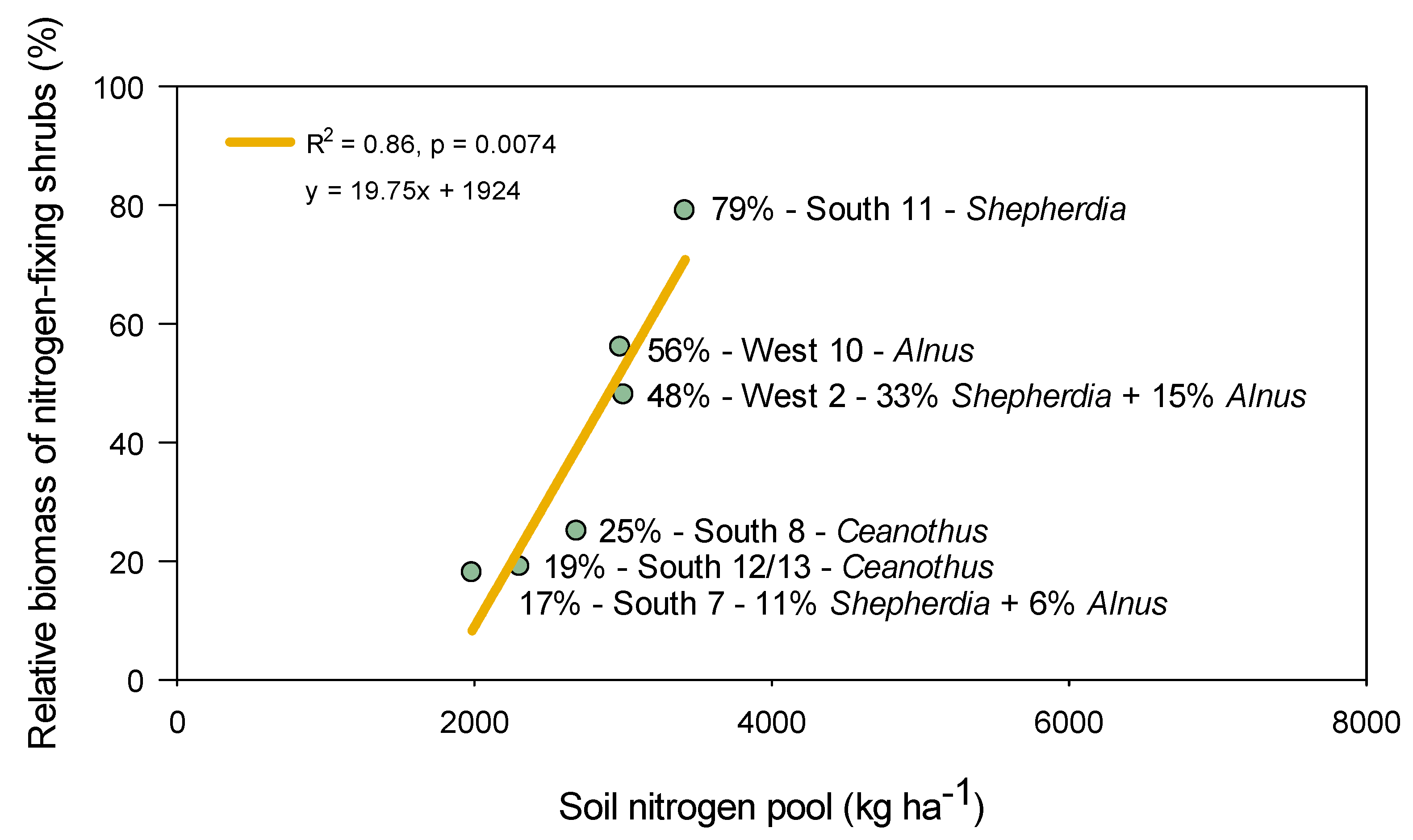
4.1. Shrub Regeneration
4.2. Larix occidentalis Regeneration
4.3. Soil Responses and C and N Pools
4.4. Management Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Turner, M.G. Disturbance and landscape dynamics in a changing world. Ecology 2010, 91, 2833–2849. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.L. Living with Fire-Sustaining Ecosystems & Livelihoods through Integrated Fire Management; The Nature Conservancy: Tallahassee, FL, USA, 2006; 36p. [Google Scholar]
- Bassman, J.H.; Johnson, J.D.; Fins, L.; Dobrowolski, J.P. Rocky Mountain ecosystems: Diversity, complexity and interactions. Tree Physiol. 2003, 23, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Long, J.N. Diversity, complexity and interactions: An overview of Rocky Mountain forest ecosystems. Tree Physiol. 2003, 23, 1091–1099. [Google Scholar] [CrossRef][Green Version]
- Agee, J.K. The landscape ecology of western forest fire regimes. Northwest Sci. 1998, 72, 24–34. [Google Scholar]
- Fischer, J.; Lindenmayer, D.B.; Manning, A.D. Biodiversity, ecosystem function, and resilience: Ten guiding principles for commodity production landscapes. Front. Ecol. Environ. 2006, 4, 80–86. [Google Scholar] [CrossRef]
- Thompson, I.; Mackey, B.; McNulty, S.; Mosseler, A. Forest Resilience, Biodiversity, and Climate Change. In A Synthesis of the Biodiversity/Resilience/Stability Relationship in Forest Ecosystems; Technical Series 43; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2009; 67p. [Google Scholar]
- Long, J.N. Emulating natural disturbance regimes as a basis for forest management: A North American view. For. Ecol. Manag. 2009, 257, 1868–1873. [Google Scholar] [CrossRef]
- Stanturf, J.A.; Palik, B.J.; Dumroese, R.K. Contemporary forest restoration: A review emphasizing function. For. Ecol. Manag. 2014, 331, 292–323. [Google Scholar] [CrossRef]
- Keane, R.E.; Agee, J.K.; Fulé, P.; Keeley, J.E.; Key, C.; Kitchen, S.G.; Miller, R.F.; Schulte, L.A. Ecological effects of large fires on US landscapes: Benefit or catastrophe? Int. J. Wildland Fire 2008, 17, 696–712. [Google Scholar] [CrossRef]
- Williams, J. Exploring the onset of high-impact mega-fires through a forest land management prism. For. Ecol. Manag. 2013, 294, 4–10. [Google Scholar] [CrossRef]
- Stanturf, J.A.; Palik, B.J.; Williams, M.I.; Dumroese, R.K.; Madsen, P. Forest restoration paradigms. J. Sustain. For. 2014, 33, S161–S194. [Google Scholar] [CrossRef]
- Keeley, J.E.; Aplet, G.H.; Christensen, N.L.; Conard, S.C.; Johnson, E.A.; Omi, P.N.; Peterson, D.L.; Swetnam, T.W. Ecological Foundations for Fire Management in North American Forest and Shrubland Ecosystems; General Technical Report PNW-GTR-779; USDA Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2009; 92p. Available online: https://www.fs.usda.gov/treesearch/pubs/32483 (accessed on 11 August 2020).
- Agee, J.K. Fire and Weather Disturbances in Terrestrial Ecosystems of the Eastern Cascades; General Technical Report PNW-GTR-320; USDA Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 1994; 52p. [CrossRef]
- Arno, S.F.; Fischer, W.C. Larix occidentalis—Fire Ecology and Fire Management. In Ecology and Management of Larix Forests: A Look Ahead; General Technical Report, GTR-INT-319; Schmidt, W.C.; McDonald, K.J. (Compiler) USDA Forest Service, Intermountain Research Station: Ogden, UT, USA, 1995; pp. 130–135. [Google Scholar]
- Schmidt, W.C.; Shearer, R.C. Larix occidentalis: A Pioneer of The North American West. In Ecology and Management of Larix Forests: A Look Ahead; General Technical Report, GTR-INT-319; Schmidt, W.C.; McDonald, K.J. (Compiler) USDA Forest Service, Intermountain Research Station: Ogden, UT, USA, 1995; pp. 33–37. [Google Scholar]
- Camp, A.; Oliver, C.; Hessburg, P.; Everett, R. Predicting late-successional fire refugia pre-dating European settlement in the Wenatchee Mountains. For. Ecol. Manag. 1997, 95, 63–77. [Google Scholar] [CrossRef]
- Shearer, R.C. Seedbed Characteristics in Western Larch Forests after Prescribed Burning; Research Paper INT-167; USDA Forest Service, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1975; 26p.
