Metrics of Growth Habit Derived from the 3D Tree Point Cloud Used for Species Determination—A New Approach in Botanical Taxonomy Tested on Dragon Tree Group Example
Abstract
:1. Introduction
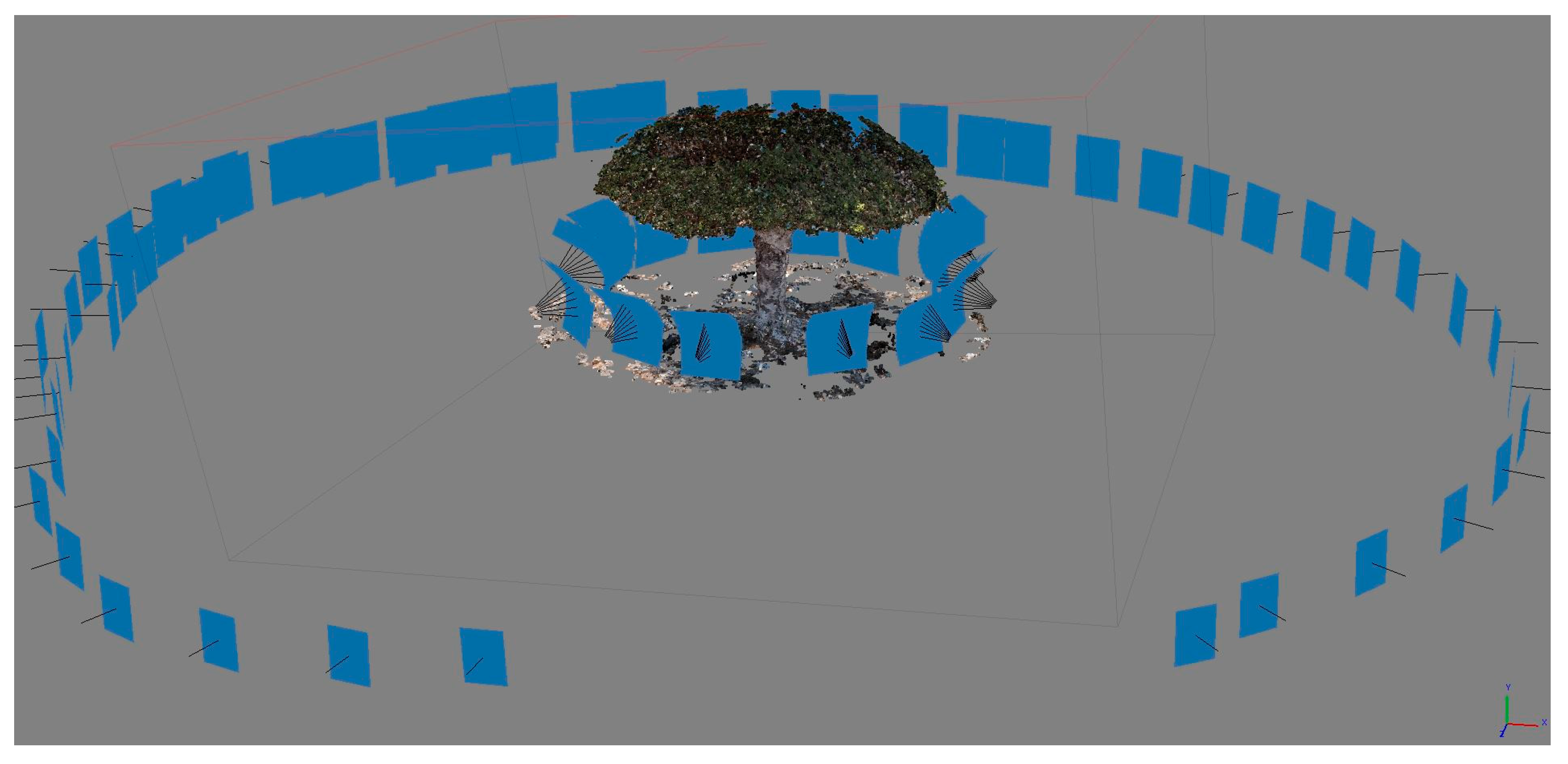
2. Material and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mabberley, D.J. The Plant-Book: A Portable Dictionary of the Higher Plants; Cambridge Press: Cambridge, UK, 1990. [Google Scholar]
- Brown, N.E. Notes on the genera Cordyline, Dracaena, Pleomele, Sansevieria, and Taetsia. Bull. Misc. Inf. (R. Bot. Gard. Kew) 1914, 8, 273–279. [Google Scholar] [CrossRef]
- Zona, S.; Álvarez De Zayas, A.; Orellana, R.; Oviedo, R.; Jestrow, B.; Francisco-Ortega, J. Dracaena L. (Asparagaceae) in the New World: Its history and Botany. Vieraea 2014, 42, 219–240. [Google Scholar]
- Marrero, A.; Almeida, S.R.; Martín-González, M. A new species of the wild Dragon Tree, Dracaena (Dracaenaceae) from Gran Canaria and its taxonomic and biogeographic implications. Bot. J. Linn. Soc. 1998, 128, 291–314. [Google Scholar]
- Benabid, A.; Cuzin, F. Populations de dragonnier (Dracaena draco L. subsp. Aigal benabid et Cuzin) au Maroc: Valeurs taxinomique, biogéographique et phytosociologique. C. R. Acad. Sci. Sci. Vie 1997, 320, 267–277. [Google Scholar] [CrossRef]
- Marrero, A.; Almeida, S.R. A new subspecies, Dracaena draco (L.) L. subsp. Caboverdeana Marrero Rodr. & R. Almeida (Dracaenaceae) from Cape Verde Island. Int. J. Geobot. Res. 2012, 2, 35–40. [Google Scholar] [CrossRef]
- Balfour, B. The dragon’s blood tree of Socotra (Dracaena cinnabari Balf. fil.). Trans. R. Soc. Edinb. 1883, 30, 619–623. [Google Scholar] [CrossRef] [Green Version]
- Maděra, P.; Volařík, D.; Patočka, Z.; Kalivodová, H.; Divín, J.; Rejžek, M.; Vybíral, J.; Lvončík, S.; Jeník, D.; Hanáček, P.; et al. Sustainable land use management needed to conserve the dragon’s blood tree of Socotra Island, a vulnerable endemic umbrella species. Sustainability 2019, 11, 3557. [Google Scholar] [CrossRef] [Green Version]
- Beyhl, F.E. Der Drachenbaum und seine Verwandtschaft: II. Der echte Drachenbaum, Dracaena cinnabari, von der Insel Sokotra. Palmengarten 1995, 59, 140–145. [Google Scholar]
- Baker, J.G. Liliaceae: Dracaena. In Flora of Tropical Africa, Hydrocharideae to Liliaceae; Thiselton-Dyer, W.T., Ed.; L. Reeve & Co.: Ashford, UK, 1898; Volume 2, pp. 436–450. [Google Scholar]
- Täckholm, V.; Drar, M. Flora of Egypt. Volume III: Angiospermae, Part Monocotyledones: Liliaceae-Musaceae, 2nd ed.; Otto Koeltz Antiquariat: Koenigstein, Germany, 1973. [Google Scholar]
- Friis, I. Forests and Forest Trees of Northeast Tropical Africa; New Bulletin Addition Series XV; Her Majesty’s Stationery Office (HMSO): Richmond, UK; Royal Botanic Gardens, Kew: Richmond, UK, 1992. [Google Scholar]
- Baker, J.G. Dracaena schizantha. In The Journal of Botany, New Series; Trimen, H., Ed.; Ranken & Co: London, UK, 1877; Volume 6, p. 71. [Google Scholar]
