Magnitude and Edaphic Controls of Nitrous Oxide Fluxes in Natural Forests at Different Scales
Abstract
:1. Introduction
2. Data and Method
2.1. Field Measurement Data Compilation
2.2. Data Analysis
3. Results
3.1. Annual N2O Fluxes and N2O Emission Budget of Global Forests and Forest Classified by Different Biotic Groups
3.2. Seasonal Variation of N2O Fluxes of Global Forests and Forest Classified by Different Biotic Groups
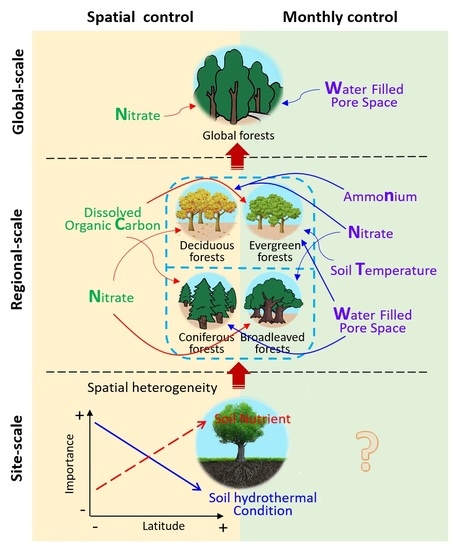
3.3. Edaphic Factors Involved in Controlling N2O Fluxes on Global, Regional, and Site Scales
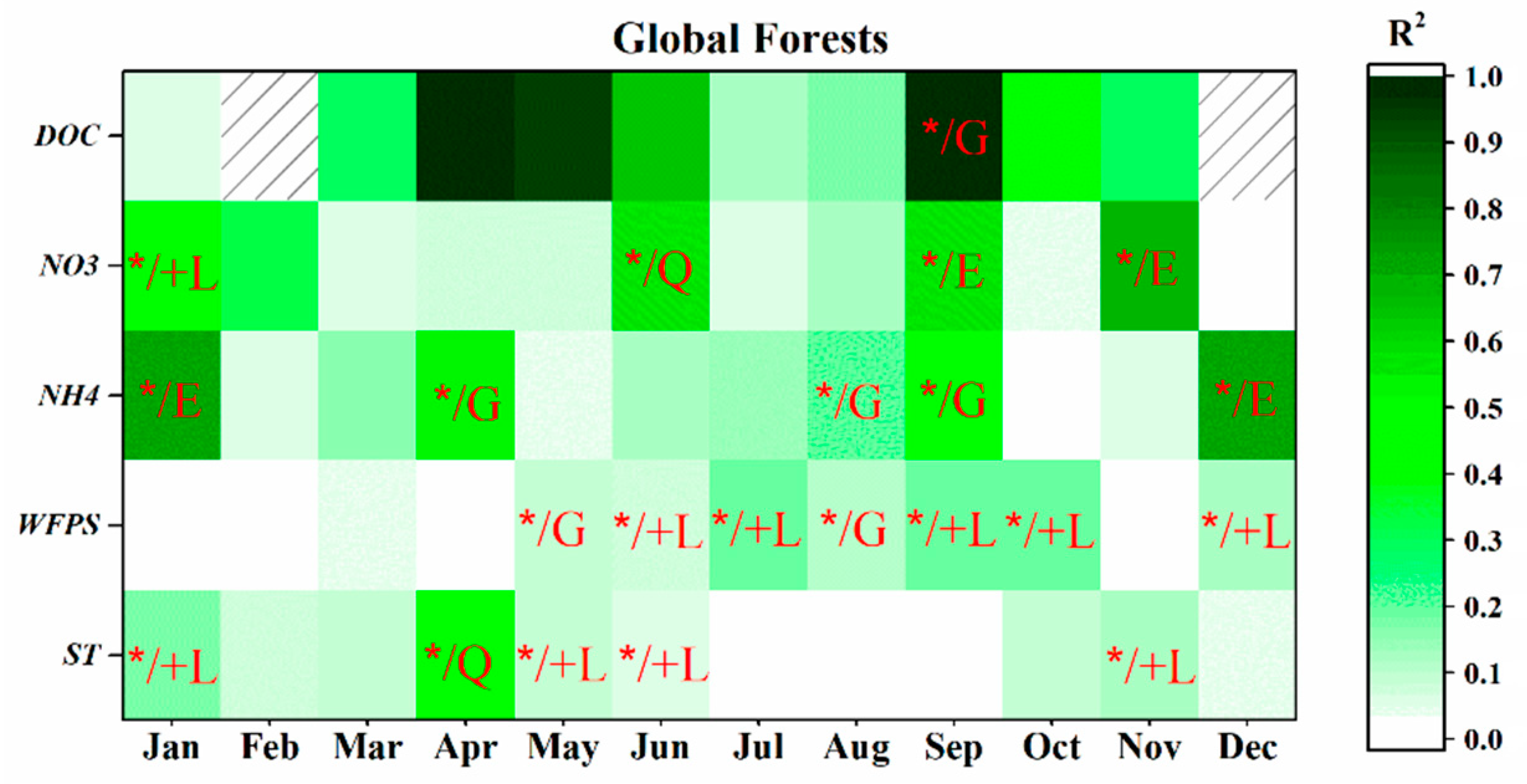
3.3.1. Global-Scale
3.3.2. Regional-Scale
3.3.3. Site-Scale
4. Discussion
4.1. Comparison of Total N2O Budget with Previous Studies
4.2. Distal Biotic Drivers of Soil N2O Flux
4.3. Comparison among Edaphic Factors Involved in Controlling N2O Fluxes on Different Scales
4.3.1. The Explanatory Power of Five Edaphic Factors for N2O Flux Varied among the Different Scales
4.3.2. Key Spatial Controls Vary at Different Scales
4.3.3. The Trend of Spatial Controls Response to N2O Fluxes Vary at Different Scales
4.3.4. Monthly Controls Vary at Different Scales
5. Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Knohl, A.; Veldkamp, E. Indirect feedbacks to rising CO2. Nature 2011, 475, 177. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciais, P.; Chris, S.; Govindasamy, B.; Bopp, L.; Brovkin, V.; Canadell, J.; Chhabra, A.; Defries, R.; Galloway, J.; Heimann, M. Carbon and Other Biogeochemical Cycles. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., et al., Eds.; Cambridge University Press: Cambridge, UK ; New York, NY, USA, 2013; pp. 465–570. [Google Scholar]
- Tian, H.; Lu, C.; Ciais, P.; Michalak, A.M.; Canadell, J.G.; Saikawa, E.; Huntzinger, D.N.; Gurney, K.R.; Sitch, S.; Zhang, B.; et al. The terrestrial biosphere as a net source of greenhouse gases to the atmosphere. Nature 2016, 531, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prentice, I.C. Terrestrial nitrogen cycle simulation with a dynamic global vegetation model. Glob. Chang. Biol. 2008, 14, 1745–1764. [Google Scholar] [CrossRef]