- Shearer, R.C.; Stickney, P.F. Natural Revegetation of Burned and Unburned Clearcuts in Western Larch Forests of Northwest Montana. In Fire and the Environment: Ecological and Cultural Perspectives; General Technical Report, SE-69; Nodvin, S.C., Waldrop, T.A., Eds.; USDA Forest Service, Southeastern Forest Experiment Station: Asheville, NC, USA, 1991; pp. 66–74. [Google Scholar] [CrossRef]
- Harmon, M.E.; Ferrell, W.K.; Franklin, J.F. Effects on carbon storage of conversion of old-growth forests to young forests. Science 1990, 247, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.; Page-Dumroese, D.S.; Keyes, C.R. Long-term soil changes from forest harvesting and residue management in the Northern Rocky Mountains. Soil Sci. Soc. Am. J. 2016, 80, 727–741. [Google Scholar] [CrossRef]
- Gough, C.M.; Vogel, C.S.; Harrold, K.H.; George, K.; Curtis, P.S. The legacy of harvest and fire on ecosystem carbon storage in a north temperate forest. Glob. Chang. Boil. 2007, 13, 1935–1949. [Google Scholar] [CrossRef]
- Powers, R.F.; Scott, D.A.; Sanchez, F.G.; Voldseth, R.A.; Page-Dumroese, D.; Elioff, J.D.; Stone, D.M. The North American long-term soil productivity experiment: Findings from the first decade of research. For. Ecol. Manag. 2005, 220, 31–50. [Google Scholar] [CrossRef]
- Bisbing, S.M.; Alaback, P.B.; DeLuca, T.H. Carbon storage in old-growth and second growth fire-dependent western larch (Larix occidentalis Nutt.) forests of the Inland Northwest, USA. For. Ecol. Manag. 2010, 259, 1041–1049. [Google Scholar] [CrossRef]
- Spies, T.A.; Franklin, J.F.; Thomas, T.B. Coarse woody debris in Douglas-fir forests of western Oregon and Washington. Ecology 1988, 69, 1689–1702. [Google Scholar] [CrossRef]
- Harmon, M.E.; Marks, B. Effects of silvicultural practices on carbon stores in Douglas-fir—western hemlock forests in the Pacific Northwest, USA: Results from a simulation model. Can. J. For. Res. 2002, 32, 863–877. [Google Scholar] [CrossRef]
- Lattimore, B.; Smith, C.T.; Titus, B.D.; Stupak, I.; Egnell, G. Environmental factors in woodfuel production: Opportunities, risks, and criteria and indicators for sustainable practices. Biomass Bioenergy 2009, 33, 1321–1342. [Google Scholar] [CrossRef]
- Thiffault, E.; Hannam, K.D.; Paré, D.; Titus, B.D.; Hazlett, P.W.; Maynard, D.G.; Brais, S. Effects of forest biomass harvesting on soil productivity in boreal and temperate forests—A review. Environ. Rev. 2011, 19, 278–309. [Google Scholar] [CrossRef]
- Page-Dumroese, D.S.; Jurgensen, M.F. Soil carbon and nitrogen pools in mid- to late-successional forest stands of the northwestern United States: Potential impact of fire. Can. J. For. Res. 2006, 36, 2270–2284. [Google Scholar] [CrossRef]
- McNabb, D.H.; Cromack, K., Jr. Effects of prescribed fire on nutrients and soil productivity. In Natural and Prescribed Fire in Pacific Northwest Forests; Walstad, J.D., Radosevich, S.R., Sandberg, D.V., Eds.; Oregon State University Press: Corvallis, OR, USA, 1990; pp. 125–142. [Google Scholar]
- Grogan, P.; Bruns, T.D.; Chapin, F.S. III. Fire effects on ecosystem nitrogen cycling in a California bishop pine forest. Oecologia 2000, 122, 537–544. Available online: https://www.jstor.org/stable/4222577 (accessed on 11 August 2020). [PubMed]
- DeBano, L.F.; Eberlein, G.E.; Dunn, P.H. Effects of burning on chaparral soils: I. Soil nitrogen. Soil Sci. Soc. Am. J. 1979, 43, 504–509. [Google Scholar] [CrossRef]
- Raison, R.J.; Khanna, P.K.; Woods, P.V. Mechanisms of element transfer to the atmosphere during vegetation fires. Can. J. For. Res. 1985, 15, 132–140. [Google Scholar] [CrossRef]
- Gillon, D.; Rapp, M. Nutrient losses during a winter low-intensity prescribed fire in a Mediterranean forest. Plant Soil 1989, 120, 69–77. [Google Scholar] [CrossRef]
- Jurgensen, M.F.; Harvey, A.E.; Graham, R.T.; Page-Dumroese, D.S.; Tonn, J.T.; Larsen, M.J.; Jain, T.B. Impacts of timber harvesting on soil organic matter, nitrogen, productivity, and health of Inland Northwest forests. For. Sci. 1997, 43, 234–251. [Google Scholar] [CrossRef]
- Powers, R.F.; Alban, D.H.; Miller, R.E.; Tiarks, A.E.; Wells, C.G.; Avers, P.E.; Cline, R.G.; Fitzgerald, R.D.; Loftus, N.S., Jr. Sustaining Site Productivity in North America Forests: Problems and Prospects. In Sustained Productivity of Forest Soils, Proceedings of the Seventh North American Forest Soils Conference, University of British Columbia, Vancouver, British Columbia, Canada, August 1998; Gessel, S.P., Lacate, D.S., Weetman, G.F., Powers, R.F., Eds.; Forestry Publications, Faculty of Forestry, University of British Columbia: Vancouver, BC, Canada, 1990; pp. 49–79. [Google Scholar]
- Burger, J.A.; Mitchem, D.O.; Scott, D.A. Field Assessment of Mine Site Quality for Establishing Hardwoods in the Appalachians. In Proceedings: American Society of Mining and Reclamation 19th Annual National Conference and International Affiliation of Land Reclamation 6th International Conference, Lexington, KY, USA, 9–13 June 2002; Barnhisel, R., Collins, M., Eds.; American Society of Mining and Reclamation: Lexington, KY, USA, 2002; pp. 226–240. [Google Scholar]
- Sanchez, F.G.; Tiarks, A.E.; Kranabetter, J.M.; Page-Dumroese, D.S.; Powers, R.F.; Sanborn, P.T.; Chapman, W.K. Effects of organic matter removal and soil compaction on fifth-year mineral soil carbon and nitrogen contents for sites across the United States and Canada. Can. J. For. Res. 2006, 36, 565–576. [Google Scholar] [CrossRef]
- Clarke, N.; Gundersen, P.; Jönsson-Belyazid, U.; Kjønaas, O.J.; Persson, T.; Sigurdsson, B.D.; Stupak, I.; Vesterdal, L. Influence of different tree-harvesting intensities on forest soil carbon stocks in boreal and northern temperate forest ecosystems. For. Ecol. Manag. 2015, 351, 9–19. [Google Scholar] [CrossRef]
- Shepherd, M.; Harrison, R.; Webb, J. Managing soil organic matter—implications for soil structure on organic farms. Soil Use Manag. 2002, 18, 284–292. [Google Scholar] [CrossRef]
- Jastrow, J. Soil aggregate formation and the accrual of particulate and mineral-associated organic matter. Soil Boil. Biochem. 1996, 28, 665–676. [Google Scholar] [CrossRef]
- Harvey, A.E.; Larsen, M.J.; Jurgensen, M.F. Ecology of Ectomycorrhizae in a Northern Rocky Mountain Forest. In Environmental Consequences of Timber Harvesting in Rocky Mountain Coniferous Forests; General Technical Report GTR-INT-90; USDA Forest Service, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1980; pp. 189–208. [Google Scholar]
- Walker, B.H.; Steffen, W.L. The Nature of Global Change. In The Terrestrial Biosphere and Global Change; Walker, B.H., Steffen, W.L., Canadell, J., Ingram, J., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 1–18. [Google Scholar]
- Rehfeldt, G.E.; Jaquish, B.C. Ecological impacts and management strategies for western larch in the face of climate-change. Mitig. Adapt. Strat. Glob. Chang. 2010, 15, 283–306. [Google Scholar] [CrossRef]
- Beaufait, W.R.; Hardy, C.E.; Fischer, W.C. Broadcast Burning in Larch-Fir Clearcuts: The Miller Creek-Newman Ridge Study; Research Paper INT-175; USDA Forest Service, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1977; 53p.
- DeByle, N.V. Clearcutting and Fire in the Larch/Douglas-fir Forests of Western Montana—A Multifaceted Research Summary; General Technical Report INT-99; USDA Forest Service, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1981; 73p.
