The Impact of Climate Variations on the Structure of Ground Beetle (Coleoptera: Carabidae) Assemblage in Forests and Wetlands
Abstract
1. Introduction
2. Materials and Methods
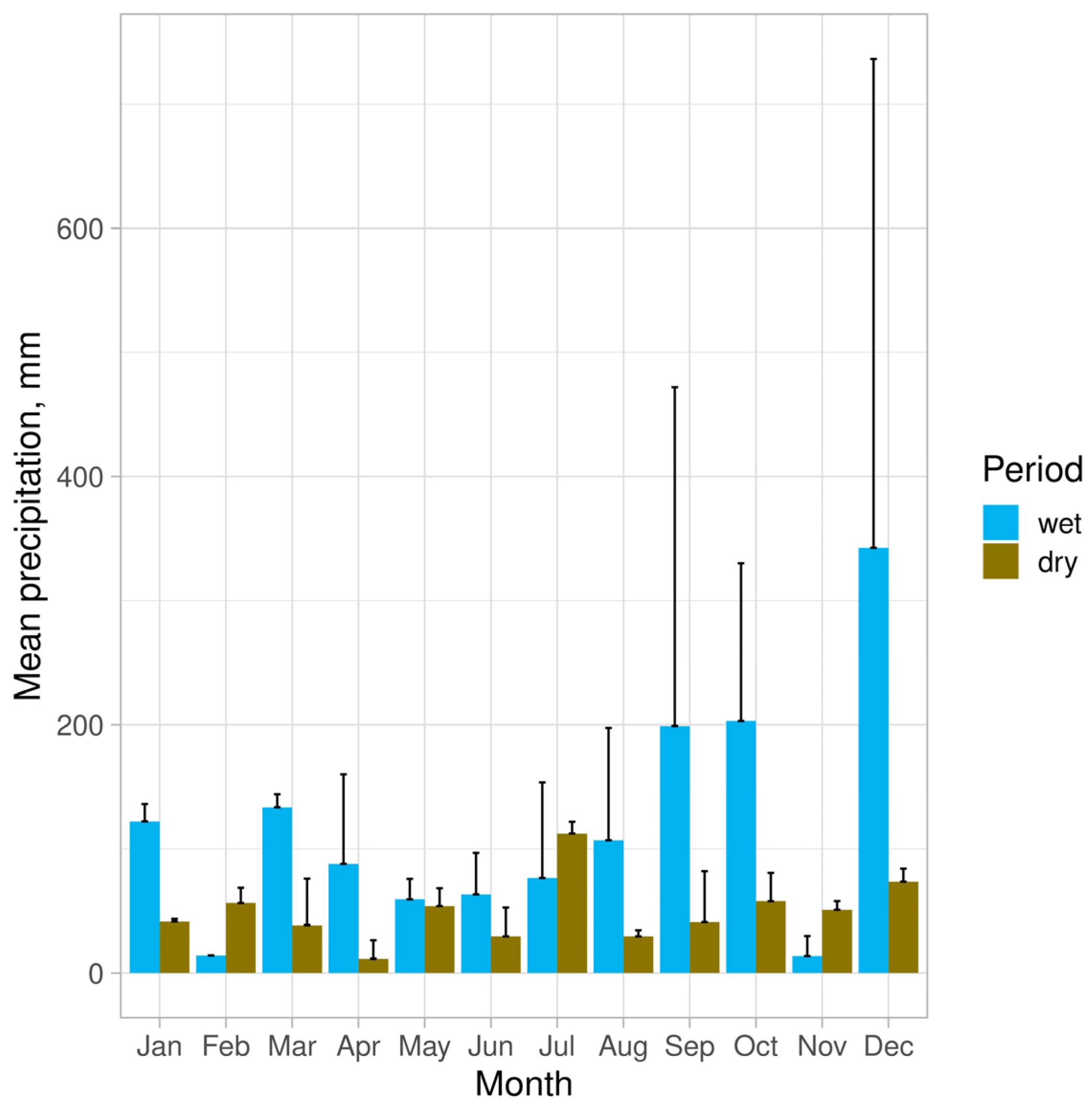
2.1. Study Area
2.2. Sampling Design
2.3. Statistical Analysis
3. Results
- Group 1: Abax ater, Pterostichus melanarius, Harpalus luteicornis, Stomis pumicatus, Harpalus winkleri, Harpalus quadripunctatus, Abax parallelus, and Pterostichus oblongopunctatus;
- Group 2: Lorocera pilicornis, Patrobus assimilis, Pterostichus anthracinus, P. diligens, P. niger, Agonum fuliginosum, P. strenuus, Amara communis, Elaphrus cupreus, P. nigrita, A. moestum, Platynus assimile, P. minor, and Carabus granulatus.