- Thulin, M. Dracaenaceae. In Flora of Somalia; Thulin, M., Ed.; Royal Botanic Gardens, Kew: Richmond, UK, 1995; Volume 4, pp. 27–30. [Google Scholar]
- Wilkin, P.; Suksathan, P.; Keeratikiat, K.; Van Welzen, P.; Wiland-Szymanska, J. A new threatened endemic species from central and northeastern Thailand, Dracaena jayniana (Asparagaceae: Tribe Nolinoideae). Kew Bull. 2012, 67, 697–705. [Google Scholar] [CrossRef]
- Zheng, D.J.; Xie, L.S.; Zhu, J.H.; Zhang, Z.L. Low genetic diversity and local adaptive divergence of Dracaena cambodiana (Liliaceae) populations associated with historical population bottlenecks and natural selection: An endangered long-lived tree endemic to Hainan Island, China. Plant Biol. 2012, 14, 828–838. [Google Scholar] [CrossRef]
- Chen, X.Q.; Turland, N.J. Dracaena vandelli ex Linnaeus. In Flora of China; Wu, Z.Y., Raven, P.H., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2000; Volume 24, pp. 215–217. [Google Scholar]
- Fan, J.Y.; Yi, T.; Sze-To, C.M.; Zhu, L.; Peng, W.L.; Zhang, Y.Z.; Zhao, Z.Z.; Chen, H.B. A Systematic review of the botanical, phytochemical and pharmacological profile of Dracaena cochinchinensis, a plant source of the ethnomedicine “Dragon’s Blood”. Molecules 2014, 19, 10650–10669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkin, P.; Suksathan, P.; Keeratikiat, K.; Van Welzen, P.; Wiland-Szymanska, J. A new species from Thailand and Burma, Dracaena kaweesakii Wilkin & Suksathan (Asparagaceae subfamily Nolinoideae). PhytoKeys 2013, 26, 101–112. [Google Scholar] [CrossRef]
- Lu, P.-L.; Morden, C.W. Phylogenetic relationships among Dracaenoid genera (Asparagaceae: Nolinoideae) inferred from Chloroplast DNA Loci. Syst. Bot. 2014, 39, 90–104. [Google Scholar] [CrossRef]
- Baker, J.G. Dracaena ombet Kotschy et Peir. In Hooker’s Icones Plantarum; Ser. 4, Part II; Thiselton-Dyer, W.T., Ed.; Dulay & Co.: London, UK, 1897; Volume 6, plate 2539. [Google Scholar]
- Donnell Smith, J. Dracaena americana. In Trees and Shrubs. Illustrations of New or Little Known Ligneous Plants; Sargent, C.S., Ed.; Houghton, Mifflin and Company: Boston, MA, USA; New York, NY, USA, 1905; Volume 1, p. 207. [Google Scholar]
- Baker, J.G. Revision of the genera and species of Asparagaceae. J. Lin. Soc. Bot. 1875, 14, 508–632. [Google Scholar] [CrossRef]
- Hyyppä, J.; Hyyppä, H.; Leckie, D.; Gougeon, F.; Yu, X.; Maltamo, M. Review of methods of small-footprint airborne laser scanning for extracting forest inventory data in boreal forests. Int. J. Remote Sens. 2008, 29, 1339–1366. [Google Scholar] [CrossRef]
- Puliti, S.; Hauglin, M.; Breidenbach, J.; Montesano, P.; Neigh, C.S.R.; Rahlf, J.; Solberg, S.; Klingenberg, T.F.; Astrup, R. Modelling above-ground biomass stock over Norway using national forest inventory data with ArcticDEM and Sentinel-2 data. Remote Sens. Environ. 2020, 236, 111501. [Google Scholar] [CrossRef]