- Zhang, K.; Zhu, Q.; Liu, J.; Wang, M.; Zhou, X.; Li, M.; Wang, K.; Ding, J.; Peng, C. Spatial and temporal variations of N2O emissions from global forest and grassland ecosystems. Agric. For. Meteorol. 2019, 266–267, 129–139. [Google Scholar] [CrossRef]
- Zhuang, Q.; Lu, Y.; Chen, M. An inventory of global N2O emissions from the soils of natural terrestrial ecosystems. Atmos. Environ. 2012, 47, 66–75. [Google Scholar] [CrossRef]
- Baggs, E.M. Soil microbial sources of nitrous oxide: Recent advances in knowledge, emerging challenges and future direction. Curr. Opin. Environ. Sustain. 2011, 3, 321–327. [Google Scholar] [CrossRef]
- Machefert, S.E.; Dise, N.B.; Goulding, K.W.T.; Whitehead, P.G. Nitrous oxide emission from a range of land uses across Europe. Hydrol. Earth Syst. Sci. Discuss. 2002, 6, 325–338. [Google Scholar] [CrossRef] [Green Version]
- Zerva, A.; Mencuccini, M. Short-term effects of clearfelling on soil CO2, CH4, and N2O fluxes in a Sitka spruce plantation. Soil Biol. Biochem. 2005, 37, 2025–2036. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef]
- Pilegaard, K.; Skiba, U.; Ambus, P.; Beier, C.; Pihlatie, M.; Vesala, T. Factors controlling regional differences in forest soil emission of nitrogen oxides (NO and N2O). Biogeosciences 2006, 3, 651–661. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Allen, G.; Dekate, C.; Kaiser, H.; Lei, Z.; Maclaren, J. getdata: A Grid Enabled Data Client for Coastal Modeling. Simul. Ser. 2006, 38, 187. [Google Scholar]
- He, Z.; Huo, S.; Ma, C.; Zhang, H.; An, D.; Xi, B.; Xu, Q.; Xia, X.; Wu, F. The Contributions of Climate Changes and Human Activities to Long-Term Variations in Lake Sediments Based on Results from Generalized Additive Models. Water Resour. Manag. 2019, 33, 1069–1085. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012; Available online: http://www.Rproject.org/bin/windows/base/old/2.2.1/ (accessed on 20 December 2010).