- Group 1: A. ater and P. oblongopunctatus;
- Group 2: P. anthracinus, P. assimile, N. germinyi, C. caraboides, and C. menetriesi;
- Group 3: P. nigrita and A. fuliginosum.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Santamarta, J.C.; Neris, J.; Rodríguez-Martín, J.; Arraiza, M.P.; López, J. Climate change and water planning: New challenges on islands environments. IERI Procedia 2014, 9, 59–63. [Google Scholar] [CrossRef]
- Azadi, F.; Ashofteh, P.-S.; Loáiciga, H.A. Reservoir water-quality projections under climate-change conditions. Water Resour. Manag. 2018, 33, 401–421. [Google Scholar] [CrossRef]
- Komolafe, A.A.; Herath, S.; Avtar, R. Methodology to assess potential flood damages in urban areas under the influence of climate change. Nat. Hazards Rev. 2018, 19, 05018001. [Google Scholar] [CrossRef]
- Pielke, R. Tracking progress on the economic costs of disasters under the indicators of the sustainable development goals. Environ. Hazards 2018, 18, 1–6. [Google Scholar] [CrossRef]
- Rötzer, T.; Rahman, M.; Moser-Reischl, A.; Pauleit, S.; Pretzsch, H. Process based simulation of tree growth and ecosystem services of urban trees under present and future climate conditions. Sci. Total. Environ. 2019, 676, 651–664. [Google Scholar] [CrossRef]
- Ruffault, J.; Martin-StPaul, N.K.; Rambal, S.; Mouillot, F. Differential regional responses in drought length, intensity and timing to recent climate changes in a Mediterranean forested ecosystem. Clim. Chang. 2012, 117, 103–117. [Google Scholar] [CrossRef]
- Tao, F.; Yokozawa, M.; Hayashi, Y.; Lin, E. Terrestrial water cycle and the impact of climate change. Ambio 2003, 32, 295–301. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef]
- Leta, O.T.; El-Kadi, A.I.; Dulai, H.; Ghazal, K.A. Assessment of climate change impacts on water balance components of Heeia watershed in Hawaii. J. Hydrol. Reg. Stud. 2016, 8, 182–197. [Google Scholar] [CrossRef]
- Barros, D.; Albernaz, A. Possible impacts of climate change on wetlands and its biota in the Brazilian Amazon. Braz. J. Biol. 2014, 74, 810–820. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Gosselink, J.G. Wetlands, 4th ed.; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Gibbs, J.P. Wetland loss and biodiversity conservation. Conserv. Biol. 2000, 14, 314–317. [Google Scholar] [CrossRef]
- Green, A.J.; El Hamzaoui, M.; El Agbani, M.A.; Franchimont, J.; Green, A.J. The conservation status of Moroccan wetlands with particular reference to waterbirds and to changes since 1978. Biol. Conserv. 2002, 104, 71–82. [Google Scholar] [CrossRef]
- Gardner, R.C.; Barchiesi, S.; Beltrame, C.; Finlayson, C.M.; Galewski, T.; Harrison, I.; Paganini, M.; Perennou, C.; Pritchard, D.; Rosenqvist, A.; et al. State of the world’s wetlands and their services to people: A compilation of recent analyses. SSRN Electron. J. 2015. [Google Scholar] [CrossRef]
- Arfanuzzaman, M.; Rahman, A.A. Sustainable water demand management in the face of rapid urbanization and ground water depletion for social-ecological resilience building. Glob. Ecol. Conserv. 2017, 10, 9–22. [Google Scholar] [CrossRef]
- Nagypál, V.; Mikó, E.; Hodúr, C. Sustainable water use considering three Hungarian dairy farms. Sustainability 2020, 12, 3145. [Google Scholar] [CrossRef]
- Yang, T.-H.; Liu, W.-C. A General overview of the risk-reduction strategies for floods and droughts. Sustainability 2020, 12, 2687. [Google Scholar] [CrossRef]
