Fine Root and Soil Nitrogen Dynamics during Stand Development Following Shifting Agriculture in Northeast India
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Vegetation Sampling
2.3. Soil Sampling and Analysis
2.4. Determinations of Available Nitrogen and N-Mineralization Rate
2.5. Sampling and Analysis of Fine Roots
2.6. Statistical Analysis
3. Results
3.1. Vegetation
3.2. Soil Physicochemical Properties
3.3. Available N Concentrations
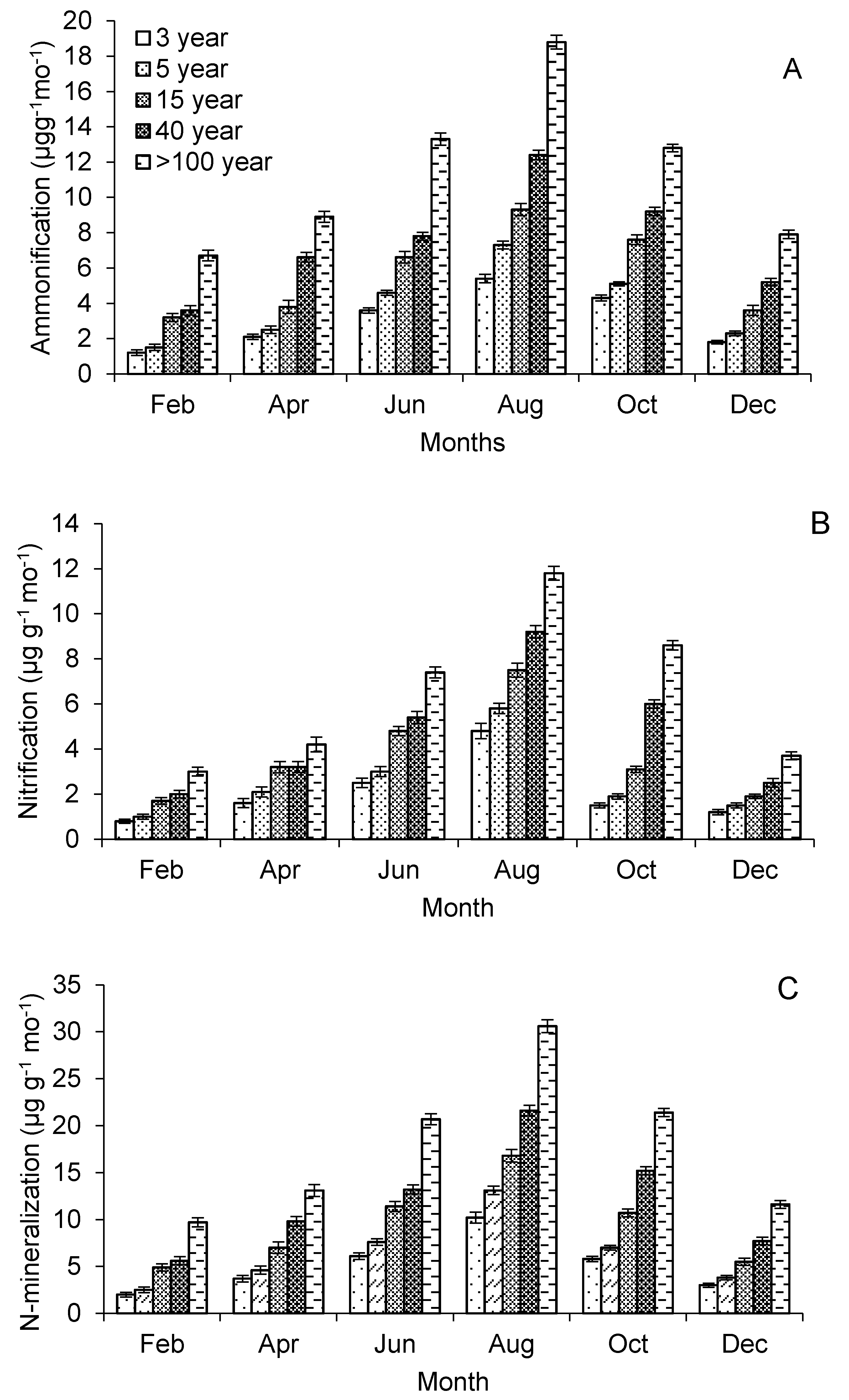
3.4. Nitrification and N-Mineralization Rate
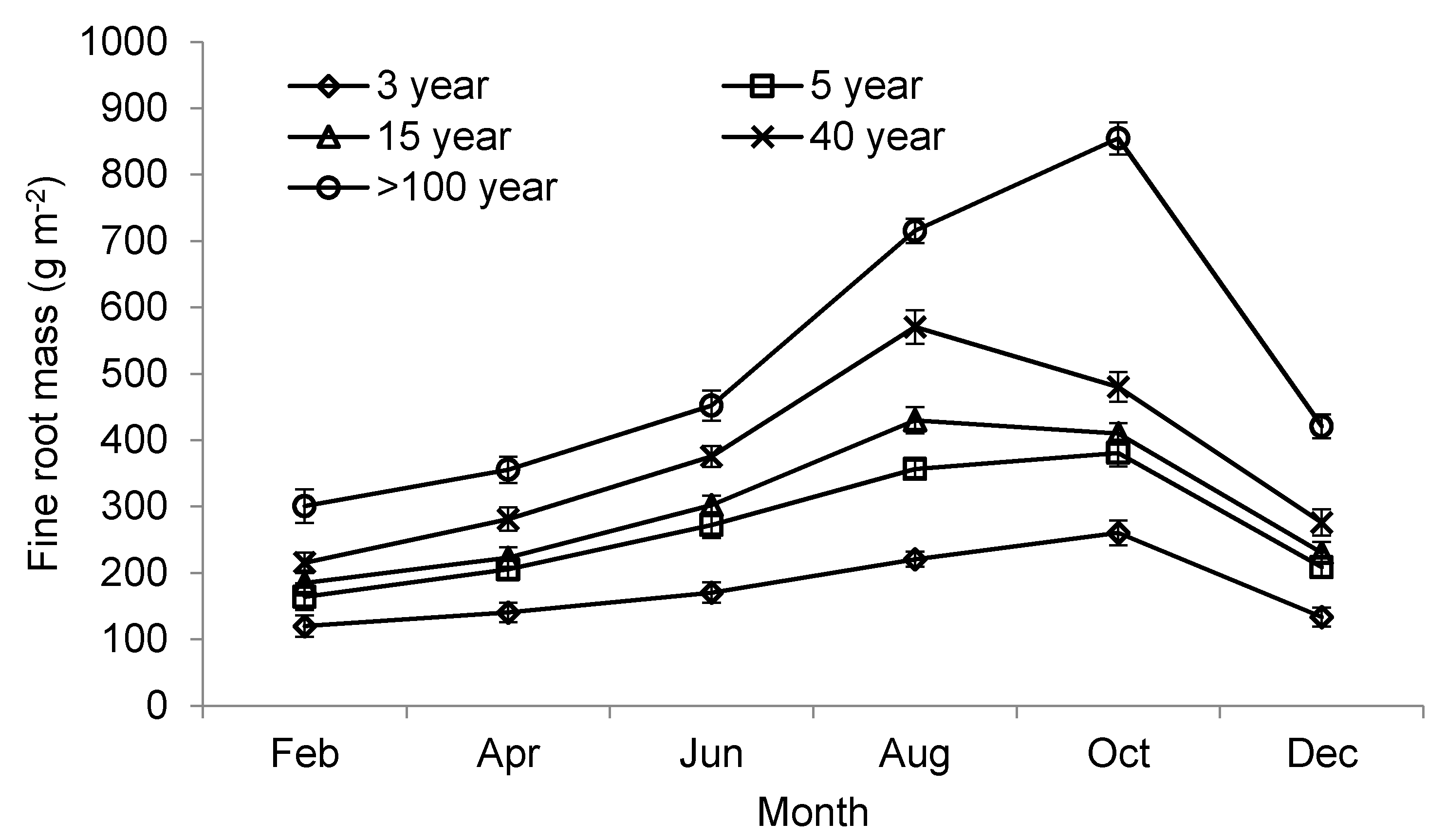
3.5. Fine Root Biomass
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tripathi, S.K.; Kushwaha, C.P.; Singh, K.P. Tropical forest and savanna ecosystems show differential impact of N and P additions on soil organic matter and aggregate structure. Glob. Chang. Biol. 2008, 14, 2572–2581. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Singh, K.P.; Singh, P.K. Temporal changes in spatial pattern of fine-root mass and nutrient concentrations in Indian bamboo savanna. Appl. Veg. Sci. 1999, 2, 229–238. [Google Scholar] [CrossRef]
- Lalnunzira, C.; Brearley, F.Q.; Tripathi, S.K. Root growth dynamics during recovery of tropical mountain forest in North-east India. J. Mt. Sci. 2019, 16, 2335–2347. [Google Scholar] [CrossRef]
- Cairns, M.F. Shifting Cultivation and Environmental Change; Routledge: Abingdon, UK, 2015. [Google Scholar]
- Grogan, P.; Lalnunmawia, F.; Tripathi, S.K. Shifting cultivation in steeply sloped regions: A review of management options and research priorities for Mizoram state, Northeast India. Agrofor. Syst. 2012, 84, 163–177. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Vanlalfakawma, D.C.; Lalnunmawia, F. Shifting cultivation on steep slopes of Mizoram, India: Impact of policy reforms. In Shifting Cultivation Policies: Balancing Environmental and Social Sustainability; Cairns, M., Ed.; CABI Publishing: Wallingford, UK, 2017; pp. 393–413. [Google Scholar]
- Kauffman, J.B.; Sanford, R.L.; Cummings, D.L.; Salcedo, I.H.; Sampaio, E.V.S.B. Biomass and nutrient dynamics associated with sash fires in Neotropical dry forests. Ecology 1993, 74, 140–151. [Google Scholar] [CrossRef]
- Filho, A.A.R.; Adams, C.; Manfredini, S.; Aguilar, R.; Neves, W.A. Dynamics of soil chemical properties in shifting cultivation systems in the tropics: A meta-analysis. Soil Use Manag. 2015, 31, 474–482. [Google Scholar] [CrossRef]