- Li, W.; Ciais, P.; MacBean, N.; Peng, S.; Defourny, P.; Bontemps, S. Major forest changes and land cover transitions based on plant functional types derived from the ESA CCI Land Cover product. Int. J. Appl. Earth Obs. Geoinf. 2016, 47, 30–39. [Google Scholar] [CrossRef]
- Raich, J.W.; Potter, C.S.; Bhagawati, D. Interannual variability in global soil respiration, 1980–1994. Glob. Chang. Biol. 2002, 8, 800–812. [Google Scholar] [CrossRef]
- Xu, X.; Tian, H.; Hui, D. Convergence in the relationship of CO2 and N2O exchanges between soil and atmosphere within terrestrial ecosystems. Glob. Chang. Biol. 2008, 14, 1651–1660. [Google Scholar] [CrossRef]
- Tian, H.; Chen, G.; Lu, C.; Xu, X.; Ren, W.; Banger, K.; Zhang, B.; Tao, B.; Pan, S.; Liu, M. Global land-atmosphere exchange of methane and nitrous oxide: Magnitude and spatiotemporal patterns. Biogeosci. Discuss. 2013, 10, 19811–19865. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Xu, X.; Liu, M.; Ren, W.; Zhang, C.; Chen, G.; Lu, C. Spatial and temporal patterns of CH4 and N2O fluxes in terrestrial ecosystems of North America during 1979–2008: Application of a global biogeochemistry model. Biogeosciences 2010, 7, 2673–2694. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Peng, C.; Wang, M.; Zhou, X.; Li, M.; Wang, K.; Ding, J.; Zhu, Q. Process-based TRIPLEX-GHG model for simulating N2O emissions from global forests and grasslands: M odel development and evaluation. J. Adv. Modeling Earth Syst. 2017, 9, 2079–2102. [Google Scholar] [CrossRef]
- Augusto, L.; De Schrijver, A.; Vesterdal, L.; Smolander, A.; Prescott, C.; Ranger, J. Influences of evergreen gymnosperm and deciduous angiosperm tree species on the functioning of temperate and boreal forests. Biol. Rev. 2015, 90, 444–466. [Google Scholar] [CrossRef]
- Binkley, D.; Giardina, C. Why do tree species affect soils? The Warp and Woof of tree-soil interactions. In Plant-Induced Soil Changes: Processes and Feedbacks; Van Breemen, N., Ed.; Springer: Dordrecht, The Netherlands, 1998; pp. 89–106. [Google Scholar] [CrossRef]
- Orwin, K.H.; Buckland, S.M.; Johnson, D.; Turner, B.L.; Smart, S.; Oakley, S.; Bardgett, R.D. Linkages of plant traits to soil properties and the functioning of temperate grassland. J. Ecol. 2010, 98, 1074–1083. [Google Scholar] [CrossRef] [Green Version]
- Fyles, J.W.; Fyles, I.H. Interaction of Douglas-fir with red alder and salal foliage litter during decomposition. Can. J. For. Res. 1993, 23, 358–361. [Google Scholar] [CrossRef]
- McTiernan, K.B.; Ineson, P.; Coward, P.A. Respiration and Nutrient Release from Tree Leaf Litter Mixtures. Oikos 1997, 78, 527–538. [Google Scholar] [CrossRef]
- Salamanca, E.F.; Kaneko, N.; Katagiri, S. Effects of leaf litter mixtures on the decomposition of Quercus serrata and Pinus densiflora using field and laboratory microcosm methods. Ecol. Eng. 1998, 10, 53–73. [Google Scholar] [CrossRef]
- Laughlin, D.C.; Leppert, J.J.; Moore, M.M.; Sieg, C.H. A multi-trait test of the leaf-height-seed plant strategy scheme with 133 species from a pine forest flora. Funct. Ecol. 2010, 24, 493–501. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.C.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Pérez-Harguindeguy, N.; et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef]