- Özerol, G.; Dolman, N.; Bormann, H.; Bressers, H.; Lulofs, K.; Böge, M. Urban water management and climate change adaptation: A self-assessment study by seven midsize cities in the North Sea Region. Sustain. Cities Soc. 2020, 55, 102066. [Google Scholar] [CrossRef]
- Parry, M.L.; Canziani, O.F.; Palutikof, J.P.; Van der Linden, P.J.; Hanson, C.E. Climate Change 2007: Impacts, Adaptation and Vulnerability; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Climate Change in Eastern Europe. Available online: https://issuu.com/zoienvironment/docs/ccee-ebook (accessed on 16 August 2019).
- Hampe, A.; Petit, R.J. Conserving biodiversity under climate change: The rear edge matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef]
- Hay, J.E.; Mimura, N. Supporting climate change vulnerability and adaptation assessments in the Asia-Pacific region: An example of sustainability science. Sustain. Sci. 2006, 1, 23–35. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Biondi, S.; Oses, R.; Acuña-Rodríguez, I.S.; Antognoni, F.; Martinez-Mosqueira, E.A.; Coulibaly, A.; Canahua-Murillo, A.; Pinto, M.; Zurita-Silva, A.; et al. Quinoa biodiversity and sustainability for food security under climate change. A review. Agron. Sustain. Dev. 2013, 34, 349–359. [Google Scholar] [CrossRef]
- Stenseth, N.C.; Mysterud, A. Climate, changing phenology, and other life history traits: Nonlinearity and match-mismatch to the environment. Proc. Natl. Acad. Sci. USA 2002, 99, 13379–13381. [Google Scholar] [CrossRef] [PubMed]
- Huntley, B.; Collingham, Y.C.; Green, R.E.; Hilton, G.M.; Rahbek, C.; Willis, S.G. Potential impacts of climatic change upon geographical distributions of birds. Ibis 2006, 148, 8–28. [Google Scholar] [CrossRef]
- Pounds, J.A.; Bustamante, M.R.; Coloma, L.A.; Consuegra, J.A.; Fogden, M.P.L.; Foster, P.N.; La Marca, E.; Masters, K.L.; Merino-Viteri, A.; Puschendorf, R.; et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nat. Cell Biol. 2006, 439, 161–167. [Google Scholar] [CrossRef]
- Platt, T.; White, G.N.; Zhai, L.; Sathyendranath, S.; Roy, S. The phenology of phytoplankton blooms: Ecosystem indicators from remote sensing. Ecol. Model. 2009, 220, 3057–3069. [Google Scholar] [CrossRef]
- Botsford, L.W.; Holland, M.D.; Samhouri, J.F.; White, J.W.; Hastings, A. Importance of age structure in models of the response of upper trophic levels to fishing and climate change. ICES J. Mar. Sci. 2011, 68, 1270–1283. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nat. Cell Biol. 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Cotton, P.A. Avian migration phenology and global climate change. Proc. Natl. Acad. Sci. USA 2003, 100, 12219–12222. [Google Scholar] [CrossRef] [PubMed]
- Hüppop, O.; Ppop, K.H. North Atlantic oscillation and timing of spring migration in birds. Proc. R. Soc. B Boil. Sci. 2003, 270, 233–240. [Google Scholar] [CrossRef]
- Assmann, T. Ground beetles and global change: First results from ongoing studies on case study species. In Proceedings of the Abstracts of the XIV European Carabidologists Meeting, Westerbork, The Netherlands, 14–18 September 2009. [Google Scholar]
- Pizzolotto, R. 30 years of carabid sampling in Italy: A data bank for studying local climate change. In Proceedings of the Abstracts of the XIV European Carabidologists Meeting, Westerbork, The Netherlands, 14–18 September 2009. [Google Scholar]