- Booth, M.S.; Stark, J.M.; Rastetter, E. Controls on nitrogen cycling in terrestrial ecosystems: A synthetic analysis of literature data. Ecol. Monogr. 2005, 75, 139–157. [Google Scholar] [CrossRef]
- Lebauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef]
- Ostertag, R.; DiManno, N.M. Detecting terrestrial nutrient limitation: A global meta-analysis of foliar nutrient concentrations after fertilization. Front. Earth Sci. 2016, 4, 23. [Google Scholar] [CrossRef]
- Walker, T.; Syers, J. The fate of phosphorus during pedogenesis. Geoderma 1976, 15, 1–19. [Google Scholar] [CrossRef]
- Vitousek, P.M. Litterfall, nutrient cycling, and nutrient limitation in tropical forests. Ecology 1984, 65, 285–298. [Google Scholar] [CrossRef]
- Davidson, E.A.; De Carvalho, C.J.R.; Figueira, A.M.; Ishida, F.Y.; Ometto, J.P.H.B.; Nardoto, G.B.; Sabá, R.T.; Hayashi, S.N.; Leal, E.C.; Vieira, I.C.G.; et al. Recuperation of nitrogen cycling in Amazonian forests following agricultural abandonment. Nature 2007, 447, 995–998. [Google Scholar] [CrossRef]
- Silver, W.L.; Miya, R.K. Global patterns in root decomposition: Comparisons of climate and litter quality effects. Oecologia 2001, 129, 407–419. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Sumida, A.; Ono, K.; Shibata, H.; Uemura, S.; Takahashi, K.; Hara, T. The effects of understorey dwarf bamboo (Sasa kurilensis) removal on soil fertility in a Betula ermanii forest of northern Japan. Ecol. Res. 2005, 21, 315–320. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. Fine root biomass, production, turnover rates, and nutrient contents in boreal forest ecosystems in relation to species, climate, fertility, and stand age: Literature review and meta-analyses. Crit. Rev. Plant Sci. 2010, 29, 204–221. [Google Scholar] [CrossRef]
- Finér, L.; Ohashi, M.; Noguchi, K.; Hirano, Y. Factors causing variation in fine root biomass in forest ecosystems. For. Ecol. Manag. 2011, 261, 265–277. [Google Scholar] [CrossRef]
- Keyes, M.R.; Grier, C.C. Above- and below-ground net production in 40-year-old Douglas-fir stands on low and high productivity sites. Can. J. For. Res. 1981, 11, 599–605. [Google Scholar] [CrossRef]
- Wapongnungsang; Tripathi, S. Fine root growth and soil nutrient dynamics during shifting cultivation in tropical semi-evergreen forests of northeast India. J. Environ. Biol. 2019, 40, 45–52. [Google Scholar] [CrossRef]
