Small-Scale Forest Structure Influences Spatial Variability of Belowground Carbon Fluxes in a Mature Mediterranean Beech Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Characteristics
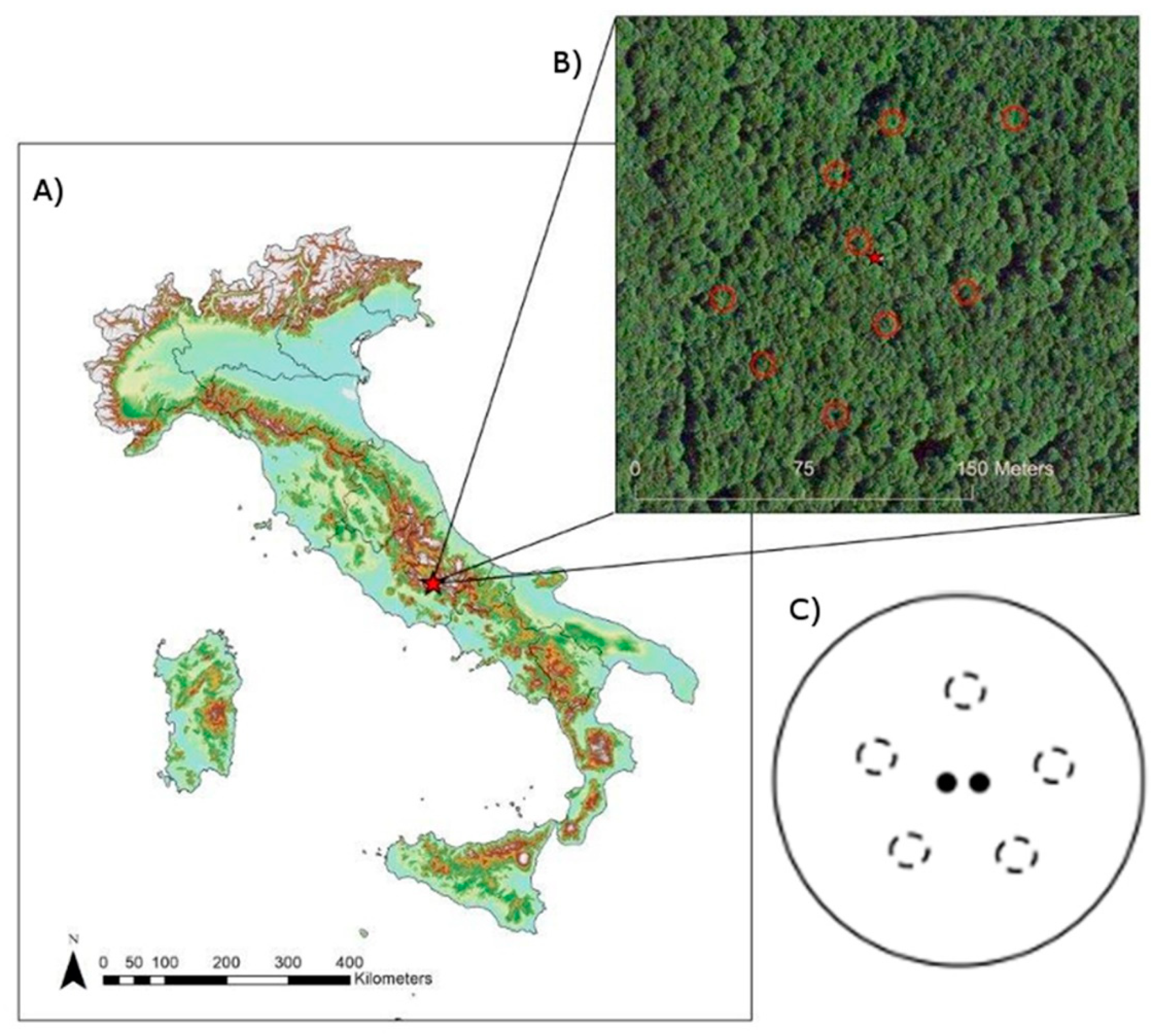
2.2. Experimental Design
2.3. Fine Root Production (FRP)
2.4. Forest Structural Parameters
2.5. Soil CO2 Efflux, Microclimatic Condition, and Soil Characteristics
2.6. Carbon/Nitrogen Concentration
2.7. Statistical Analysis
3. Results
3.1. Fine Root Production and Its Drivers
3.2. Spatial Variability of Soil Respiration
4. Discussion
4.1. Fine Root Production and Its Contribution to NPP
4.2. Spatial Variability of Soil Respiration and FRP
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bonan, G.B. Forests and Climate Change: Forcings, Feedbacks, and the Climate Benefits of Forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bombi, P.; D’Andrea, E.; Rezaie, N.; Cammarano, M.; Matteucci, G. Which climate change path are we following? Bad news from Scots pine. PLoS ONE 2017, 12, e0189468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Quéré, C.; Raupach, M.R.; Canadell, J.G.; Marland, G.; Bopp, L.; Ciais, P.; Conway, T.J.; Doney, S.C.; Feely, R.A.; Foster, P.; et al. Trends in the sources and sinks of carbon dioxide. Nat. Geosci. 2009, 2, 831–836. [Google Scholar] [CrossRef]
- Collalti, A.; Prentice, I.C. Is NPP proportional to GPP? Waring’s hypothesis 20 years on. Tree Physiol. 2019, 39, 1473–1483. [Google Scholar] [CrossRef]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef]
- Santantonio, D.; Grace, J.C. Estimating fine-root production and turnover from biomass and decomposition data: A compartment–flow model. Can. J. For. Res. 1987, 17, 900–908. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.S.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Hanson, P.J.; Edwards, N.T.; Garten, C.T.; Andrews, J.A. Separating root and soil microbial contributions to soil respiration: A review of methods and observations. Biogeochemistry 2000, 48, 115–146. [Google Scholar] [CrossRef]
- Gaudinski, J.; Trumbore, S.; Davidson, E.; Cook, A.; Markewitz, D.; Richter, D. The age of fine-root carbon in three forests of the eastern United States measured by radiocarbon. Oecologia 2001, 129, 420–429. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Baldocchi, D.D. Spatial-temporal variation in soil respiration in an oak-grass savanna ecosystem in California and its partitioning into autotrophic and heterotrophic components. Biogeochemistry 2005, 73, 183–207. [Google Scholar] [CrossRef]
- Davidson, E.A.; Richardson, A.D.; Savage, K.E.; Hollinger, D.Y. A distinct seasonal pattern of the ratio of soil respiration to total ecosystem respiration in a spruce-dominated forest. Glob. Chang. Biol. 2006, 12, 230–239. [Google Scholar] [CrossRef]