- Boix, D.; Batzer, D.P. Invertebrate assemblages and their ecological controls across the world’s freshwater wetlands. In Invertebrates in Freshwater Wetlands; Batzer, D., Boix, D., Eds.; Springer International Publishing, Springer Science and Business Media LLC: Cham, Switzerland; Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2016; pp. 601–639. [Google Scholar]
- Ramey, T.L.; Richardson, J.S. Terrestrial invertebrates in the riparian zone: Mechanisms underlying their unique diversity. BioScience 2017, 67, 808–819. [Google Scholar] [CrossRef]
- Sasakawa, K. Notes on the reproductive ecology and description of the preimaginal morphology of Elaphrus sugai Nakane, the most endangered species of Elaphrus Fabricius (Coleoptera: Carabidae) ground beetle worldwide. PLoS ONE 2016, 11, e0159164. [Google Scholar] [CrossRef]
- Pearce, J.; Venier, L.A. The use of ground beetles (Coleoptera: Carabidae) and spiders (Araneae) as bioindicators of sustainable forest management: A review. Ecol. Indic. 2006, 6, 780–793. [Google Scholar] [CrossRef]
- Kirichenko, M.B.; Babko, R.V. The Structure of the Assemblage of Ground Beetles (Coleoptera: Cicindelidae, Carabidae) in the Vakalivshchyna Tract. Collection of Works: To the 40th Anniversary of Biological Station; SSPU im. A.S. Makarenka: Sumy, Ukraine, 2008; pp. 53–59. (In Ukraine) [Google Scholar]
- Babko, R.V.; Kirichenko, M.B.; Deryzemlia, A.M. Spatial structure of populations of Carabus granulatus and Carabus cancellatus (Coleoptera, Carabidae) in a moist deciduous forest on the Left Bank Forest-Steppe (Ukraine). In Proceedings of the Biodiversity and Sustainability of Living Systems: Materials of the XIII International Scientific and Practical Environmental Conference, Belgorod, Russia, 6–11 October 2014; Belgorod NRU BelGU: Belgorod, Russia, 2014; pp. 17–18. [Google Scholar]
- Pogoda i Klimat. Available online: http://www.pogodaiklimat.ru/history/33275_2.htm. (accessed on 5 August 2020).
- World Weather. Available online: https://world-weather.ru/archive (accessed on 16 August 2019).
- Hůrka, K. Carabidae of the Czech and Slovak Republics; Illustrated key; Kabourek: Zlín, Czech Republic, 1996. [Google Scholar]
- Müller-Motzfeld, G. Adephaga 1: Carabidae (Laufkäfer). In Die Käfer Mitteleuropas, Band 2; Freude, H., Harde, K.W., Lohse, G.A., Klausnitzer, B., Eds.; Spektrum-Verlag: Berlin/Heidelberg, Germany, 2004; pp. 3–502. [Google Scholar]
- Turin, H. De Nederlandse Loopkevers. Verspreiding en Oecologie (Coleoptera: Carabidae). Nederlandse Fauna 3; Nationaal Natuurhistorisch Museum Naturalis, KNNV Uitgeverij & EIS-Nederland: Leiden, The Netherlands, 2000. [Google Scholar]
- Lindroth, C.H. Ground Beetles (Carabidae) of Fennoscandia: A Zoogeographic Study. Part I. Specific Knowledge Regarding the Species; Intercept: Andover, UK, 1992. [Google Scholar]
- Ribera, I.; Doleґdec, S.; Downie, I.S.; Foster, G.N. Effect of land disturbance and stress on species traits of ground beetle assemblages. Ecology 2001, 82, 1112–1129. [Google Scholar] [CrossRef]
- Kulkarni, S.; Dosdall, L.M.; Willenborg, C.J. The role of ground beetles (Coleoptera: Carabidae) in weed seed consumption: A review. Weed Sci. 2015, 63, 355–376. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2018. Available online: https://www.r-project.org/ (accessed on 13 February 2012).