- Hertel, D.; Leuschner, C.; Harteveld, M.; Wiens, M. Fine root mass, distribution and regeneration in disturbed primary forests and secondary forests of the moist tropics. In The Stability of Tropical Rainforest Margins: Linking Ecological, Economic and Social Constraints of Land Use and Conservation; Tscharntke, T., Leuschner, C., Zeller, M., Guhardja, E., Bidin, A., Eds.; Springer: Berlin, Germany, 2007; pp. 87–106. [Google Scholar]
- Brearley, F.Q. Below-ground secondary succession in tropical forests of Borneo. J. Trop. Ecol. 2011, 27, 413–420. [Google Scholar] [CrossRef][Green Version]
- Lalnunzira, C.; Tripathi, S.K. Leaf and root production, decomposition and carbon and nitrogen fluxes during stand development in tropical moist forests, north-east India. Soil Res. 2018, 56, 306. [Google Scholar] [CrossRef]
- Champion, H.G.; Seth, S.K. A Revised Survey of the Forest Types of India; Manager of Publications, Government of India: New Delhi, India, 1968. [Google Scholar]
- Misra, R. Ecology Work Book; Oxford & IBH Publishing Co.: New Delhi, India, 1968. [Google Scholar]
- Bouyoucos, G.J. Directions for making mechanical analyses of soils by the hydrometer method. Soil Sci. 1936, 42, 225–230. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice-Hall Inc.: Englewood Cliffs, NJ, USA, 1958. [Google Scholar]
- Wetzel, R.G.; Likens, G.E. Limnological Analyses; W. B. Saunders Company: Philadelphia, PA, USA, 1979. [Google Scholar]
- Eno, C.F. Nitrate production in the field by incubating the soil in polyethylene bags. Soil Sci. Soc. Am. J. 1960, 24, 277–279. [Google Scholar] [CrossRef]
- Persson, H. Root dynamics in a young Scots pine stand in central Sweden. Oikos 1978, 30, 508. [Google Scholar] [CrossRef]
- McClaugherty, C.; Aber, J.D.; Melillo, J.M. The role of fine roots in the organicmatter and nitrogen budgets of two forested ecosystems. Ecology 1982, 63, 1481–1490. [Google Scholar] [CrossRef]
- Vogt, K.; Grier, C.; Vogt, D. Production, Turnover, and Nutrient Dynamics of Above- and Belowground Detritus of World Forests. Adv. Ecol. Res. 1986, 15, 303–377. [Google Scholar] [CrossRef]
- Arunachalam, A.; Pandey, H.N.; Tripathi, R.S.; Maithani, K. Biomass and production of fine and coarse roots during regrowth of a disturbed subtropical humid forest in north-east India. Vegetatio 1996, 123, 73–80. [Google Scholar] [CrossRef]