- Mikita, T.; Janata, P.; Surový, P. Forest stand inventory based on combined aerial and terrestrial close-range photogrammetry. Forests 2016, 7, 165. [Google Scholar] [CrossRef] [Green Version]
- Wallace, L.; Lucieer, A.; Malenovský, Z.; Darren, T.; Vopěnka, P. Assessment of forest structure using two UAV techniques: A comparison of airborne laser scanning and structure from motion (SfM) point clouds. Forests 2016, 7, 62. [Google Scholar] [CrossRef] [Green Version]
- Dassot, M.; Constant, T.; Fournier, M. The use of terrestrial LiDAR technology in forest science: Application fields, benefits and challenges. Ann. For. Sci. 2011, 68, 959–974. [Google Scholar] [CrossRef] [Green Version]
- Lowe, D.G. Method and Apparatus for Identifying Scale Invariant Features in an Image and Use of Same for Locating an Object in an Image. U.S. Patent 6,711,293, 23 March 2004. [Google Scholar]
- Kangas, A.; Gobakken, T.; Puliti, S.; Hauglin, M.; Næsset, E. Value of airborne laser scanning and digital aerial photogrammetry data in forest decision making. Silva Fenn. 2018, 52, 19. [Google Scholar] [CrossRef] [Green Version]
- Hrůza, P.; Mikita, T.; Tyagur, N.; Krejza, Z.; Cibulka, M.; Procházková, A.; Patočka, Z. Detecting forest road wearing course damage using different methods of remote sensing. Remote Sens. 2018, 10, 492. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Guo, Q.; Su, Y.; Tao, S.; Zhao, K.; Xu, G. Retrieving the gap fraction, element clumping index, and leaf area index of individual trees using single-scan data from a terrestrial laser scanner. ISPRS J. Photogramm. Remote Sens. 2017, 130, 308–316. [Google Scholar] [CrossRef]
- Hosoi, F.; Omasa, K. Voxel-Based 3-D modeling of individual trees for estimating leaf area density using high-resolution portable scanning Lidar. IEEE Trans. Geosci. Remote Sens. 2006, 44, 3610–3618. [Google Scholar] [CrossRef]
- Hubálková, I.; Maděra, P.; Volařík, D. Growth dynamics of Dracaena cinnabari under controlled conditions as the most effective way to protect endangered species. Saudi J. Biol. Sci. 2017, 24, 1445–1452. [Google Scholar] [CrossRef] [Green Version]
- Adolt, R.; Habrová, H.; Maděra, P. Crown age estimation of a monocotyledonous tree species Dracaena cinnabari using logistic regression. Trees Struct. Funct. 2012, 26, 1287–1298. [Google Scholar] [CrossRef]
- Bauwens, S.; Fayolle, A.; Fleury, S.; Ndjele, L.; Mengal, C.; Lejeune, P. Terrestrial photogrammetry: A non-destructive method for modelling irregularly shaped tropical tree trunks. Methods Ecol. Evol. 2017. [Google Scholar] [CrossRef]
- Trochta, J.; Krůček, M.; Vrška, T.; Král, K. 3D Forest: An application for descriptions of three-dimensional forest structures using terrestrial LiDAR. PLoS ONE 2017, 12, e0176871. [Google Scholar] [CrossRef] [Green Version]
- Preparata, F.P.; Hong, S.J. Convex hulls of finite sets of points in two and three dimensions. Commun. ACM 1977, 20, 87–93. [Google Scholar] [CrossRef]