- Quested, H.; Eriksson, O.; Fortunel, C.; Garnier, E. Plant traits relate to whole-community litter quality and decomposition following land use change. Funct. Ecol. 2007, 21, 1016–1026. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, K.; Lyu, Z.; Zhu, J. Microbial groups and their functions control the decomposition of coniferous litter: A comparison with broadleaved tree litters. Soil Biol. Biochem. 2019, 133, 196–207. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, J.; Wang, S.; Cai, Z.; Wang, L. Effects of temperature change and tree species composition on N2O and NO emissions in acidic forest soils of subtropical China. J. Environ. Sci. 2014, 26, 617–625. [Google Scholar] [CrossRef]
- Rivallan, M.; Ricchiardi, G.; Bordiga, S.; Zecchina, A. Adsorption and reactivity of nitrogen oxides (NO2, NO, N2O) on Fe–zeolites. J. Catal. 2009, 264, 104–116. [Google Scholar] [CrossRef]
- Wang, S.; Ruan, H.; Han, Y. Effects of microclimate, litter type, and mesh size on leaf litter decomposition along an elevation gradient in the Wuyi Mountains, China. Ecol. Res. 2010, 25, 1113–1120. [Google Scholar] [CrossRef]
- Ellsworth, P.V.; Sternberg, L.S.L. Biochemical effects of salinity on oxygen isotope fractionation during cellulose synthesis. New Phytol. 2014, 202, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Berg, B.; Xie, Z. Leaf habit of tree species does not strongly predict leaf litter decomposition but alters climate-decomposition relationships. Plant Soil 2017, 419, 363–376. [Google Scholar] [CrossRef]
- Han, W.X.; Fang, J.Y.; Reich, P.B.; Ian Woodward, F.; Wang, Z.H. Biogeography and variability of eleven mineral elements in plant leaves across gradients of climate, soil and plant functional type in China. Ecol. Lett. 2011, 14, 788–796. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H.; Wang, J.; Cui, L.; Tian, D.; Wang, J.; Zhang, X.; Yan, L.; Yan, Z.; Zhang, K. Plant biomass and soil organic carbon are main factors influencing dry-season ecosystem carbon rates in the coastal zone of the Yellow River Delta. PLoS ONE 2019, 14, e0210768. [Google Scholar] [CrossRef]
- Szukics, U.; Abell, G.C.J.; Hödl, V.; Mitter, B.; Sessitsch, A.; Hackl, E.; Zechmeister-Boltenstern, S. Nitrifiers and denitrifiers respond rapidly to changed moisture and increasing temperature in a pristine forest soil. FEMS Microbiol. Ecol. 2010, 72, 395–406. [Google Scholar] [CrossRef]
- Xue, K.; Yuan, M.M.; Shi, Z.J.; Qin, Y.; Deng, Y.; Cheng, L.; Wu, L.; He, Z.; Van Nostrand, J.D.; Bracho, R.; et al. Tundra soil carbon is vulnerable to rapid microbial decomposition under climate warming. Nat. Clim. Chang. 2016, 6, 595–600. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.E.; Myrold, D.D.; Bottomley, P.J. Temperature affects the kinetics of nitrite oxidation and nitrification coupling in four agricultural soils. Soil Biol. Biochem. 2019, 136, 107523. [Google Scholar] [CrossRef]
- Saggar, S.; Jha, N.; Deslippe, J.; Bolan, N.S.; Luo, J.; Giltrap, D.L.; Kim, D.G.; Zaman, M.; Tillman, R.W. Denitrification and N2O:N2 production in temperate grasslands: Processes, measurements, modelling and mitigating negative impacts. Sci. Total Environ. 2013, 465, 173–195. [Google Scholar] [CrossRef]
- Pärn, J.; Verhoeven, J.T.A.; Butterbach-Bahl, K.; Dise, N.B.; Ullah, S.; Aasa, A.; Egorov, S.; Espenberg, M.; Järveoja, J.; Jauhiainen, J.; et al. Nitrogen-rich organic soils under warm well-drained conditions are global nitrous oxide emission hotspots. Nat. Commun. 2018, 9, 1135. [Google Scholar] [CrossRef] [Green Version]
- Veber, G.; Kull, A.; Villa, J.A.; Maddison, M.; Paal, J.; Oja, T.; Iturraspe, R.; Pärn, J.; Teemusk, A.; Mander, Ü. Greenhouse gas emissions in natural and managed peatlands of America: Case studies along a latitudinal gradient. Ecol. Eng. 2018, 114, 34–45. [Google Scholar] [CrossRef]