- Upadhaya, K.; Pandey, H.N.; Law, P.S.; Tripathi, R.S. Dynamics of fine and coarse roots and nitrogen mineralization in a humid subtropical forest ecosystem of northeast India. Biol. Fertil. Soils 2005, 41, 144–152. [Google Scholar] [CrossRef]
- Barbhuiya, A.R.; Arunachalam, A.; Pandey, H.N.; Khan, M.L.; Arunachalam, K. Fine root dynamics in undisturbed and disturbed stands of a tropical wet evergreen forest in northeast India. Trop. Ecol. 2012, 53, 69–79. [Google Scholar]
- Yuan, Z.Y.; Chen, H.Y. Fine root dynamics with stand development in the boreal forest. Funct. Ecol. 2012, 26, 991–998. [Google Scholar] [CrossRef]
- Jagodziński, A.M.; Kałucka, I. Fine root biomass and morphology in an age-sequence of post-agricultural Pinus sylvestris L. stands. Dendrobiology 2011, 66, 71–84. [Google Scholar]
- Harris, W.F.; Kinerson, R.S., Jr.; Edwards, N.T. Comparison of belowground biomass of natural deciduous forest and loblolly pine plantations. In The Belowground Ecosystem: A Synthesis of Plant-Associated Processes; Marshall, J.K., Ed.; Colorado State University: Fort Collins, CO, USA, 1977; pp. 29–38. [Google Scholar]
- Srivastava, S.K.; Singh, K.P.; Upadhyay, R.S. Fine root growth dynamics in teak (Tectona grandis Linn. F.). Can. J. For. Res. 1986, 16, 1360–1364. [Google Scholar] [CrossRef]
- Maycock, C.R.; Congdon, R.A. Fine root biomass and soil N and P in north Queensland rain forests. Biotropica 2000, 32, 185–190. [Google Scholar] [CrossRef]
- Powers, J.S.; Treseder, K.K.; Lerdau, M.T. Fine roots, arbuscular mycorrhizal hyphae and soil nutrients in four neotropical rain forests: Patterns across large geographic distances. New Phytol. 2004, 165, 913–921. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. A global analysis of fine root production as affected by soil nitrogen and phosphorus. Proc. Royal Soc. Lond. Ser. B Biol. Sci. 2012, 279, 3796–3802. [Google Scholar] [CrossRef]
- Phillips, R.P.; Fahey, T.J. Fertilization effects on fineroot biomass, rhizosphere microbes and respiratory fluxes in hardwood forest soils. New Phytol. 2007, 176, 655–664. [Google Scholar] [CrossRef]
- Aber, J.D.; Melillo, J.M.; Nadelhoffer, K.J.; McClaugherty, C.; Pastor, J. Fine root turnover in forest ecosystems in relation to quantity and form of nitrogen availability: A comparison of two methods. Oecologia 1985, 66, 317–321. [Google Scholar] [CrossRef]