- Hopkins, F.; Gonzalez-Meler, M.A.; Flower, C.E.; Lynch, D.J.; Czimczik, C.; Tang, J.; Subke, J.A. Ecosystem-level controls on root-rhizosphere respiration. New Phytol. 2013, 199, 339–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mccormack, M.L.; Gaines, K.P.; Pastore, M.; Eissenstat, D.M. Early season root production in relation to leaf production among six diverse temperate tree species. Plant Soil 2015, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Hertel, D.; Strecker, T.; Muller-Haubold, H.; Leuschner, C. Fine root biomass and dynamics in beech forests across a precipitation gradient—Is optimal resource partitioning theory applicable to water-limited mature trees? J. Ecol. 2013, 101, 1183–1200. [Google Scholar] [CrossRef]
- Maeght, J.-L.; Gonkhamdee, S.; Clément, C.; Isarangkool Na Ayutthaya, S.; Stokes, A.; Pierret, A. Seasonal Patterns of Fine Root Production and Turnover in a Mature Rubber Tree (Hevea brasiliensis Müll. Arg.) Stand- Differentiation with Soil Depth and Implications for Soil Carbon Stocks. Front. Plant Sci. 2015, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Saiz, G.; Green, C.; Butterbach-Bahl, K.; Kiese, R.; Avitabile, V.; Farrell, E.P. Seasonal and spatial variability of soil respiration in four Sitka spruce stands. Plant Soil 2006, 287, 161–176. [Google Scholar] [CrossRef]
- Shibistova, O.; Lloyd, J.; Evgrafova, S.; Savushkina, N.; Zrazhevskaya, G.; Arneth, A.; Knohl, A.; Kolle, O.; Schulze, E.D. Seasonal and spatial variability in soil CO2 efflux rates for a central Siberian Pinus sylvestris forest. Tellus 2002, 54, 552–567. [Google Scholar] [CrossRef]
- Sevanto, S.; Dickman, L.T. Where does the carbon go?-Plant carbon allocation under climate change. Tree Physiol. 2015, 35, 581–584. [Google Scholar] [CrossRef] [Green Version]
- Shugart, H.H.; Saatchi, S.; Hall, F.G. Importance of structure and its measurement in quantifying function of forest ecosystems. J. Geophys. Res. Biogeosciences 2010, 115, 1–16. [Google Scholar] [CrossRef]
- D’Andrea, E.; Micali, M.; Sicuriello, F.; Cammarano, M.; Ferlan, M.; Skudnik, M.; Mali, B.; Čater, M.; Simončič, P.; De Cinti, B.; et al. Improving carbon sequestration and stocking as a function of forestry. Ital. J. Agron. 2016, 11, 56–60. [Google Scholar]
- Collalti, A.; Trotta, C.; Keenan, T.F.; Ibrom, A.; Bond-lamberty, B.; Grote, R.; Vicca, S.; Reyer, C.P.O. Thinning Can Reduce Losses in Carbon Use Efficiency and Carbon Stocks in Managed Forests Under Warmer Climate. J. Adv. Model. Earth Syst. 2018, 2427–2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bombi, P.; Gnetti, V.; D’Andrea, E.; De Cinti, B.; Vigna Taglianti, A.; Bologna, M.A.; Matteucci, G. Identifying priority sites for insect conservation in forest ecosystems at high resolution: The potential of LiDAR data. J. Insect Conserv. 2019, 23, 689–698. [Google Scholar] [CrossRef]