- Firestone, M.K.; Davidson, E.A. Microbiological basis of NO and N2O production and consumption in soil. Exch. Trace Gases Terr. Ecosyst. Atmos. 1989, 47, 7–21. [Google Scholar]
- Lovett, G.M.; Weathers, K.C.; Arthur, M.A.; Schultz, J.C. Nitrogen cycling in a northern hardwood forest: Do species matter? Biogeochemistry 2004, 67, 289–308. [Google Scholar] [CrossRef]
- Näsholm, T.; Kielland, K.; Ganeteg, U. Uptake of organic nitrogen by plants. New Phytol. 2009, 182, 31–48. [Google Scholar] [CrossRef]
- Mukai, M.; Sawada, Y.; Kitayama, K.; Aiba, S.-I. Productivity and morphological traits of fine roots in forest ecosystems along an elevation gradient of Yakushima Island. J. For. Res. 2019, 24, 35–41. [Google Scholar] [CrossRef]
- Hu, H.; Chen, D.; He, J.-Z. Microbial Regulation of Terrestrial Nitrous Oxide Formation: Understanding the Biological Pathways for Prediction of Emission Rates. FEMS Microbiol. Rev. 2015, 39, 729–749. [Google Scholar] [CrossRef]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J.C. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- Wrage, N.; Velthof, G.L.; Laanbroek, H.J.; Oenema, O. Nitrous oxide production in grassland soils: Assessing the contribution of nitrifier denitrification. Soil Biol. Biochem. 2004, 36, 229–236. [Google Scholar] [CrossRef]
- Banerjee, S.; Helgason, B.; Wang, L.; Winsley, T.; Ferrari, B.C.; Siciliano, S.D. Legacy effects of soil moisture on microbial community structure and N2O emissions. Soil Biol. Biochem. 2016, 95, 40–50. [Google Scholar] [CrossRef]
- Phillips, R.; McMillan, A.; Palmada, T.; Dando, J.; Giltrap, D. Temperature effects on N2O and N2 denitrification end-products for a New Zealand pasture soil. N. Z. J. Agric. Res. 2015, 58, 89–95. [Google Scholar] [CrossRef]
- Brumme, R.; Borken, W. N2O Emission from Temperate Beech Forest Soils. In Functioning and Management of European Beech Ecosystems; Brumme, R., Khanna, P.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 353–367. [Google Scholar] [CrossRef]
- Keller, M.; Kaplan, W.A.; Wofsy, S.C.; Da Costa, J.M. Emissions of N2O from tropical forest soils: Response to fertilization with NH4+, NO3−, and PO43−. J. Geophys. Res. Atmos. 1988, 93, 1600–1604. [Google Scholar] [CrossRef]
- Li, C.; Frolking, S.; Butterbach-Bahl, K. Carbon Sequestration in Arable Soils is Likely to Increase Nitrous Oxide Emissions, Offsetting Reductions in Climate Radiative Forcing. Clim. Chang. 2005, 72, 321–338. [Google Scholar] [CrossRef]
- Robertson, K.; Klemedtsson, L. Assessment of denitrification in organogenic forest soil by regulating factors. Plant Soil 1996, 178, 49–57. [Google Scholar] [CrossRef]
- Chen, S.; Wang, F.; Zhang, Y.; Qin, S.; Wei, S.; Wang, S.; Hu, C.; Liu, B. Organic carbon availability limiting microbial denitrification in the deep vadose zone. Environ. Microbiol. 2018, 20, 980–992. [Google Scholar] [CrossRef] [Green Version]
- McCarty, G.W.; Bremner, J.M. Availability of organic carbon for denitrification of nitrate in subsoils. Biol. Fertil. Soils 1992, 14, 219–222. [Google Scholar] [CrossRef]
- Peterson, M.E.; Curtin, D.; Thomas, S.; Clough, T.J.; Meenken, E.D. Denitrification in vadose zone material amended with dissolved organic matter from topsoil and subsoil. Soil Biol. Biochem. 2013, 61, 96–104. [Google Scholar] [CrossRef]