- Singh, J.S.; Raghubanshi, A.S.; Srivastava, S.C. Microbial biomass acts as a source of plant nutrients in dry tropical forest and savanna. Nature 1989, 338, 499–500. [Google Scholar] [CrossRef]
- Roy, S.; Singh, J. Seasonal and spatial dynamics of plant-available N and P pools and N-mineralization in relation to fine roots in a dry tropical forest habitat. Soil Biol. Biochem. 1995, 27, 33–40. [Google Scholar] [CrossRef]
- Hertel, D.; Harteveld, M.A.; Leuschner, C. Conversion of a tropical forest into agroforest alters the fine root-related carbon flux to the soil. Soil Biol. Biochem. 2009, 41, 481–490. [Google Scholar] [CrossRef]
- Singh, S.B.; Mishra, B.P.; Tripathi, S.K. Recovery of plant diversity and soil nutrients during stand development in subtropical forests of Mizoram, Northeast India. Biodiversitas 2015, 16, 205–212. [Google Scholar] [CrossRef]
- Singha, D.; Tripathi, S.K. Variations in fine root growth during age chronosequence of moist tropical forest following shifting cultivation in Mizoram, northeast India. Trop. Ecol. 2017, 58, 769–779. [Google Scholar]
- Liu, B.; Li, H.; Zhu, B.; Koide, R.T.; Eissenstat, D.M.; Guo, D. Complementarity in nutrient foraging strategies of absorptive fine roots and arbuscular mycorrhizal fungi across 14 co-existing subtropical tree species. New Phytol. 2015, 208, 125–136. [Google Scholar] [CrossRef]
- Amazonas, N.T.; Martinelli, L.A.; Piccolo, M.D.C.; Rodrigues, R.R. Nitrogen dynamics during ecosystem development in tropical forest restoration. For. Ecol. Manag. 2011, 262, 1551–1557. [Google Scholar] [CrossRef]
- Mylliemngap, W.; Nath, D.; Barik, S.K. Changes in vegetation and nitrogen mineralization during recovery of a montane subtropical broadleaved forest in North-eastern India following anthropogenic disturbance. Ecol. Res. 2015, 31, 21–38. [Google Scholar] [CrossRef]
- Winbourne, J.B.; Feng, A.; Reynolds, L.; Piotto, D.; Hastings, M.G.; Porder, S. Nitrogen cycling during secondary succession in Atlantic Forest of Bahia, Brazil. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Rice, E.L.; Pancholy, S.K. Inhibition of nitrification by climax ecosystems. Am. J. Bot. 1972, 59, 1033–1040. [Google Scholar] [CrossRef]
- Walley, F.; Van Kessel, C.; Pennock, D. Landscape-scale variability of N mineralization in forest soils. Soil Biol. Biochem. 1996, 28, 383–391. [Google Scholar] [CrossRef]
- Sahrawat, K.L. Factors affecting nitrification in soils. Commun. Soil Sci. Plant Anal. 2008, 39, 1436–1446. [Google Scholar] [CrossRef]