- Lé, S.; Josse, J.; Husson, F. FactoMineR: AnRPackage for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M. Community Ecology Package. R Package Version 2.5-5. Available online: https://CRAN.R-project.org/package=vegan (accessed on 20 July 2020).
- Legendre, P.; Legendre, L. Numerical Ecology; Developments in Environmental Modelling, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R package version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 20 July 2020).
- Slowikowski, K. Ggrepel: Automatically Position Non-Overlapping Text Labels with ’ggplot2’ R package version 0.8.0. Available online: https://CRAN.R-project.org/package=ggrepel (accessed on 14 August 2019).
- Hocking, T.D. Directlabels: Direct Labels for Multicolor Plots. R package version 2018.05.22. Available online: https://CRAN.R-project.org/package=directlabels (accessed on 14 August 2019).
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning; Springer International Publishing, Springer Science and Business Media LLC: New York, NY, USA, 2013. [Google Scholar]
- McCarthy, B.C.; Magurran, A.E. Measuring biological diversity. J. Torrey Bot. Soc. 2004, 131, 277. [Google Scholar] [CrossRef]
- Paschetta, M.; Giachino, P.; Isaia, M. Taxonomic relatedness of spider and carabid assemblages in a wetland ecosystem. Zool. Stud. 2012, 51, 1175–1187. [Google Scholar]
- Species Diversity and Richness III; Pisces Conservation Ltd, IRC House: Pennington, Lymington, UK, 2004.
- Baselga, A. Separating the two components of abundance-based dissimilarity: Balanced changes in abundance vs. abundance gradients. Methods Ecol. Evol. 2013, 4, 552–557. [Google Scholar] [CrossRef]
- Šustek, Z. Characteristics of humidity requirements and relations to vegetation cover of selected Central-European carabids (Col., Carabidae). In Proceedings of the Hodnocení Stavu a Vývoje Lesních Geobiocenóz, Brno, Czech Republic, 15–16 October 2004; Polehla, P., Ed.; Geobiocenologické Spisy: Brno, Czech Republic, 2004; pp. 210–214. [Google Scholar]
- Vician, V.; Svitok, M.; Michalková, E.; Lukáčik, I.; Stašiov, S. Influence of tree species and soil properties on ground beetle (Coleoptera: Carabidae) communities. Acta Oecologica 2018, 91, 120–126. [Google Scholar] [CrossRef]
- Nepstad, D.C.; Moutinho, P.; Dias-Filho, M.B.; Davidson, E.A.; Cardinot, G.; Markewitz, D.; Figueiredo, R.D.O.; Vianna, N.; Chambers, J.Q.; Guerreiros, J.B.; et al. The effects of partial throughfall exclusion on canopy processes, aboveground production, and biogeochemistry of an Amazon forest. J. Geophys. Res. Space Phys. 2002, 107, 8085. [Google Scholar] [CrossRef]
- Dijk, T.S. On the relationship between food, reproduction and survival of two carabid beetles: Calathus melanocephalus and Pterostichus versicolor. Ecol. Èntomol. 1994, 19, 263–270. [Google Scholar] [CrossRef]