- Maděra, P.; Slach, T.; Úradníček, L.; Lacina, J.; Černušáková, L.; Friedl, M.; Řepka, R.; Buček, A. Tree shape and form in ancient coppice woodlands. J. Landsc. Ecol. 2017, 10, 49–62. [Google Scholar] [CrossRef] [Green Version]
- Du, S.; Lindenbergh, R.; Ledoux, H.; Stoter, J.; Nan, J. AdTree: Accurate, detailed, and automatic Modelling of laser-scanned trees. Remote Sens. 2019, 11, 2074. [Google Scholar] [CrossRef] [Green Version]
- Tu, Y.H.; Phinn, S.; Johansen, K.; Robson, A.; Wu, D. Optimising drone flight planning for measuring horticultural tree crop structure. ISPRS J. Photogramm. Remote Sens. 2020, 160, 83–96. [Google Scholar] [CrossRef] [Green Version]
- Manohar, Y.; Bharat, L. Identification of trees and their trunks from mobile laser scanning data of roadway scenes. Int. J. Remote Sens. 2020, 41, 1233–1258. [Google Scholar] [CrossRef]
- Qinan, L.; Huaguo, H.; Jingxu, W.; Kan, H.; Yangyang, L. Detection of pine shoot beetle (PSB) stress on pine forests at individual tree level using UAV-based hyperspectral imagery and lidar. Remote Sens. 2019, 11, 2540. [Google Scholar] [CrossRef] [Green Version]
- Holmgren, J.; Tulldahl, M.; Nordlöf, J.; Willén, E.; Olsson, H. Mobile laser scanning for estimating tree stem diameter using segmentation and tree spine calibration. Remote Sens. 2019, 11, 2781. [Google Scholar] [CrossRef] [Green Version]
- Thies, M.; Pfeifer, N.; Winterhalder, D.; Gorte, B.G.H. Three-Dimensional reconstruction of stems for assessment of taper, sweep and lean based on laser scanning of standing trees. Scand. J. For. Res. 2004, 19, 571–581. [Google Scholar] [CrossRef]
- Guerra-Hernández, J.; Cosenza, D.N.; Cardil, A.; Silva, C.A.; Botequim, B.; Soares, P.; Silva, M.; González-Ferreiro, E.; Díaz-Varela, R.A. Predicting Growing stock volume of eucalyptus plantations using 3-D point clouds derived from UAV imagery and ALS data. Forests 2019, 10, 905. [Google Scholar] [CrossRef] [Green Version]
- Yun, T.; Cao, L.; An, F.; Chen, B.; Xue, L.; Li, W.; Pincebourde, S.; Smith, M.J.; Eichhorn, M.P. Simulation of multi-platform LiDAR for assessing total leaf area in tree crowns. Agric. For. Meteorol. 2019, 276–277, 107610. [Google Scholar] [CrossRef]
- Meesuk, V.; Vojinovic, Z.; Mynettac, A.; Abdullah, A.F. Urban flood modelling combining top-view LiDAR data with ground-view SfM observations. Adv. Water Resour. 2015, 75, 105–117. [Google Scholar] [CrossRef]
- Gergeľová, M.; Kuzevičová, Ž.; Labant, S.; Gašinec, J.; Kuzevič, Š.; Unucka, J.; Liptai, P. Evaluation of selected sub-elements of spatial data quality on 3D flood event modeling: Case study of Prešov City, Slovakia. Appl. Sci. 2020, 10, 820. [Google Scholar] [CrossRef] [Green Version]
- Mokroš, M.; Liang, X.; Surový, P.; Valent, P.; Čerňava, J.; Chudý, F.; Tunák, D.; Saloň, Š.; Merganič, J. Evaluation of close-range photogrammetry image collection methods for estimating tree diameters. Int. J. Geo Inf. 2018, 7, 93. [Google Scholar]