- Shearer, R.C.; Stickney, P.F.; VanDenburg, J.H.; Wirt, S.E. A Long-Term Management and Research Partnership Facilitates Ecosystem Management Opportunities in a Montana Western Larch Forest. In Silviculture: From the Cradle of Forestry to Ecosystem Management: Proceedings of the National Silviculture Workshop; General Technical Report, SE-88; Foley, L.H. (Compiler) USDA Forest Service, Southeastern Forest Experiment Station: Asheville, NC, USA, 1994; pp. 194–200. [Google Scholar] [CrossRef]
- Soil Survey Staff, USDA Natural Resources Conservation Service. Official Soil Series Descriptions. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/main/soils/survey/ (accessed on 13 July 2020).
- Eyre, F.H. (Ed.) Forest Cover Types of the United States and Canada; Society of American Foresters: Bethesda, MD, USA, 1980; 148p. [Google Scholar]
- Pfister, R.D.; Kovalchik, B.; Arne, S.; Presby, R. Forest Habitat Types of Montana; General Technical Report INT-34; USDA Forest Service, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1977; 174p. Available online: https://www.fs.usda.gov/treesearch/pubs/41077 (accessed on 11 August 2020).
- Stickney, P.F.; Campbell, R.B., Jr. Data Base for Early Postfire Succession in Northern Rocky Mountain Forests; General Technical Report RMRS-GTR-61CD; USDA Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 2000. [CrossRef]
- Beaufait, W.R. An integrating device for evaluating prescribed fire. For. Sci. 1966, 12, 27–29. [Google Scholar] [CrossRef]
- Latham, P.A.; Shearer, R.C.; O’Hara, K.L. Miller Creek Demonstration Forest—A Forest Born of Fire: A Field Guide; General Technical Report RMRS-GTR-7; USDA Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 1998; 68p. [CrossRef]
- U. S. Department of Agriculture. National Fire-danger Rating Handbook; Handbook 5109.1; USDA Forest Service: Washington, DC, USA, 1964.
- DeByle, N.V. Broadcast burning of logging residues and the water repellency of soils. Northwest Sci. 1973, 47, 77–87. [Google Scholar]
- Shearer, R.C.; Schmidt, J.A.; Stickney, P.F. Development of Natural and Planted Conifer Regeneration and Forest Succession of Young Stands Following Clearcutting and Burning Treatments, within the Western Larch Forest Cover Type, Miller Creek Demonstration Forest, Tally Lake Ranger District, Flathead National Forest; Study Plan INT-4151-021; USDA Forest Service, Intermountain Research Station: Missoula, MT, USA, 1991.
- Bonham, C.D. Measurements for Terrestrial Vegetation; John Wiley & Sons Inc.: New York, NY, USA, 1989; 338p. [Google Scholar]
- Brown, J.K. Estimating shrub biomass from basal stem diameters. Can. J. For. Res. 1976, 6, 153–158. [Google Scholar] [CrossRef]
- Integrated Taxonomic Information System (ITIS) Online Database. Available online: https://www.itis.gov (accessed on 13 July 2020).
- Cairns, M.A.; Brown, S.; Helmer, E.H.; Baumgardner, G.A. Root biomass allocation in the world’s upland forests. Oecologia 1997, 111, 1–11. [Google Scholar] [CrossRef]
- Brown, J.K. Handbook for Inventorying Downed Woody Material; General Technical Report INT-GTR-16; USDA Forest Service, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1974; 24p. [Google Scholar]
- Jurgensen, M.F.; Larsen, M.J.; Harvey, A.E. A Soil Sampler for Steep, Rocky Slopes; Research Note INT-RN-217; USDA Forest Service, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1977; 5p. [Google Scholar]
- Page-Dumroese, D.S.; Brown, R.E.; Jurgensen, M.F.; Mroz, G.D. Comparison of methods for determining bulk densities of rocky forest soils. Soil Sci. Soc. Am. J. 1999, 63, 379–383. [Google Scholar] [CrossRef]
- Triska, F.J.; Cromack, K., Jr. The Role of Woody Debris in Forests and Streams. In Forests: Fresh Perspectives from Ecosystem Analysis; Waring, R.H., Ed.; Oregon State University Press: Corvallis, OR, USA, 1979; pp. 171–190. [Google Scholar]
- Reinhardt, E.D.; Crookston, N.L. (Eds.) The Fire and Fuels Extension to the Forest Vegetation Simulator; General Technical Report RMRS-GTR-116; USDA Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 2003; 209p. [CrossRef]
- Dixon, G.E. (Compiler) Essential FVS: A User’s Guide to the Forest Vegetation Simulator; Internal Report (12 August 2011 revision); USDA Forest Service, Forest Management Service Center: Fort Collins, CO, USA, 2002; 244p. Available online: https://www.fs.fed.us/fmsc/fvs/documents/userguides.shtml (accessed on 5 April 2017).
- Keyser, C.E. (Compiler) Northern Idaho/Inland Empire (NI/IE) Variants Overview—Forest Vegetation Simulator; Internal Report (4 October 2011 revision); USDA Forest Service, Forest Management Service Center: Fort Collins, CO, USA, 2008; 49p. Available online: https://www.fs.fed.us/fmsc/fvs/documents/userguides.shtml (accessed on 5 April 2017).
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar]
- Cromack, K., Jr.; Miller, R.E.; Anderson, H.W.; Helgerson, O.T.; Smith, R.B. Soil carbon and nutrients in a coastal Oregon Douglas-fir plantation with red alder. Soil Sci. Soc. Am. J. 1999, 63, 232–239. [Google Scholar] [CrossRef]
- Harrison, R.B.; Adams, A.B.; Licata, C.; Flaming, B.; Wagoner, G.L.; Carpenter, P.; Vance, E.D. Quantifying deep-soil and coarse-soil fractions. Soil Sci. Soc. Am. J. 2003, 67, 1602–1606. [Google Scholar] [CrossRef]
- Whitney, N.; Zabowski, D. Total soil nitrogen in the coarse fraction and at depth. Soil Sci. Soc. Am. J. 2004, 68, 612–619. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 3 January 2020).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.Y. Extension of multiple range tests to group means with unequal numbers of replications. Biometrics 1956, 12, 307–310. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Erickson, H.E.; Soto, P.; Johnson, D.W.; Roath, B.; Hunsaker, C. Effects of vegetation patches on soil nutrient pools and fluxes within a mixed-conifer forest. For. Sci. 2005, 51, 211–220. [Google Scholar] [CrossRef]
- Bormann, B.T.; Darbyshire, R.L.; Homann, P.S.; Morrissette, B.A.; Little, S.N. Managing early succession for biodiversity and long-term productivity of conifer forests in southwestern Oregon. For. Ecol. Manag. 2015, 340, 114–125. [Google Scholar] [CrossRef]
- Schrempp, T.V.; Rachlow, J.L.; Johnson, T.R.; Shipley, L.A.; Long, R.A.; Aycrigg, J.L.; Hurley, M.A. Linking forest management to moose population trends: The role of the nutritional landscape. PLoS ONE 2019, 14, e0219128. [Google Scholar] [CrossRef]
- Lyon, L.J.; Stickney, P.F. Early Vegetal Succession Following Large Northern Rocky Mountain Wildfires. In Proceedings of the Montana Tall Timbers Fire Ecology Conference and Fire and Land Management Symposium; 14. Tall Timbers Research Station: Tallahassee, FL, USA, 1976; pp. 355–375. [Google Scholar]
- Lentile, L.B.; Morgan, P.; Hudak, A.T.; Bobbitt, M.J.; Lewis, S.A.; Smith, A.M.S.; Robichaud, P.R. Post-fire burn severity and vegetation response following eight large wildfires across the western United States. Fire Ecol. 2007, 3, 91–108. [Google Scholar] [CrossRef]
- Crotteau, J.S.; Varner, J.M.; Ritchie, M.W. Post-fire regeneration across a fire severity gradient in the southern Cascades. For. Ecol. Manag. 2013, 287, 103–112. [Google Scholar] [CrossRef]
- Chase, C.W.; Kimsey, M.J.; Shaw, T.M.; Coleman, M.D. The response of light, water, and nutrient availability to pre-commercial thinning in dry inland Douglas-fir forests. For. Ecol. Manag. 2016, 363, 98–109. [Google Scholar] [CrossRef]
- Mueggler, W.F. Ecology of seral shrub communities in the cedar-hemlock zone of Northern Idaho. Ecol. Monogr. 1965, 35, 165–185. [Google Scholar] [CrossRef]
- Irwin, L.L.; Peek, J.M. Shrub production and biomass trends following five logging treatments within the cedar-hemlock zone of northern Idaho. For. Sci. 1979, 25, 415–426. [Google Scholar] [CrossRef]
- Alldredge, M.W.; Peek, J.M.; Wall, W.A. Shrub community development and annual productivity trends over a 100-year period on an industrial forest of Northern Idaho. For. Ecol. Manag. 2001, 152, 259–273. [Google Scholar] [CrossRef]
- Jang, W.; Keyes, C.R.; Page-Dumroese, D.S. Recovery and diversity of the forest shrub community 38 years after biomass harvesting in the northern Rocky Mountains. Biomass Bioenergy 2016, 92, 88–97. [Google Scholar] [CrossRef]
- Alaback, P.B. Dynamics of understory biomass in Sitka spruce—western hemlock forests of Southeast Alaska. Ecology 1982, 63, 1932–1948. [Google Scholar] [CrossRef]
- Oliver, C.D. Forest development in North America following major disturbances. For. Ecol. Manag. 1980, 3, 153–168. [Google Scholar] [CrossRef]
- Habeck, R.J. Menziesia ferruginea. In Fire Effects Information System; USDA Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory: Missoula, MT, USA, 1992; Available online: https://www.fs.fed.us/database/feis/plants/shrub/menfer/all.html (accessed on 16 July 2020).