- Holland, J.; Thomas, C.; Birkett, T.; Southway, S. Spatio-temporal distribution and emergence of beetles in arable fields in relation to soil moisture. Bull. Èntomol. Res. 2007, 97, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.A.; Richardson, M.J.; González, G.; Shiels, A.B.; Srivastava, D.S. A canopy trimming experiment in Puerto Rico: The response of litter invertebrate communities to canopy loss and debris deposition in a tropical forest subject to hurricanes. Ecosystems 2010, 13, 286–301. [Google Scholar] [CrossRef]
- Facey, S.L.; Fidler, D.B.; Rowe, R.C.; Bromfield, L.M.; Nooten, S.S.; Staley, J.T.; Ellsworth, D.; Johnson, S.N. Atmospheric change causes declines in woodland arthropods and impacts specific trophic groups. Agric. For. Èntomol. 2016, 19, 101–112. [Google Scholar] [CrossRef]
- Ivanova, K.O.; Kosheleva, T.N. Comparative analysis of structure of species dominance of marine Nematoda assemblages (Sevastopol Bays). Optim. Prot. Ecosyst. 2012, 7, 209–216. (In Russian) [Google Scholar]
- Purtauf, T.; Dauber, J.; Wolters, V. The response of carabids to landscape simplification differs between trophic groups. Oecologia 2004, 142, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, S.; Hannig, K.; Schirmel, J. Losing uniqueness-shifts in carabid species composition during dry grassland and heathland succession. Anim. Conserv. 2013, 16, 661–670. [Google Scholar] [CrossRef]
- Vanbergen, A.J.; Woodcock, B.A.; Koivula, M.; Niemelä, J.; Kotze, D.J.; Bolger, T.; Golden, V.; Dubs, F.; Boulanger, G.; Serrano, J.; et al. Trophic level modulates carabid beetle responses to habitat and landscape structure: A pan-European study. Ecol. Èntomol. 2010, 35, 226–235. [Google Scholar] [CrossRef]
- Grant, J.A.; Villani, M.G. Soil Moisture effects on entomopathogenic nematodes. Environ. Èntomol. 2003, 32, 80–87. [Google Scholar] [CrossRef]
- Saska, P.; Martinková, Z.; Honěk, A. Temperature and rate of seed consumption by ground beetles (Carabidae). Biol. Control. 2010, 52, 91–95. [Google Scholar] [CrossRef]





| Species and Their Codes | Occupancy of Sites (%) | Trophic Requirement * | ||
|---|---|---|---|---|
| Wet | Drought | |||
| Hygrophilous | ||||
| Abax parallelopipedus Piller et Mitterpacher, 1783 | Ab.ater | 93 | 85 | o |
| Abax parallelus Duftschmid, 1812 | Ab.parallelus | 27 | 46 | o |
| Agonum fuliginosum Panzer, 1809 | Ag.fuliginos | 7 | 38 | c |
| Agonum micans Nicolai, 1822 | Ag.micans | 7 | - | c |
| Agonum moestum Duftschmid, 1812 | Ag.moestum | 27 | - | c |
| Badister dorsiger Duftschmid, 1812 | Ba.dorsiger | 7 | - | c |
| Carabus granulatus Linnaeus, 1758 | Ca.granulatus | 80 | 77 | c |
| Carabus menetriesi Faldermann, 1827 | Ca.menetriesi | - | 15 | c |