- Robertson, G.P.; Vitousek, P.M. Nitrification potentials in primary and secondary succession. Ecology 1981, 62, 376–386. [Google Scholar] [CrossRef]
- Pajares, S.; Bohannan, B.J.M. Ecology of nitrogen fixing, nitrifying, and denitrifying microorganisms in tropical forest soils. Front. Microbiol. 2016, 7, 1045. [Google Scholar] [CrossRef]


| Stand Age (Years) | |||||
|---|---|---|---|---|---|
| 3 | 5 | 15 | 40 | NF | |
| Tree density (no. ha−1) | 399 ± 36 a | 933 ± 88 b | 1690 ± 124 c | 2290 ± 169 d | 4290 ± 281 e |
| Tree basal area (m2 ha−1) | 2.1 ± 0.5 a | 6.9 ± 0.8 b | 12.3 ± 1.3 c | 41.4 ± 4.8 d | 64.7 ± 6.2 e |
| Herb density (no. m−2) | 39.1 ± 3.4 b | 35.4 ± 3.6 b | 33.9 ± 4.2 b | 28.2 ± 2.9 a | 27.6 ± 3.1a |
| Stand Age (Years) | |||||
|---|---|---|---|---|---|
| 3 | 5 | 15 | 40 | NF | |
| Soil temperature (°C) * | 20.6 ± 1.1 a | 20.4 ± 0.9 a | 19.8 ± 1.2 a | 19.1 ± 0.7 a | 18.7 ± 1.0 a |
| Bulk density (g cm−3) | 0.97 ± 0.03 a | 0.92 ± 0.03 b | 0.80 ± 0.02 c | 0.80 ± 0.05 c | 0.69 ± 0.05 d |
| Textural class | Sandy Loam | Loamy Sand | Loamy Sand | Loamy sand | Loamy Sand |
| Moisture content (%) * | 29.7 ± 1.1 a | 30.1 ± 1.6 a | 30.4 ± 0.8 a | 31.8 ± 1.2 a | 41.3 ± 1.2 b |
| pH in water | 4.6 ± 0.2 a | 4.8 ± 0.4 a | 4.8 ± 0.4 a | 4.7 ± 0.1 a | 4.5 ± 0.2 a |
| C (mg g–1) * | 23.4 ± 0.3 a | 28.2 ± 0.3 a | 26.5 ± 0.3 a | 27.3 ± 0.3 a | 44.1 ± 0.4 b |
| N (mg g–1) * | 2.3 ± 0.02 a | 2.4 ± 0.02 a | 2.0 ± 0.03 a | 2.2 ± 0.02 a | 3.3 ± 0.3 b |
| C:N ratio | 10.2 ± 0.6 a | 11.8 ± 1.3 a | 13.2 ± 0.9 a | 12.4 ± 1.1 a | 13.4 ± 0.9 a |
| Stand Age (Years) | |||||
|---|---|---|---|---|---|
| 3 | 5 | 15 | 40 | NF | |
| NH4-N (μg g−1) * | 5.7 ± 0.5 a | 7.4 ± 0.6 ab | 10.2 ± 0.6 b | 12.4 ± 0.5 b | 16.1 ± 0.8 c |
| NO3-N (μg g−1) * | 3.3 ± 0.4 a | 3.8 ± 0.3 ab | 4.8 ± 0.4 ab | 5.6 ± 0.5 bc | 6.7 ± 0.6 c |
| NH4-N/NO3-N | 1.7 ± 0.1 a | 1.9 ± 0.05 ab | 2.1 ± 0.04 b | 2.2 ± 0.1 b | 2.4 ± 0.1 c |
| Ammonification (μg g−1 month−1) * | 3.1 ± 0.1 a | 3.9 ± 0.1 a | 5.7 ± 0.3 ab | 7.5 ± 0.2 b | 11.4 ± 0.3 c |
| Nitrification (μg g−1 month−1) * | 2.1 ± 0.2 a | 2.6 ± 0.1 a | 3.7 ± 0.2 a | 4.7 ± 0.3 ab | 6.5 ± 0.3 b |
| N-mineralization (μg g−1 month−1) | 5.2 ± 0.3 a | 6.5 ± 0.2 a | 9.4 ± 0.5 b | 12.2 ± 0.5 b | 17.9 ± 0.6 c |
| Ammonification: nitrification | 1.5 ± 0.2 a | 1.5 ± 0.2 a | 1.5 ± 0.2 a | 1.6 ± 0.1 a | 1.8 ± 0.1 a |