- Simonin, K.A. Vaccinium membranaceum. In Fire Effects Information System; USDA Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory: Missoula, MT, USA, 2000; Available online: https://www.fs.fed.us/database/feis/plants/shrub/vacmem/all.html (accessed on 16 July 2020).
- Uchytil, R.J. Alnus viridis subsp. sinuata. In Fire Effects Information System; USDA Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory: Missoula, MT, USA, 1989; Available online: https://www.fs.fed.us/database/feis/plants/shrub/alnvirs/all.html (accessed on 15 July 2020).
- Wilcots, M.; Taylor, B.; Kuprewicz, E.K.; Menge, D.N.L. Small traits with big consequences: How seed traits of nitrogen-fixing plants might influence ecosystem nutrient cycling. Oikos 2019, 128, 668–679. [Google Scholar] [CrossRef]
- Anderson, M.D. Salix scouleriana. In Fire Effects Information System; USDA Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory: Missoula, MT, USA, 2001; Available online: https://www.fs.fed.us/database/feis/plants/tree/salsco/all.html (accessed on 15 July 2020).
- Walkup, C.J. Shepherdia canadensis. In Fire Effects Information System; USDA Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory: Missoula, MT, USA, 1991; Available online: https://www.fs.fed.us/database/feis/plants/shrub/shecan/all.html (accessed on 15 July 2020).
- Walls, L.; Zamora, B.A. Nitrogen-fixing Nodule Characterization and Morphology of Four Species in the Northern Intermountain Region. In Shrubland Ecosystem Genetics and Biodiversity: Proceedings; Proceedings, RMRS-P-21; McArthur, E.D., Fairbanks, D.J., Eds.; USDA Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 2001; pp. 295–301. Available online: https://www.fs.usda.gov/treesearch/pubs/44600 (accessed on 15 July 2020).
- Rosner, L.S.; Harrington, J.T. Optimizing acid scarification and stratification combinations for russet buffaloberry seeds. Native Plants, J. 2003, 4, 81–86. [Google Scholar] [CrossRef]
- Johnstone, J.F. Response of boreal plant communities to variations in previous fire-free interval. Int. J. Wildland Fire 2006, 15, 497–508. [Google Scholar] [CrossRef]
- Gratkowski, H.J. Seeds of Ceanothus velutinus var. laevigatus T. & G. Ph.D. Dissertation, Oregon State University, Corvallis, OR, USA, 1962; 122p. [Google Scholar]
- Noste, N.V.; Bushey, C.L. Fire Response of Shrubs of Dry Forest Habitat Types in Montana and Idaho; General Technical Report INT-239; USDA Forest Service, Intermountain Research Station: Ogden, UT, USA, 1987; 22p. [Google Scholar]
- Stickney, P.F. Data Base for Post-Fire Succession, First 6 to 9 Years, in Montana Larch-Fir Forests; General Technical Report INT-62; USDA Forest Service, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1980; 133p.
- Youngberg, C.T.; Wollum, A.G., II. Nitrogen accretion in developing Ceanothus velutinus stands. Soil Sci. Soc. Am. J. 1976, 40, 109–112. [Google Scholar] [CrossRef]
- Newland, J.A.; DeLuca, T.H. Influence of fire on native nitrogen-fixing plants and soil nitrogen status in ponderosa pine—Douglas-fir forests in western Montana. Can. J. For. Res. 2000, 30, 274–282. [Google Scholar] [CrossRef]
- Rhoades, C.; Binkley, D.; Oskarsson, H.; Stottlemyer, R. Soil nitrogen accretion along a floodplain terrace chronosequence in northwest Alaska: Influence of the nitrogen-fixing shrub Shepherdia canadensis. Écoscience 2008, 15, 223–230. [Google Scholar] [CrossRef]
- Zavitkovski, J.; Newton, M. Ecological importance of snowbrush Ceanothus velutinus in the Oregon Cascades. Ecology 1968, 49, 1134–1145. [Google Scholar] [CrossRef]
- Hendrickson, O.Q.; Burgess, D. Nitrogen-fixing plants in a cut-over lodgepole pine stand of southern British Columbia. Can. J. For. Res. 1989, 19, 936–939. [Google Scholar] [CrossRef]
- Busse, M.D. Suitability and use of the 15N-isotope dilution method to estimate nitrogen fixation by actinorhizal shrubs. For. Ecol. Manag. 2000, 136, 85–95. [Google Scholar] [CrossRef]
- Yelenik, S.; Perakis, S.; Hibbs, D. Regional constraints to biological nitrogen fixation in post-fire forest communities. Ecology 2013, 94, 739–750. [Google Scholar] [CrossRef]
- Freund, S.M.; Soper, F.; Poulson, S.R.; Selmants, P.C.; Sullivan, B.W. Actinorhizal species influence plant and soil nitrogen status of semiarid shrub-dominated ecosystems in the western Great Basin, USA. J. Arid. Environ. 2018, 157, 48–56. [Google Scholar] [CrossRef]
- Png, G.K.; Lambers, H.; Kardol, P.; Turner, B.L.; Wardle, D.A.; Laliberté, E. Biotic and abiotic plant–soil feedback depends on nitrogen-acquisition strategy and shifts during long-term ecosystem development. J. Ecol. 2019, 107, 142–153. [Google Scholar] [CrossRef]
- Hibbs, D.E.; Cromack, K., Jr. Actinorhizal Plants in Pacific Northwest Forests. In The Biology of Frankia and Actinorhizal Plants; Schwintzer, C.R., Tjepkema, J.D., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 343–363. [Google Scholar]
- Walker, L.R. Nitrogen fixers and species replacements in primary succession. In Primary Succession on Land; Miles, J., Walton, W.H.D., Eds.; Blackwell Scientific Publications: Oxford, UK, 1993; pp. 249–272. [Google Scholar]
- Shen, C.; Nelson, A.S. Natural conifer regeneration patterns in temperate forests across the Inland Northwest, USA. Ann. For. Sci. 2018, 75, 54. [Google Scholar] [CrossRef]
- Artley, D.K.; Shearer, R.C.; Steele, R.W. Effects of Burning Moist Fuels on Seedbed Preparation in Cutover Western Larch Forests; Research Paper INT-211; USDA Forest Service, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1978; 16p.
- Niinemets, Ü.; Valladares, F. Tolerance to shade, drought, and waterlogging of temperate northern hemisphere trees and shrubs. Ecol. Monogr. 2006, 76, 521–547. [Google Scholar] [CrossRef]
- Oswald, B.P.; Neuenschwander, L.F. Microsite variability and safe site description for western larch germination and establishment. Bull. Torrey Bot. Club 1993, 120, 148–156. [Google Scholar] [CrossRef]
- Schmidt, W.C. Seedbed Treatments Influence Seedling Development in Western Larch Forests; Research Note INT-93; UDSA Forest Service, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1969; 7p.