| Cychrus caraboides Linnaeus, 1758 | Cy.caraboides | - | 8 | c |
| Elaphrus cupreus Duftschmid, 1812 | El.cupreus | 40 | 23 | c |
| Loricera pilicornis Fabricius, 1775 | Lo.pilicornis | 13 | 31 | c |
| Notiophilus palustris Duftschmid, 1812 | No.palustris | 33 | 8 | c |
| Oodes helopioides Fabricius, 1792 | Oo.helopioides | 33 | 15 | c |
| Oxypselaphus obscurum Herbst, 1784 | Ox.obscurum | 7 | - | c |
| Patrobus atrorufus Stroem, 1768 | Pa.atrorufus | 7 | 15 | c |
| Platynus assimile Paykull, 1790 | Pl.assimile | 33 | 23 | c |
| Pterostichus anthracinus Illiger, 1798 | Pt.anthracinus | 7 | 15 | c |
| Pterostichus diligens Sturm, 1824 | Pt.diligens | 7 | 8 | c |
| Pterostichus minor Gyllenhal, 1827 | Pt.minor | 13 | 8 | c |
| Pterostichus niger Schaller, 1783 | Pt.niger | 7 | 38 | c |
| Pterostichus nigrita Paykull, 1790 | Pt.nigrita | 60 | 62 | c |
| Pterostichus strenuus Panzer, 1797 | Pt.strenuus | 7 | 15 | c |
| Stomis pumicatus Panzer, 1796 | St.pumicatus | 27 | 8 | c |
| Mesophilous | ||||
| Amara communis Panzer, 1797 | Am.communis | 7 | - | g |
| Anisodactylus signatus Panzer, 1797 | An.signatus | 7 | - | g |
| Asaphidion flavipes Linnaeus, 1761 | As.flavipes | 7 | - | c |
| Carabus cancellatus Illiger, 1798 | Ca.cancellatus | 13 | - | c |
| Carabus glabratus Paykull, 1790 | Ca.glabratus | - | 8 | c |
| Harpalus latus Linnaeus, 1758 | Ha.latus | - | 15 | g |
| Harpalus luteicornis Duftschmid, 1812 | Ha.luteicornis | 13 | 8 | g |
| Harpalus quadripunctatus Dejean, 1829 | Ha.quadripun | 7 | 8 | g |
| Pterostichus melanarius Illiger, 1798 | Pt.melanarius | 27 | 54 | o |
| Pterostichus oblongopunctatus Fabricius, 1787 | Pt.oblongop | 40 | 54 | |
| Xerophilous | ||||
| Harpalus xanthopus winkleri Schauberger, 1923 | Ha.winkleri | 13 | - | g |
| Notiophilus germinyi Fauvel in Grenier, 1863 | No.germinyi | - | 8 | c |
| Pseudoophonus rufipes De Geer, 1774 | Ps.rufipes | - | 23 | g |
| Species | Periods | Shared Species | Total No. Species | |
|---|---|---|---|---|
| Wet | Dry | |||
| Hygro-preference/Feeding Group (in Adult): | ||||
| Hygrophilous: | 23 | |||
| Carnivorous | 19 | 16 | 14 | 21 |
| Omnivorous | 2 | 2 | 2 | 2 |
| Granivorous | 0 | 0 | 0 | 0 |
| Mesophilous: | 10 | |||
| Carnivorous | 3 | 2 | 1 | 4 |
| Omnivorous | 1 | 1 | 1 | 1 |
| Granivorous | 4 | 3 | 2 | 5 |
| Xerophilous: | 3 | |||
| Carnivorous | 0 | 1 | 0 | 1 |
| Omnivorous | 0 | 0 | 0 | 0 |
| Granivorous | 1 | 1 | 0 | 2 |
| Total no. species | 30 | 27 | 21 | 36 |
| Habitats | Periods | Shared Species | Total No. Species | |
|---|---|---|---|---|
| Wet | Dry | |||
| Bottom of ravine | 21 | 22 | 17 | 27 |
| Slope of ravine | 12 | 11 | 6 | 17 |
| Forest on top slope | 15 | 9 | 8 | 16 |
| Traits | Group 1 | Group 2 | Group 3 | Total Trait |