- Senbayram, M.; Chen, R.; Budai, A.; Bakken, L.; Dittert, K. N2O emission and the N2O/(N2O+N2) product ratio of denitrification as controlled by available carbon substrates and nitrate concentrations. Agric. Ecosyst. Environ. 2012, 147, 4–12. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, G.; Li, F.; Zhou, G.; Yang, G.; Fang, K.; Liu, L.; Qin, S.; Zhang, D.; Yang, Y. Soil Temperature Dynamics Modulate N2O Flux Response to Multiple Nitrogen Additions in an Alpine Steppe. J. Geophys. Res. Biogeosci. 2018, 123, 3308–3319. [Google Scholar] [CrossRef]
- Davidson, E.; Keller, M.; Erickson, H.; Verchot, L.; Veldkamp, E. Testing a Conceptual Model of Soil Emissions of Nitrous and Nitric Oxides. Biosciences 2000, 50, 667–680. [Google Scholar] [CrossRef]
- Reddy, N.; Crohn, D.M. Effects of soil salinity and carbon availability from organic amendments on nitrous oxide emissions. Geoderma 2014, 235–236, 363–371. [Google Scholar] [CrossRef]
- Wang, R.; Chen, J.M.; Luo, X.; Black, A.; Arain, A. Seasonality of leaf area index and photosynthetic capacity for better estimation of carbon and water fluxes in evergreen conifer forests. Agric. For. Meteorol. 2019, 279, 107708. [Google Scholar] [CrossRef]
- Rapp, M.; Santa Regina, I.; Rico, M.; Gallego, H.A. Biomass, nutrient content, litterfall and nutrient return to the soil in Mediterranean oak forests. For. Ecol. Manag. 1999, 119, 39–49. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. Fine Root Biomass, Production, Turnover Rates, and Nutrient Contents in Boreal Forest Ecosystems in Relation to Species, Climate, Fertility, and Stand Age: Literature Review and Meta-Analyses. Crit. Rev. Plant Sci. 2010, 29, 204–221. [Google Scholar] [CrossRef]
- Mori, T.; Lu, X.; Aoyagi, R.; Mo, J. Reconsidering the phosphorus limitation of soil microbial activity in tropical forests. Funct. Ecol. 2018, 32, 1145–1154. [Google Scholar] [CrossRef] [Green Version]
- Qin, H.; Xing, X.; Tang, Y.; Hou, H.; Yang, J.; Shen, R.; Zhang, W.; Liu, Y.; Wei, W. Linking soil N2O emissions with soil microbial community abundance and structure related to nitrogen cycle in two acid forest soils. Plant Soil 2019, 435, 95–109. [Google Scholar] [CrossRef]








| Estimated Method | Period | Global Budget (Tg N) | Reference |
|---|---|---|---|
| Empirical climate driven soil respiration model | 2000–2008 | 6.99 | [18] |
| Artificial neural network | 2000 | 1.3 | [7] |
| Process-based model (DLEM) | 1981–2010 | 4.28 | [19] |
| Process-based model (TRIPLEX-GHG) | 1992–2015 | 3.62 | [6] |
| Literature survey | 2000 | 4.45–4.69 | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Wu, H.; Li, M.; Yan, Z.; Li, Y.; Wang, J.; Zhang, X.; Yan, L.; Kang, X. Magnitude and Edaphic Controls of Nitrous Oxide Fluxes in Natural Forests at Different Scales. Forests 2020, 11, 251. https://doi.org/10.3390/f11030251
Zhang K, Wu H, Li M, Yan Z, Li Y, Wang J, Zhang X, Yan L, Kang X. Magnitude and Edaphic Controls of Nitrous Oxide Fluxes in Natural Forests at Different Scales. Forests. 2020; 11(3):251. https://doi.org/10.3390/f11030251
Chicago/Turabian StyleZhang, Kerou, Haidong Wu, Mingxu Li, Zhongqing Yan, Yong Li, Jinzhi Wang, Xiaodong Zhang, Liang Yan, and Xiaoming Kang. 2020. "Magnitude and Edaphic Controls of Nitrous Oxide Fluxes in Natural Forests at Different Scales" Forests 11, no. 3: 251. https://doi.org/10.3390/f11030251
APA StyleZhang, K., Wu, H., Li, M., Yan, Z., Li, Y., Wang, J., Zhang, X., Yan, L., & Kang, X. (2020). Magnitude and Edaphic Controls of Nitrous Oxide Fluxes in Natural Forests at Different Scales. Forests, 11(3), 251. https://doi.org/10.3390/f11030251