- Steed, J.E.; Goeking, S.A. Western Larch Regeneration Responds More Strongly to Site and Indirect Climate Factors than to Direct Climate Factors. Forest 2020, 11, 482. [Google Scholar] [CrossRef]
- Neary, D.G.; Klopatek, C.C.; DeBano, L.F.; Ffolliott, P.F. Fire effects on belowground sustainability: A review and synthesis. For. Ecol. Manag. 1999, 122, 51–71. [Google Scholar] [CrossRef]
- Harmon, M.E.; Hua, C. Coarse Woody Debris Dynamics in Two Old-Growth Ecosystems. Bioscience 1991, 41, 604–610. [Google Scholar] [CrossRef]
- Tinker, D.B.; Knight, D.H. Coarse woody debris following fire and logging in Wyoming lodgepole pine forests. Ecosystems 2000, 3, 472–483. [Google Scholar] [CrossRef]
- Wirth, C.; Schulze, E.-D.; Lühker, B.; Grigoriev, S.; Siry, M.; Hardes, G.; Ziegler, W.; Backor, M.; Bauer, G.; Vygodskaya, N. Fire and site type effects on the long-term carbon and nitrogen balance in pristine Siberian Scots pine forests. Plant. Soil 2002, 242, 41–63. [Google Scholar] [CrossRef]
- Tonn, J.R.; Jurgensen, M.F.; Graham, R.T.; Harvey, A.E. Nitrogen-fixing Processes in Western Larch Ecosystems. In Ecology and Management of Larix Forests: A Look Ahead; General Technical Report, GTR-INT-319; Schmidt, W.C., McDonald, K.J., Eds.; USDA Forest Service, Intermountain Research Station: Ogden, UT, USA, 1995; p. 327333. [Google Scholar]
- Harvey, A.E.; Larsen, M.J.; Jurgensen, M.F. Comparative distribution of ectomycorrhizae in soils of three western Montana forest habitat types. For. Sci. 1979, 25, 350–358. [Google Scholar] [CrossRef]
- Binkley, D. Connecting soils with forest productivity. In Proceedings: Management and Productivity of Western‒Montane Forest Soils; General Technical Report, INT-GTR-280; Harvey, A.E.; Neuenschwander, L.F. (Compiler) USDA Forest Service, Intermountain Research Station: Ogden, UT, USA, 1991; pp. 66–69. [Google Scholar] [CrossRef]
- Kurz, W.A.; Apps, M.J. A 70-year retrospective analysis of carbon fluxes in the Canadian forest sector. Ecol. Appl. 1999, 9, 526–547. [Google Scholar] [CrossRef]
- Keegan, C.E., III.; Blatner, K.A.; Wichman, D.P. Use and Value of Western Larch as a Commercial Timber Species. In Ecology and Management of Larix Forests: A Look Ahead; General Technical Report GTR-INT-319; Schmidt, W.C.; McDonald, K.J. (Compiler) USDA Forest Service, Intermountain Research Station: Ogden, UT, USA, 1995; pp. 155–157. [Google Scholar]
- Drever, C.R.; Peterson, G.; Messier, C.; Bergeron, Y.; Flannigan, M. Can forest management based on natural disturbances maintain ecological resilience? Can. J. For. Res. 2006, 36, 2285–2299. [Google Scholar] [CrossRef]
- Millar, C.I.; Stephenson, N.L.; Stephens, S.L. Climate change and forests of the future: Managing in the face of uncertainty. Ecol. Appl. 2007, 17, 2145–2151. [Google Scholar] [CrossRef]
- Klenk, N.; Bull, G.; Cohen, D. What is the “END” (emulation of natural disturbance) in forest ecosystem management? An open question. Can. J. Forest Res. 2008, 38, 2159–2168. [Google Scholar] [CrossRef]
- Johnson, D.; Murphy, J.D.; Walker, R.F.; Glass, D.W.; Miller, W.W. Wildfire effects on forest carbon and nutrient budgets. Ecol. Eng. 2007, 31, 183–192. [Google Scholar] [CrossRef]
- Dumroese, R.K.; Jurgensen, M.F.; Page-Dumroese, D.S. Soil and Vegetation Responses to 1967‒1968 Disturbances on the Miller Creek Demonstration Forest: Thirty Year Data; USDA Forest Service Research Data Archive: Fort Collins, CO, USA, 2020. [CrossRef]
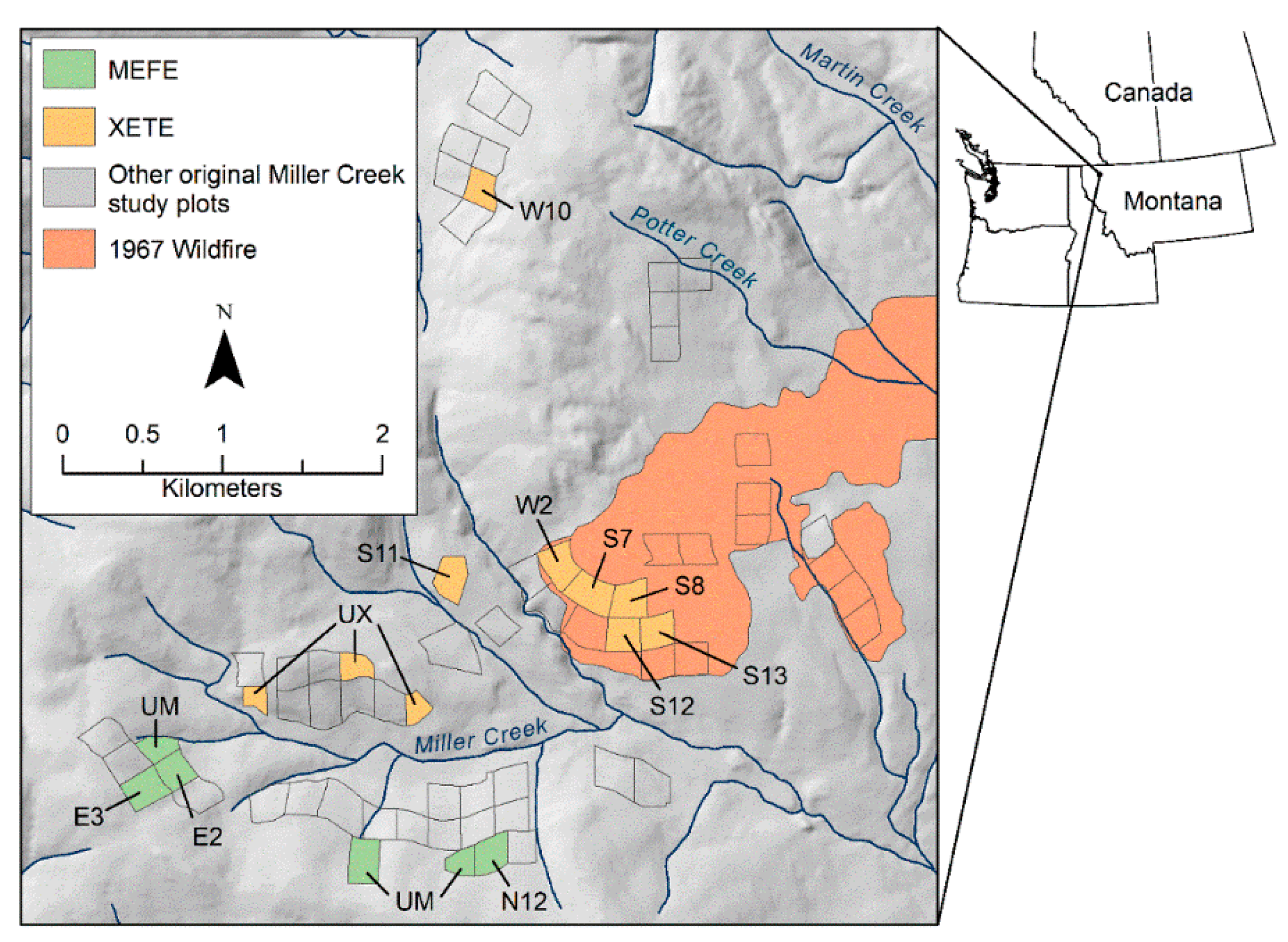