- Rezaie, N.; D’Andrea, E.; Bräuning, A.; Matteucci, G.; Bombi, P.; Lauteri, M. Do atmospheric CO2 concentration increase, climate and forest management affect iWUE of common beech? Evidences from carbon isotope analyses in tree rings. Tree Physiol. 2018, 1975, 1110–1126. [Google Scholar] [CrossRef] [Green Version]
- Søe, A.R.B.; Buchmann, N. Spatial and temporal variations in soil respiration in relation to stand structure and soil parameters in an unmanaged beech forest. Tree Physiol. 2005, 25, 1427–1436. [Google Scholar] [CrossRef]
- Collalti, A.; Tjoelker, M.G.; Hoch, G.; Mäkelä, A.; Guidolotti, G.; Heskel, M.; Petit, G.; Ryan, M.G.; Battipaglia, G.; Matteucci, G.; et al. Plant respiration: Controlled by photosynthesis or biomass? Glob. Chang. Biol. 2019. [Google Scholar] [CrossRef]
- Collalti, A.; Marconi, S.; Ibrom, A.; Trotta, C.; Anav, A.; D’Andrea, E.; Matteucci, G.; Montagnani, L.; Gielen, B.; Mammarella, I.; et al. Validation of 3D-CMCC Forest Ecosystem Model (v. 5. 1) against eddy covariance data for 10 European forest sites. Geosci. Model Dev. 2016, 9, 479–504. [Google Scholar] [CrossRef] [Green Version]
- Chiti, T.; Papale, D.; Smith, P.; Dalmonech, D.; Matteucci, G.; Yeluripati, J.; Rodeghiero, M.; Valentini, R. Predicting changes in soil organic carbon in mediterranean and alpine forests during the Kyoto Protocol commitment periods using the CENTURY model. Soil Use Manag. 2010, 26, 475–484. [Google Scholar] [CrossRef]
- Mainiero, R.; Kazda, M. Depth-related fine root dynamics of Fagus sylvatica during exceptional drought. For. Ecol. Manag. 2006, 237, 135–142. [Google Scholar] [CrossRef]
- Ostonen, I.; Lohmus, K.; Pajuste, K. Fine root biomass, production and its proportion of NPP in a fertile middle-aged Norway spruce forest: Comparison of soil core and ingrowth core methods. For. Ecol. Manag. 2005, 212, 264–277. [Google Scholar] [CrossRef]
- Scarascia-Mugnozza, G.; Bauer, G.A.; Persson, H.; Matteucci, G.; Masci, A. Tree Biomass, Growth and Nutrient Pools. In Carbon and Nitrogen Cycling in European Forest Ecosystems; Schulze, E.-D., Ed.; Springer: Berlin, Germany, 2000; pp. 49–62. [Google Scholar]
- Huang, R.; Zhu, H.; Liu, X.; Liang, E.; Grießinger, J.; Wu, G.; Li, X.; Bräuning, A. Does increasing intrinsic water use efficiency (iWUE) stimulate tree growth at natural alpine timberline on the southeastern Tibetan Plateau? Glob. Planet. Chang. 2017, 148, 217–226. [Google Scholar] [CrossRef]