|---|---|---|---|---|
| Feeding group (in adult): | ||||
| Carnivorous | 2/1 | 13/5 | 0/2 | 15/8 |
| Omnivorous | 3/1 | 0/0 | 0/0 | 3/1 |
| Granivorous | 3/0 | 1/0 | 0/0 | 4/0 |
| Hygro-preference: | ||||
| Hygrophilous | 3/1 | 13/4 | 0/2 | 16/7 |
| Mesophilous | 4/1 | 1/1 | 0/0 | 5/1 |
| Xerophilous | 1/0 | 0/1 | 0/0 | 1/1 |
| Total in each group | 8/2 | 14/5 | 0/2 | 22/9 |
| Site | Humidity in Dry Period | Predicted by Lasso | Predicted by PCR |
|---|---|---|---|
| drybot1 | 4 | 6 | 7 |
| drybot2 | 4 | 6 | 7 |
| drybot3 | 4 | 6 | 7 |
| drybot4 | 4 | 6 | 6 |
| drybot5 | 4 | 6 | 7 |
| drybot6 | 4 | 6 | 7 |
| drybot7 | 5 | 7 | 7 |
| drybot8 | 5 | 6 | 7 |
| dryforest1 | 2 | 6 | 6 |
| dryforest2 | 2 | 5 | 5 |
| dryforest3 | 2 | 4 | 5 |
| dryslope1 | 2 | 6 | 5 |
| dryslope2 | 2 | 6 | 5 |
| Wet Period | Drought Period | Bray | Balanced (B) | Gradient (G) | B to G Ratio |
|---|---|---|---|---|---|
| bank-3 | bot8 | 0.9395 | 0.8890 | 0.0505 | B |
| bank-2 | bot7 | 0.7910 | 0.7409 | 0.0501 | B |
| forest-2 | forest-2 | 0.4577 | 0.3750 | 0.0827 | B |
| bot5-1 | bot4 | 0.8188 | 0.5455 | 0.2732 | B > G |
| bot20-3 | bot3 | 0.7362 | 0.4545 | 0.2817 | B > G |
| bot20-2 | bot2 | 0.6469 | 0.4791 | 0.1677 | B > G |
| bot5-2 | bot5 | 0.5228 | 0.3823 | 0.1404 | B > G |
| forest-1 | forest-1 | 0.8846 | 0.4286 | 0.4559 | B ≈ G |
| forest-3 | forest-3 | 0.5969 | 0.2964 | 0.3006 | B ≈ G |
| slope-2 | slope-2 | 0.3476 | 0.1667 | 0.1809 | B ≈ G |
| slope-1 | slope-1 | 0.6403 | 0.1249 | 0.5154 | B < G |
| bank-1 | bot6 | 0.8991 | 0.0688 | 0.8304 | G |
| bot20-1 | bot1 | 0.7373 | 0 | 0.7373 | G |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirichenko-Babko, M.; Danko, Y.; Musz-Pomorksa, A.; Widomski, M.K.; Babko, R. The Impact of Climate Variations on the Structure of Ground Beetle (Coleoptera: Carabidae) Assemblage in Forests and Wetlands. Forests 2020, 11, 1074. https://doi.org/10.3390/f11101074
Kirichenko-Babko M, Danko Y, Musz-Pomorksa A, Widomski MK, Babko R. The Impact of Climate Variations on the Structure of Ground Beetle (Coleoptera: Carabidae) Assemblage in Forests and Wetlands. Forests. 2020; 11(10):1074. https://doi.org/10.3390/f11101074
Chicago/Turabian StyleKirichenko-Babko, Marina, Yaroslav Danko, Anna Musz-Pomorksa, Marcin K. Widomski, and Roman Babko. 2020. "The Impact of Climate Variations on the Structure of Ground Beetle (Coleoptera: Carabidae) Assemblage in Forests and Wetlands" Forests 11, no. 10: 1074. https://doi.org/10.3390/f11101074
APA StyleKirichenko-Babko, M., Danko, Y., Musz-Pomorksa, A., Widomski, M. K., & Babko, R. (2020). The Impact of Climate Variations on the Structure of Ground Beetle (Coleoptera: Carabidae) Assemblage in Forests and Wetlands. Forests, 11(10), 1074. https://doi.org/10.3390/f11101074