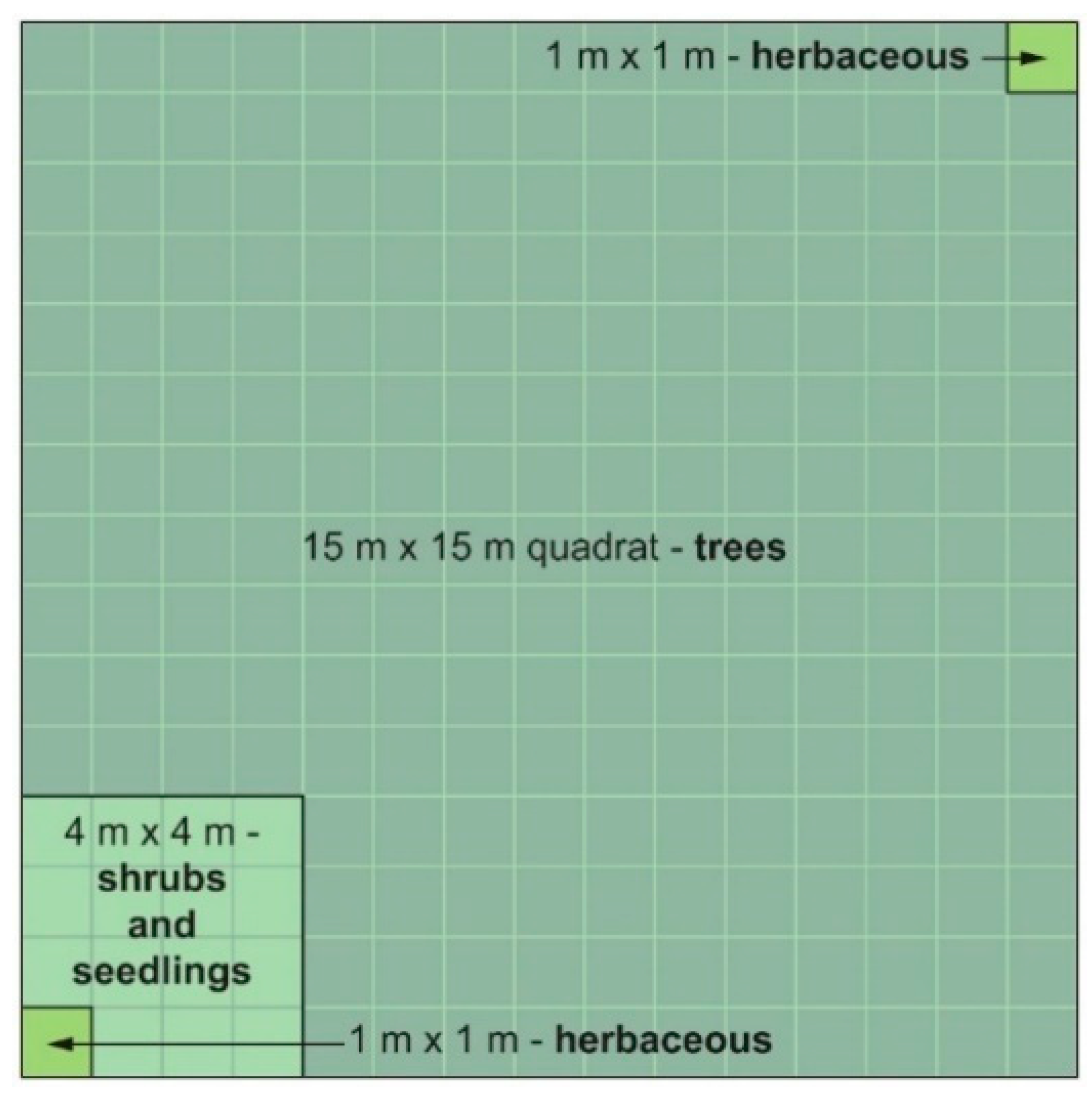


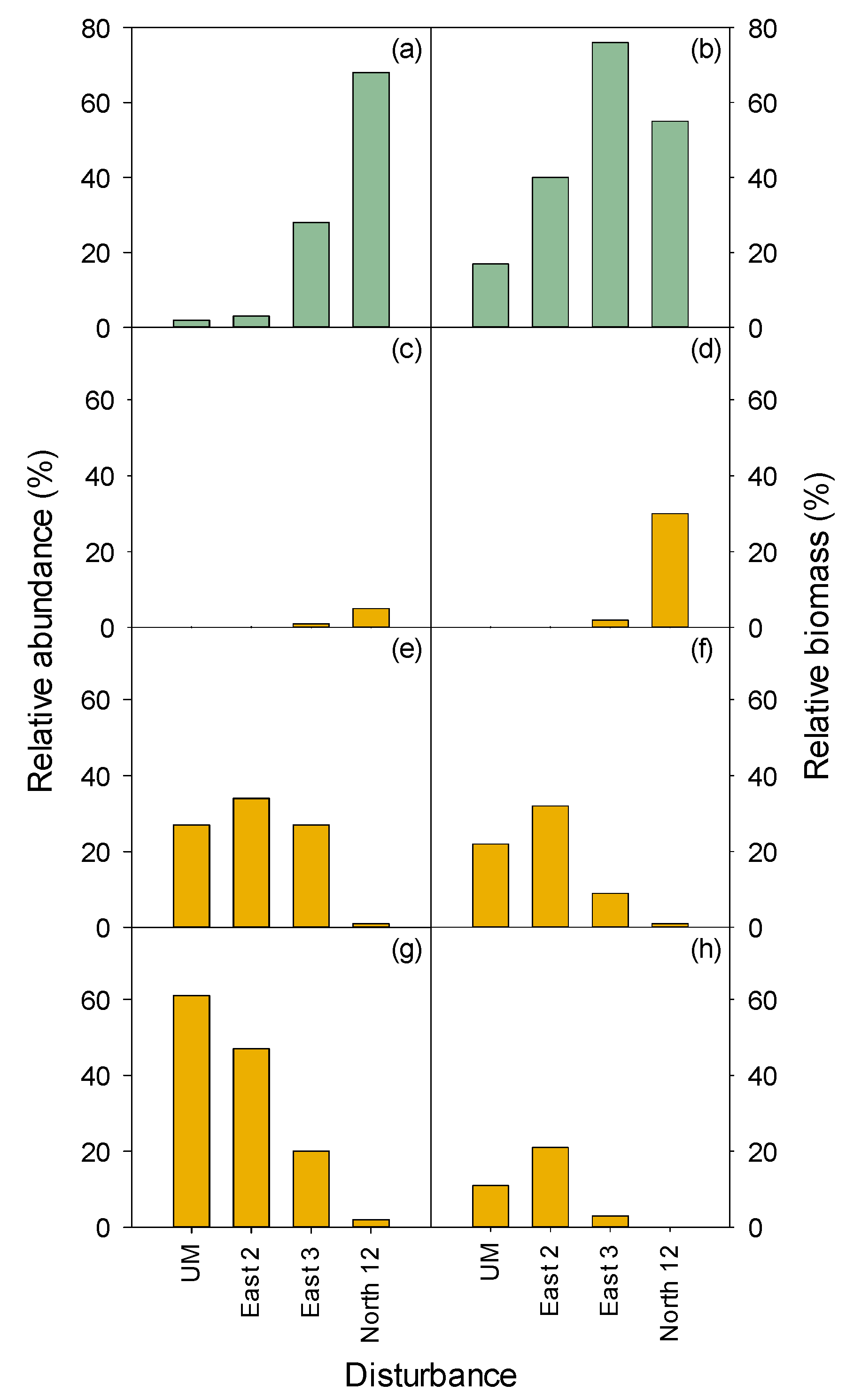
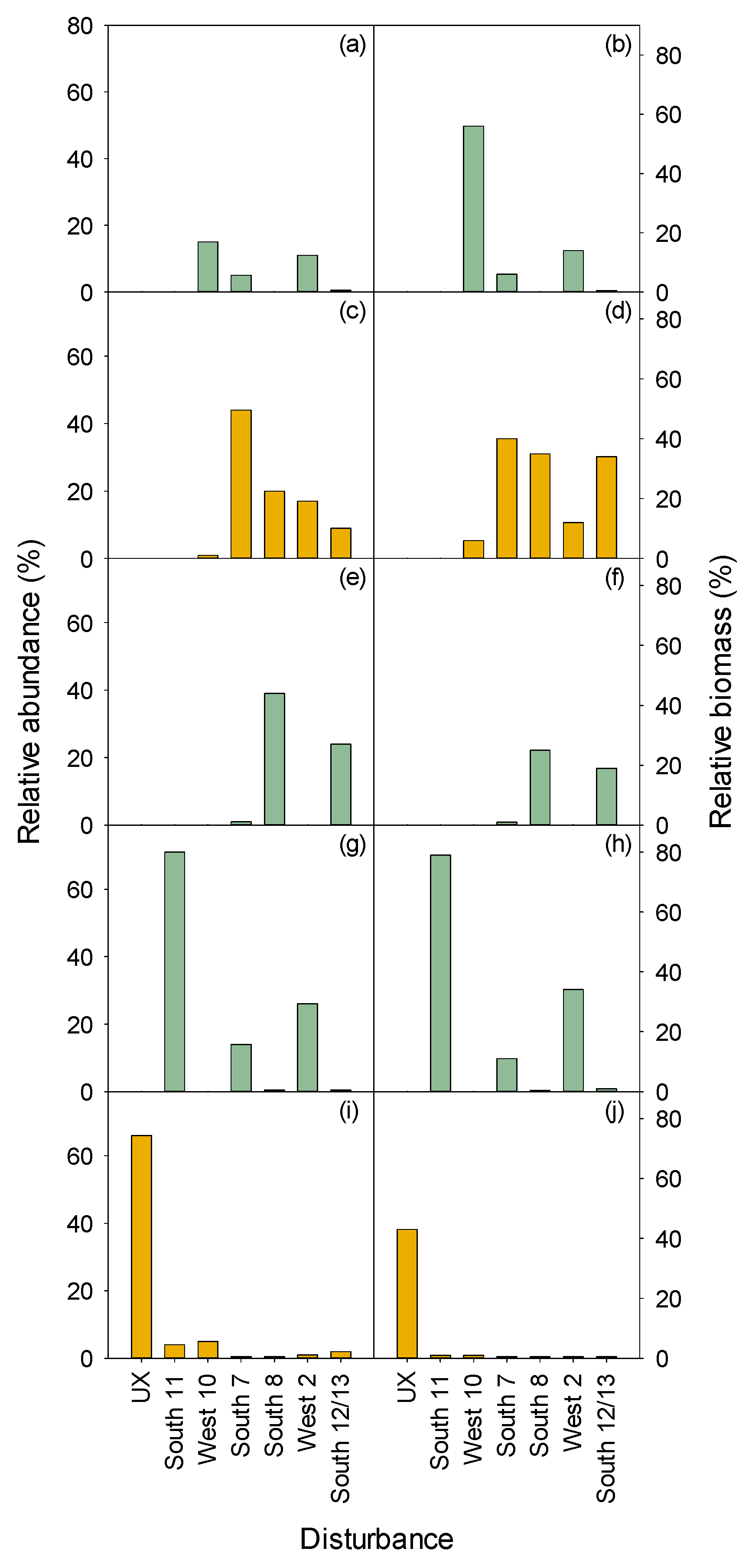
| 1967–1968 Disturbance(s) | 1996 Measurements | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase a and Disturbances | Original Designation | Clearcut Harvest and Slashed (1967) b,c | Prescribed Fire b | Wildfire (1967) b,c,d | Forest Floor e | Water Loss from Cans d | Experimental Units | Soil Cores | |||
| Pre-burn | Post-burn | ||||||||||
| --(Mg ha−1)-- | (g) | (n) | (n) | ||||||||
| Menziesia ferruginea | |||||||||||
| Harvest (H) | East 2 | E2 | Oct | No | No | 103.0 | 103.0 | ‒ | 10 | 16 | |
| H + Prescribed Fire (P) | East 3 | E3 | October‒November | 7 August 1968 | No | 94.9 | 40.4 | 940 | 10 | 16 | |
| HP | North 12 | N12 | February‒March | 3 August 1967 | No | 26.7 | 2.3 | 1062 | 10 | 16 | |
| Control (C) | UM f | UM | No | No | No | ‒ | ‒ | ‒ | 30 | 48 | |
| Xerophyllum tenax | |||||||||||
| H | South 11 | S11 | Yes | No | No | 51.5 | 51.5 | ‒ | 6 | 16 | |
| HP | West 10 | W10 | September‒October | 16 July 1968 | No | 46.9 | 22.2 | 519 | 10 | 16 | |
| H + Wildfire (W) | South 7 | S7 | May | No | 23 August | 47.2 | 13.2 | 1382 j | 10 | 16 | |
| HPW | South 8 | S8 | April‒May | 8 August 1967 | 23 August | 44.3 | 7.1 h | 784 k | 10 | 16 | |
| HW | West 2 | W2 | March‒May | No | 23 August | 48.3 | 1.2 | 1473 j | 10 | 16 | |
| W | South 12/13 | S12/13 | No | No | 23 August | 46.6 | <0.5 i | ‒ | 12 | 32 | |
| C | UX g | UX | No | No | No | ‒ | ‒ | ‒ | 30 | 48 | |
| Form | Genus, Species, and Authority z | ||
|---|---|---|---|
| Tree/seedling | |||
| Abies lasiocarpa (Hook.) Nutt. | |||
| Larix occidentalis Nutt. | |||
| Picea engelmannii Parry ex Engelm. | |||
| Pinus contorta Douglas ex Loudon | |||
| Pinus monticola Douglas ex D. Don | |||
| Populus tremuloides Michx. | |||
| Populus trichocarpa (Torr. & A. Gray ex Hook.) Brayshaw | |||
| Pseudotsuga menziesii (Mirb.) Franco var. glauca (Mayr) Franco | |||
| Taxus brevifolia Nutt. | |||
| Shrub | |||
| Low y | |||
| Berberis repens L. | |||
| Cornus canadensis L | |||
| Paxistima myrsinites (Pursh) Raf. | |||
| Ribes lacustre (Pers.) Poir. | |||
| Rosa gymnocarpa Nutt. | |||
| Rubus parviflorus Nutt. | |||
| Spiraea betulifolia Pall. | |||
| Symphoricarpos albus (L.) S.F. Blake | |||
| Vaccinium membranaceum Douglas ex Torr. | |||
| Medium y | |||
| Ceanothus velutinus Douglas ex Hook. | |||
| Lonicera utahensis S. Watson | |||
| Juniperus communis L. | |||
| Menziesia ferruginea Sm. | |||
| Shepherdia canadensis (L.) Nutt. | |||
| High y | |||
| Acer glabrum Torr. | |||
| Alnus viridis (Chaix) DC. ssp. sinuata (Regel) A. Löve & D. Löve | |||
| Amelanchier alnifolia (Nutt.) Nutt. ex M. Roem. | |||
| Salix scouleriana Barratt ex Hook. | |||
| Dead | ― | ||
| MEFE | XETE | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| East 2 | East 3 | North 12 | Undisturbed MEFE | South 11 | West 10 | South 7 | South 8 | West 2 | South 12/13 | Undisturbed XETE | ||
| Form | Treatment | H | HP | HP | C | H | HP | HW | HPW | HW | W | C |
| Shrub | ||||||||||||
| Acer glabrum | 2000 | 625 | 1875 | 312 | 156 | 146 | ||||||
| Alnus viridisssp.sinuata | 3875 | 18,625 | 44,062 | 250 | 9250 | 1750 | 5000 | 104 | 62 | |||
| Amelanchier alnifolia | 562 | 188 | 417 | 1000 | 125 | 52 | ||||||
| Ceanothus velutinus | 188 | 22,375 | 15,625 | |||||||||
| Lonicera utahensis | 500 | 1188 | 604 | 5062 | 750 | 52 | 62 | |||||
| Menziesia ferruginea | 38,438 | 18,125 | 375 | 3646 | ||||||||