- Piermattei, L.; Karel, W.; Wang, D.; Wieser, M.; Mokroš, M.; Surový, P.; Koreň, M.; Tomaštík, J.; Pfeifer, N.; Hollaus, M. Terrestrial structure from motion photogrammetry for deriving forest inventory data. Remote Sens. 2019, 11, 950. [Google Scholar] [CrossRef] [Green Version]
- Mokroš, M.; Výbošťok, J.; Tomaštík, J.; Grznárová, A.; Valent, P.; Slavík, M.; Merganič, J. High precision individual tree diameter and perimeter estimation from close-range photogrammetry. Forests 2018, 9, 696. [Google Scholar] [CrossRef] [Green Version]
- Surový, P.; Yoshimoto, A.; Panagiotidis, D. Accuracy of reconstruction of the tree stem surface using terrestrial close-range photogrammetry. Remote Sens. 2016, 8, 123. [Google Scholar] [CrossRef] [Green Version]
- Dandois, J.P.; Ellis, E.C. Remote sensing of vegetation structure using computer vision. Remote Sens. 2010, 2, 1157–1176. [Google Scholar] [CrossRef] [Green Version]
- Mlambo, R.; Woodhouse, I.H.; Gerard, F.; Anderson, K. Structure from motion (SfM) photogrammetry with drone data: A low cost method for monitoring greenhouse gas emissions from forests in developing countries. Forests 2017, 8, 68. [Google Scholar] [CrossRef] [Green Version]










| Species | Number of Recorded Trees | Correctly Created Point Clouds |
|---|---|---|
| D. serrulata | 25 | 18 |
| D. cinnabari | 30 | 17 |
| D. draco | 16 | 11 |
| D. ombet | 10 | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vahalík, P.; Drápela, K.; Procházková, A.; Patočka, Z.; Balková, M.; Šenfeldr, M.; Lengálová, K.; Kalivodová, H.; Vaníčková, L.; Ehrenbergerová, L.; et al. Metrics of Growth Habit Derived from the 3D Tree Point Cloud Used for Species Determination—A New Approach in Botanical Taxonomy Tested on Dragon Tree Group Example. Forests 2020, 11, 272. https://doi.org/10.3390/f11030272
Vahalík P, Drápela K, Procházková A, Patočka Z, Balková M, Šenfeldr M, Lengálová K, Kalivodová H, Vaníčková L, Ehrenbergerová L, et al. Metrics of Growth Habit Derived from the 3D Tree Point Cloud Used for Species Determination—A New Approach in Botanical Taxonomy Tested on Dragon Tree Group Example. Forests. 2020; 11(3):272. https://doi.org/10.3390/f11030272
Chicago/Turabian StyleVahalík, Petr, Karel Drápela, Andrea Procházková, Zdeněk Patočka, Marie Balková, Martin Šenfeldr, Klára Lengálová, Hana Kalivodová, Lucie Vaníčková, Lenka Ehrenbergerová, and et al. 2020. "Metrics of Growth Habit Derived from the 3D Tree Point Cloud Used for Species Determination—A New Approach in Botanical Taxonomy Tested on Dragon Tree Group Example" Forests 11, no. 3: 272. https://doi.org/10.3390/f11030272
APA StyleVahalík, P., Drápela, K., Procházková, A., Patočka, Z., Balková, M., Šenfeldr, M., Lengálová, K., Kalivodová, H., Vaníčková, L., Ehrenbergerová, L., Lvončík, S., & Maděra, P. (2020). Metrics of Growth Habit Derived from the 3D Tree Point Cloud Used for Species Determination—A New Approach in Botanical Taxonomy Tested on Dragon Tree Group Example. Forests, 11(3), 272. https://doi.org/10.3390/f11030272