- Alberti, G.; Vicca, S.; Inglima, I.; Belelli-Marchesini, L.; Genesio, L.; Miglietta, F.; Marjanovic, H.; Martinez, C.; Matteucci, G.; D’Andrea, E.; et al. Soil C:N stoichiometry controls carbon sink partitioning between above-ground tree biomass and soil organic matter in high fertility forests. iForest-Biogeosciences For. 2014, 8. [Google Scholar] [CrossRef] [Green Version]
- Hendriks, C.; Bianchi, F. Root density and root biomass in pure and mixed forest stands of Douglas-fir and Beech. Neth. J. Agric. Sci. 1995, 43, 321–331. [Google Scholar]
- Mao, Z.; Jourdan, C.; Bonis, M.; Pailler, F.; Rey, H.; Saint-andré, L.; Stokes, A. Modelling root demography in heterogeneous mountain forests and applications for slope stability analysis. Plant Soil 2013, 357–382. [Google Scholar] [CrossRef]
- Guo, D.L.; Mitchell, R.J.; Hendricks, J.J. Fine root branch orders respond differentially to carbon source-sink manipulations in a longleaf pine forest. Oecologia 2004, 140, 450–457. [Google Scholar] [CrossRef]
- Schuur, E.A.G.; Trumbore, S.E. Partitioning sources of soil respiration in boreal black spruce forest using radiocarbon. Glob. Chang. Biol. 2006, 12, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Vargas, R.; Trumbore, S.E.; Allen, M.F. Evidence of old carbon used to grow new fine roots in a tropical forest. New Phytol. 2009, 182, 710–718. [Google Scholar] [CrossRef]
- Scartazza, A.; Moscatello, S.; Matteucci, G.; Battistelli, A.; Brugnoli, E. Seasonal and inter-annual dynamics of growth, non-structural carbohydrates and C stable isotopes in a Mediterranean beech forest. Tree Physiol. 2013, 33, 730–742. [Google Scholar] [CrossRef] [Green Version]
- D’Andrea, E.; Rezaie, N.; Battistelli, A.; Kuhlmann, I.; Matteucci, G.; Moscatello, S.; Proietti, S.; Scartazza, A.; Trumbore, S.; Muhr, J. Winter’s bite: Beech trees survive complete defoliation due to spring late-frost damage by mobilizing old C reserves. New Phytol. 2019, 224, 625–631. [Google Scholar] [CrossRef]
- Reich, P.B. Key canopy traits drive forest productivity. Proc Biol. Sci. 2012, 279, 2128–2134. [Google Scholar] [CrossRef] [Green Version]
- Clark, D.B.; Paulo, C.; Oberbauer, S.F.; Ryan, G. First direct landscape-scale measurement of tropical rain forest Leaf Area Index, a key driver of global primary productivity. Ecol Lett. 2008, 11, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, Q.; Cihlar, J.; Bauhus, J.; Price, D.T. Estimating fine-root biomass and production of boreal and cool temperate forests using aboveground measurements: A new approach. Plant Soil 2004, 265, 31–46. [Google Scholar] [CrossRef]
- Finér, L.; Ohashi, M.; Noguchi, K.; Hirano, Y. Factors causing variation in fine root biomass in forest ecosystems. For. Ecol. Manag. 2011, 261, 265–277. [Google Scholar] [CrossRef]