| Paxistima myrsinites | 8750 | 11,125 | 938 | 1167 | 27,938 | 125 | ||||||
| Ribes lacustre | 2062 | 625 | 1438 | 312 | 21 | |||||||
| Rosa gymnocarpa | 562 | 688 | 375 | 938 | 1562 | 167 | ||||||
| Rubus parviflorus | 3500 | 500 | 4000 | 229 | 2688 | |||||||
| Salix scouleriana | 188 | 3250 | 812 | 15,312 | 11,750 | 7562 | 5990 | |||||
| Shepherdia canadensis | 875 | 21 | 20,312 | 5000 | 62 | 11,438 | 208 | |||||
| Spiraea betulifolia | 62 | 188 | 2500 | 1667 | 8812 | 1562 | 4375 | 4750 | 13,646 | 42 | ||
| Symphoricarpos albus | 62 | 1000 | 1125 | 521 | ||||||||
| Vaccinium membranaceum | 53,938 | 13,250 | 1125 | 5562 | 1042 | 3000 | 125 | 62 | 562 | 1458 | 1042 | |
| Seedling | ||||||||||||
| Abies lasiocarpa | 125 | 1125 | 4500 | 1208 | 812 | 125 | 62 | 250 | 156 | 1104 | ||
| Larix occidentalis | 62 | 625 | 438 | 312 | 438 | 417 | ||||||
| Picea engelmannii | 62 | 2688 | 2250 | 62 | 812 | 250 | 125 | 312 | 156 | 21 | ||
| Pinus contorta | 1375 | 688 | 250 | |||||||||
| Populus tremuloides | 125 | 875 | 1875 | 250 | 52 | |||||||
| Pseudotsuga menziesii | 250 | 312 | 625 | 188 | 312 | 250 | 312 | 250 | 52 | 83 | ||
| Taxus brevifolia | 167 | 188 | 1271 | |||||||||
| Tree | ||||||||||||
| Abies lasiocarpa | 391 | 329 | 18 | 853 | 489 | 111 | 4 | 4 | 18 | 883 | ||
| Larix occidentalis | 9 | 200 | 53 | 49 | 7 | 1231 | 253 | 133 | 444 | 404 | 108 | |
| Picea engelmannii | 80 | 1249 | 98 | 459 | 227 | 53 | 71 | 191 | 78 | 308 | ||
| Pinus contorta | 13 | 40 | 16 | 15 | 9 | 147 | 58 | 80 | 1985 | 154 | ||
| Populus tremuloides | 18 | 4 | 9 | 9 | ||||||||
| Populus trichocarpa | 9 | 9 | 9 | 15 | ||||||||
| Pseudotsuga menziesii | 58 | 293 | 18 | 105 | 22 | 542 | 76 | 76 | 280 | 100 | 323 | |
| Taxus brevifolia | 76 | |||||||||||
| Biomass (Mg ha−1) | Carbon (Mg ha−1) | Nitrogen (kg ha−1) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MEFE | E2 | E3 | N12 | UM | E2 | E3 | N12 | UM | E2 | E3 | N12 | UM | |||||||||
| Treatment | H | HP | HP | C | H | HP | HP | C | H | HP | HP | C | |||||||||
| Vegetation | |||||||||||||||||||||
| Trees | 23 | 26 | 4 | 227 | 11 | 13 | 2 | 110 | 111 | 160 | 24 | 992 | |||||||||
| Seedlings | <1 | 4 | 3 | 1 | <1 | 2 | 1 | <1 | 3 | 33 | 30 | 9 | |||||||||
| Shrubs | 5 | 9 | 19 | 1 | 2 | 4 | 9 | 5 | 43 | 68 | 137 | 6 | |||||||||
| Herbaceous | 1 | 1 | 1 | <1 | <1 | <1 | <1 | <1 | 12 | 9 | 10 | 4 | |||||||||
| Roots | |||||||||||||||||||||
| Coarse | 6 | 7 | 1 | 59 | 3 | 3 | <1 | 29 | 28 | 42 | 6 | 258 | |||||||||
| Fine | 58 | 96 | 52 | 226 | 21 | 30 | 15 | 84 | 376 | 654 | 230 | 807 | |||||||||
| Total | 94 | 142 | 80 | 514 | 38 | 52 | 28 | 228 | 573 | 966 | 437 | 2076 | |||||||||
| Woody Residue | 4 | 4 | 4 | 19 | 2 | 2 | 2 | 9 | 14 | 12 | 14 | 62 | |||||||||
| Soil | |||||||||||||||||||||
| Forest floor | 189 | 180 | 116 | 139 | 102 | 98 | 42 | 74 | 4738 | 3797 | 1519 | 2277 | |||||||||
| Soil wood | 58 | 23 | 34 | 69 | 32 | 10 | 26 | 38 | 579 | 418 | 804 | 959 | |||||||||
| Mineral soil | 218 | 198 | 183 | 155 | 67 | 44 | 37 | 40 | 2513 | 1577 | 1323 | 1487 | |||||||||
| Total | 465 | 401 | 333 | 363 | 201 | 152 | 105 | 152 | 7830 | 5792 | 3646 | 4723 | |||||||||
| Total pool | 563 | 547 | 418 | 896 | 241 | 206 | 135 | 389 | 8417 | 6770 | 4083 | 6861 | |||||||||
| XETE | S11 | W10 | S7 | S8 | W2 | S12/13 | UX | S11 | W10 | S7 | S8 | W2 | S12/13 | UX | S11 | W10 | S7 | S8 | W2 | S12/13 | UX |
| Treatment | H | HP | HW | HPW | HW | W | C | H | HP | HW | HPW | HW | W | C | H | HP | HW | HPW | HW | W | C |
| Vegetation | |||||||||||||||||||||