| Fine Root Parameter | Stand Age (Years) | |||||
|---|---|---|---|---|---|---|
| Diameter (mm) | 3 | 5 | 15 | 40 | >100 | |
| Biomass (g m−2) | <0.5 | 34.4 ± 3.0 a | 49.0 ± 6.6 b | 38.5 ± 11.0 a | 45.0 ± 14.1 b | 87.1 ± 12.6 c |
| 0.5–2 | 68.3 ± 13.3 a | 101.9 ± 10.8 a | 134.9 ± 12.8 b | 169.8 ± 14.5 b | 242.4 ± 20.3 c | |
| Necromass (g m−2) | <0.5 | 40.9 ± 4.4 a | 64.8 ± 7.8 b | 56.1 ± 9.88 b | 84.7 ± 13.3 c | 103.9 ± 11.4 d |
| 0.5–2 | 30.7 ± 5.7 a | 49.0 ± 8.2 a | 67.3 ± 10.9 b | 66.9 ± 9.7 b | 83.2 ± 6.8 c | |
| Total mass (g m−2) | <2 | 174.3 ± 15.1 a | 264.7 ± 16.4 a | 296.8 ± 16.8 a | 366.4 ± 19.4 b | 516.6 ± 21.2 b |
| Very fine (<0.5 mm): total (<2 mm) biomass ratio | NA | 0.33 ± 0.03 b | 0.32 ± 0.03 b | 0.22 ± 0.02 a | 0.21 ± 0.02 a | 0.26 ± 0.01 a |
| Production (g m−2 year−1) | <2 | 140.4 ± 7.2 a | 216.7 ± 12.5 ab | 244.9 ± 14.7 b | 355.1 ± 24.1 c | 553.8 ± 27.2 c |
| Turnover (year−1) | <2 | 0.81 ± 0.07 a | 0.82 ± 0.06 a | 0.83 ± 0.11 a | 0.97 ± 0.13 b | 1.07 ± 0.09 b |
| Stand Age (Years) | Soil Property | ||||
|---|---|---|---|---|---|
| Soil Moisture | Total Mineral N | Nitrification | Ammonification | N-Mineralization | |
| 3 | 0.535 | −0.619 | 0.487 | 0.882 * | 0.727 |
| 5 | 0.732 | −0.683 | 0.616 | 0.908 * | 0.803 * |
| 15 | 0.729 | −0.665 | 0.739 | 0.972 ** | 0.898 * |
| 40 | 0.499 | −0.643 | 0.978 ** | 0.977 ** | 0.981 ** |
| NF | 0.689 | −0.705 | 0.827 * | 0.727 | 0.773 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singha, D.; Brearley, F.Q.; Tripathi, S.K. Fine Root and Soil Nitrogen Dynamics during Stand Development Following Shifting Agriculture in Northeast India. Forests 2020, 11, 1236. https://doi.org/10.3390/f11121236
Singha D, Brearley FQ, Tripathi SK. Fine Root and Soil Nitrogen Dynamics during Stand Development Following Shifting Agriculture in Northeast India. Forests. 2020; 11(12):1236. https://doi.org/10.3390/f11121236
Chicago/Turabian StyleSingha, Dipendra, Francis Q. Brearley, and Shri Kant Tripathi. 2020. "Fine Root and Soil Nitrogen Dynamics during Stand Development Following Shifting Agriculture in Northeast India" Forests 11, no. 12: 1236. https://doi.org/10.3390/f11121236
APA StyleSingha, D., Brearley, F. Q., & Tripathi, S. K. (2020). Fine Root and Soil Nitrogen Dynamics during Stand Development Following Shifting Agriculture in Northeast India. Forests, 11(12), 1236. https://doi.org/10.3390/f11121236