- Helmisaari, H.; Derome, J.; Nöjd, P.; Kukkola, M. Fine root biomass in relation to site and stand characteristics in Norway spruce and Scots pine stands. Tree Physiol. 2007, 27, 1493–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichstein, M.; Rey, A.; Freibauer, A.; Tenhunen, J.; Valentini, R.; Banza, J.; Casals, P.; Cheng, Y.; Gru, J.M.; Irvine, J.; et al. Modeling temporal and large-scale spatial variability of soil respiration from soil water availability, temperature and vegetation productivity indices. Glob. Biogeochem. Cycles 2003, 17, 320. [Google Scholar] [CrossRef]
- Ngao, J.; Epron, D.; Delpierre, N.; Bréda, N.; Granier, A.; Longdoz, B. Spatial variability of soil CO2 efflux linked to soil parameters and ecosystem characteristics in a temperate beech forest. Agric. For. Meteorol. 2012, 154–155, 136–146. [Google Scholar] [CrossRef]
- Addo-Danso, S.D.; Prescott, C.E.; Smith, A.R. Methods for estimating root biomass and production in forest and woodland ecosystem carbon studies: A review. For. Ecol. Manag. 2016, 359, 332–351. [Google Scholar] [CrossRef]
- Gill, R.A.; Jackson, R.B. Global patterns of root turnover for terrestrial ecosystems. New Phytol. 2000, 147, 13–31. [Google Scholar] [CrossRef]
- Norby, R.J.; Jackson, R.B. Root dynamics and global change: Seeking an ecosystem perspective. New Phytol. 2000, 147, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Meier, I.C.; Leuschner, C. Belowground drought response of European beech: Fine root biomass and carbon partitioning in 14 mature stands across a precipitation gradient. Glob. Chang. Biol. 2008, 14, 2081–2095. [Google Scholar] [CrossRef]
- Bloom, A.J.; Chapin, F.S.; Mooney, H.A. Resource Limitation in Plants-An Economic Analogy. Annu. Rev. Ecol. Syst. 1985, 16, 363–392. [Google Scholar] [CrossRef]
- Merganičová, K.; Merganič, J.; Lehtonen, A.; Vacchiano, G.; Sever, M.Z.O.; Augustynczik, A.L.D.; Grote, R.; Kyselová, I.; Mäkelä, A.; Yousefpour, R.; et al. Forest carbon allocation modelling under climate change. Tree Physiol. 2019, 39, 1937–1960. [Google Scholar] [CrossRef] [PubMed]
- Katayama, A.; Kume, T.; Komatsu, H.; Ohashi, M.; Nakagawa, M. Effect of forest structure on the spatial variation in soil respiration in a Bornean tropical rainforest. Agric. For. Meteorol. 2009, 149, 1666–1673. [Google Scholar] [CrossRef]
- Kosugi, Y.; Mitani, T.; Itoh, M.; Noguchi, S.; Tani, M.; Matsuo, N.; Takanashi, S.; Ohkubo, S.; Rahim Nik, A. Spatial and temporal variation in soil respiration in a Southeast Asian tropical rainforest. Agric. For. Meteorol. 2007, 147, 35–47. [Google Scholar] [CrossRef]