| Trees | 12 | 33 | 38 | 28 | 49 | 46 | 185 | 6 | 16 | 18 | 14 | 24 | 22 | 89 | 76 | 146 | 223 | 188 | 309 | 256 | 917 |
| Seedlings | 0 | 1 | 1 | 1 | 1 | <1 | 2 | 0 | 1 | 1 | <1 | <1 | <1 | 1 | 0 | 9 | 11 | 6 | 7 | 3 | 11 |
| Shrubs | 3 | 4 | 4 | 5 | 5 | 7 | <1 | 1 | 2 | 2 | 2 | 2 | 3 | <1 | 40 | 33 | 25 | 42 | 47 | 55 | <1 |
| Herbaceous | 5 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | <1 | 1 | 1 | 36 | 14 | 18 | 15 | 7 | 11 | 13 |
| Roots | |||||||||||||||||||||
| Coarse | 3 | 8 | 10 | 7 | 5 | 12 | 48 | 2 | 4 | 5 | 4 | 6 | 6 | 23 | 20 | 38 | 58 | 49 | 80 | 67 | 238 |
| Fine | 49 | 45 | 105 | 41 | 138 | 102 | 148 | 15 | 14 | 30 | 14 | 41 | 32 | 61 | 211 | 201 | 466 | 193 | 743 | 560 | 474 |
| Total | 72 | 94 | 84 | 84 | 206 | 168 | 384 | 26 | 38 | 57 | 35 | 74 | 64 | 176 | 387 | 441 | 801 | 491 | 1193 | 952 | 1654 |
| Woody Residue | 4 | 4 | 1 | 5 | 4 | 9 | 13 | 2 | 2 | <1 | 2 | 2 | 4 | 6 | 12 | 10 | 3 | 11 | 10 | 23 | 39 |
| Soil | |||||||||||||||||||||
| Forest floor | 89 | 51 | 33 | 55 | 34 | 45 | 131 | 57 | 28 | 17 | 34 | 29 | 29 | 70 | 1794 | 936 | 514 | 1386 | 936 | 884 | 2010 |
| Soil wood | 5 | 59 | 0 | 5 | 0 | 8 | 17 | 4 | 37 | 0 | 2 | 0 | 5 | 11 | 138 | 664 | 0 | 122 | 0 | 142 | 258 |
| Mineral soil | 122 | 131 | 150 | 125 | 227 | 118 | 118 | 34 | 29 | 36 | 32 | 46 | 29 | 26 | 1485 | 1382 | 1469 | 1361 | 2068 | 1279 | 1109 |
| Total | 217 | 241 | 184 | 186 | 261 | 171 | 266 | 95 | 94 | 53 | 68 | 75 | 63 | 107 | 3417 | 2982 | 1983 | 2869 | 3004 | 2305 | 3377 |
| Total pool | 292 | 339 | 344 | 274 | 471 | 348 | 664 | 123 | 134 | 110 | 150 | 151 | 131 | 289 | 3813 | 3434 | 2787 | 3371 | 4207 | 3208 | 5070 |
| Nitrogen Concentration (%) | |||
|---|---|---|---|
| Basal Diameter | Seedlings | Shrubs | |
| <2 cm | Wood | 0.43 (67) | 0.56 (163) |
| >2 cm | Wood | 0.45 (69) | 0.48 (34) |
| p-value | 0.2899 | 0.0015 | |
| <2 cm | Foliage | 1.37 (72) | 1.86 (169) |
| >2 cm | Foliage | 1.32 (65) | 2.01 (37) |
| p-value | 0.4450 | 0.0703 | |
| Nitrogen Concentration (%) | ||
|---|---|---|
| Species | Wood | Foliage |
| Alnus viridis ssp. sinuata | 0.78 (25) a | 2.60 (26) y |
| Ceanothus velutinus | 0.92 (19) a | 1.97 (19) x |
| Shepherdia canadensis | 1.49 (23) b | 2.45 (24) y |
| p-value | <0.0001 | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumroese, R.K.; F. Jurgensen, M.; Page-Dumroese, D.S. Vegetative and Edaphic Responses in a Northern Mixed Conifer Forest Three Decades after Harvest and Fire: Implications for Managing Regeneration and Carbon and Nitrogen Pools. Forests 2020, 11, 1040. https://doi.org/10.3390/f11101040
Dumroese RK, F. Jurgensen M, Page-Dumroese DS. Vegetative and Edaphic Responses in a Northern Mixed Conifer Forest Three Decades after Harvest and Fire: Implications for Managing Regeneration and Carbon and Nitrogen Pools. Forests. 2020; 11(10):1040. https://doi.org/10.3390/f11101040
Chicago/Turabian StyleDumroese, R. Kasten, Martin F. Jurgensen, and Deborah S. Page-Dumroese. 2020. "Vegetative and Edaphic Responses in a Northern Mixed Conifer Forest Three Decades after Harvest and Fire: Implications for Managing Regeneration and Carbon and Nitrogen Pools" Forests 11, no. 10: 1040. https://doi.org/10.3390/f11101040
APA StyleDumroese, R. K., F. Jurgensen, M., & Page-Dumroese, D. S. (2020). Vegetative and Edaphic Responses in a Northern Mixed Conifer Forest Three Decades after Harvest and Fire: Implications for Managing Regeneration and Carbon and Nitrogen Pools. Forests, 11(10), 1040. https://doi.org/10.3390/f11101040