- Rodeghiero, M.; Cescatti, A. Spatial variability and optimal sampling strategy of soil respiration. For. Ecol. Manage. 2008, 255, 106–112. [Google Scholar] [CrossRef]
- Sotta, E.D.; Meir, P.; Malhi, Y.; Nobre, A.D.; Hodnett, M.; Grace, J. Soil CO2 efflux in a tropical forest in the Central Amazon. Glob. Chang. Biol. 2004, 10, 601–617. [Google Scholar] [CrossRef]
- Matteucci, G.; Dore, S.; Stivanello, S.; Rebmann, C.; Buchmann, N. Soil Respiration in Beech and Spruce Forests in Europe: Trends, Controlling Factors, Annual Budgets and Implications for the Ecosystem Carbon Balance. Ecol. Stud. 2000, 142, 217–236. [Google Scholar]
- Guidolotti, G.; Rey, A.; D’Andrea, E.; Matteucci, G.; De Angelis, P. Effect of environmental variables and stand structure on ecosystem respiration components in a Mediterranean beech forest. Tree Physiol. 2013, 33, 960–972. [Google Scholar] [CrossRef] [Green Version]



| Max | Min | Mean | CV | |
|---|---|---|---|---|
| Forest Structure Parameter | ||||
| N tree plot−1 | 24 | 5 | 14 | 0.42 |
| Basal area (m2) | 0.37 | 0.21 | 0.30 | 0.20 |
| Dmax (cm) | 46.90 | 20.90 | 31.99 | 0.25 |
| LAI (m2 m−2) | 7.13 | 5.33 | 6.10 | 0.10 |
| Soil Parameters | ||||
| T soil (°C) | 9.39 | 8.59 | 8.92 | 0.03 |
| SWC (%) | 34.56 | 14.73 | 23.61 | 0.23 |
| SMN (%) | 1.32 | 0.44 | 0.82 | 0.35 |
| SMC (%) | 17.16 | 7.35 | 10.58 | 0.33 |
| SOC (%) | 28.04 | 16.14 | 21.18 | 0.18 |
| SON (%) | 1.91 | 1.14 | 1.46 | 0.17 |
| Litter Amount (g m−2) | 265.48 | 191.22 | 232.14 | 0.10 |
| Litter N (%) | 2.02 | 1.75 | 1.86 | 0.05 |
| Litter C (%) | 41.49 | 36.14 | 38.92 | 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Andrea, E.; Guidolotti, G.; Scartazza, A.; De Angelis, P.; Matteucci, G. Small-Scale Forest Structure Influences Spatial Variability of Belowground Carbon Fluxes in a Mature Mediterranean Beech Forest. Forests 2020, 11, 255. https://doi.org/10.3390/f11030255
D’Andrea E, Guidolotti G, Scartazza A, De Angelis P, Matteucci G. Small-Scale Forest Structure Influences Spatial Variability of Belowground Carbon Fluxes in a Mature Mediterranean Beech Forest. Forests. 2020; 11(3):255. https://doi.org/10.3390/f11030255
Chicago/Turabian StyleD’Andrea, Ettore, Gabriele Guidolotti, Andrea Scartazza, Paolo De Angelis, and Giorgio Matteucci. 2020. "Small-Scale Forest Structure Influences Spatial Variability of Belowground Carbon Fluxes in a Mature Mediterranean Beech Forest" Forests 11, no. 3: 255. https://doi.org/10.3390/f11030255
APA StyleD’Andrea, E., Guidolotti, G., Scartazza, A., De Angelis, P., & Matteucci, G. (2020). Small-Scale Forest Structure Influences Spatial Variability of Belowground Carbon Fluxes in a Mature Mediterranean Beech Forest. Forests, 11(3), 255. https://doi.org/10.3390/f11030255






