Long-Term Wood Micro-Density Variation in Alpine Forests at Central México and Their Spatial Links with Remotely Sensed Information
Abstract
:1. Introduction
2. Materials and Methods
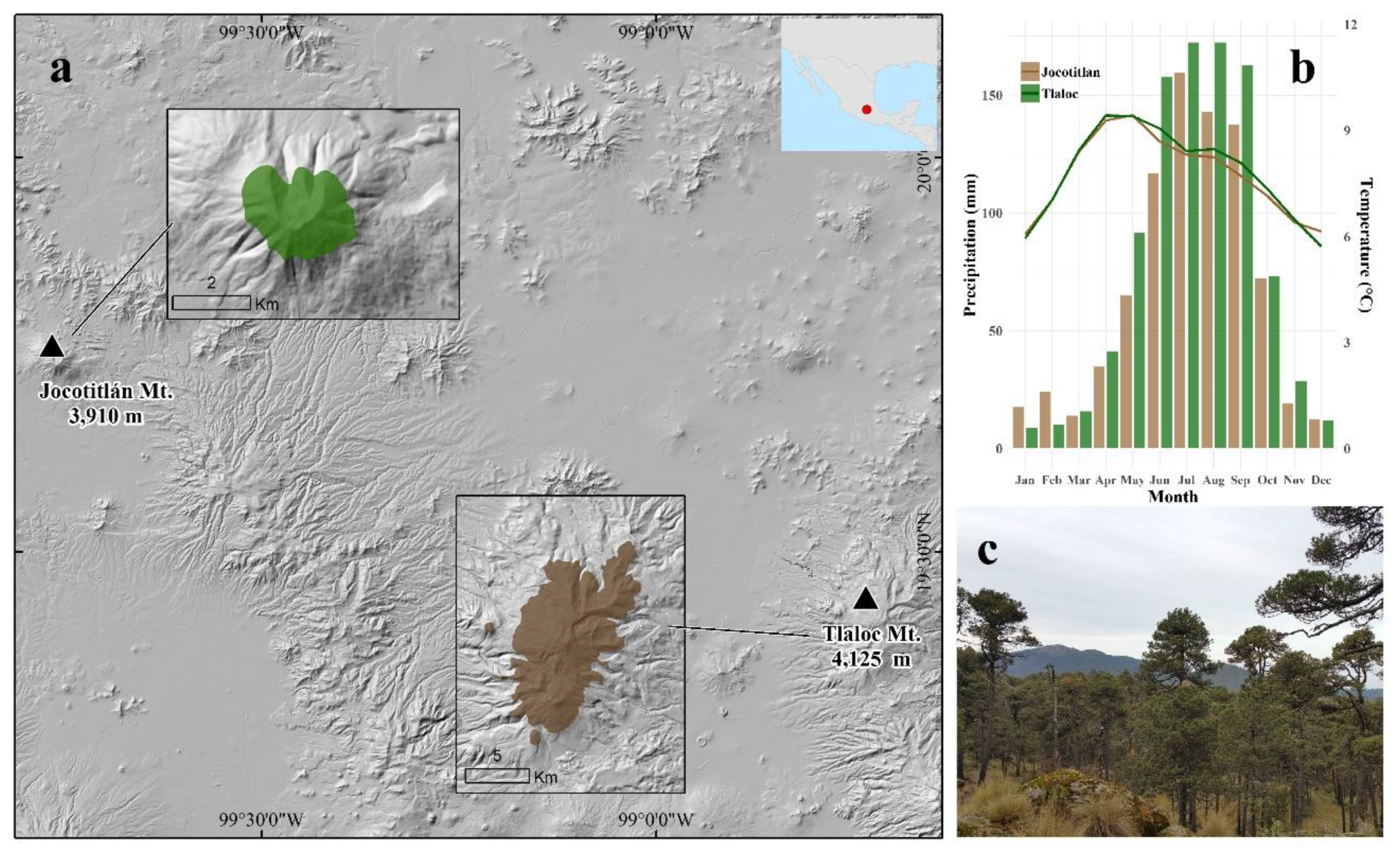
2.1. Study Sites
2.2. Sample Collection and Density Measurements
2.3. Mountain and Topographic Gradient Effect on Density Profiles
2.4. Unbiased Trends of Density Profiles and Temporal Stability
2.5. Climatic Influence on Tree-Ring Wood Density Variables
2.6. Linking Remotely Sensed Variables from MODIS to Wood Density
3. Results
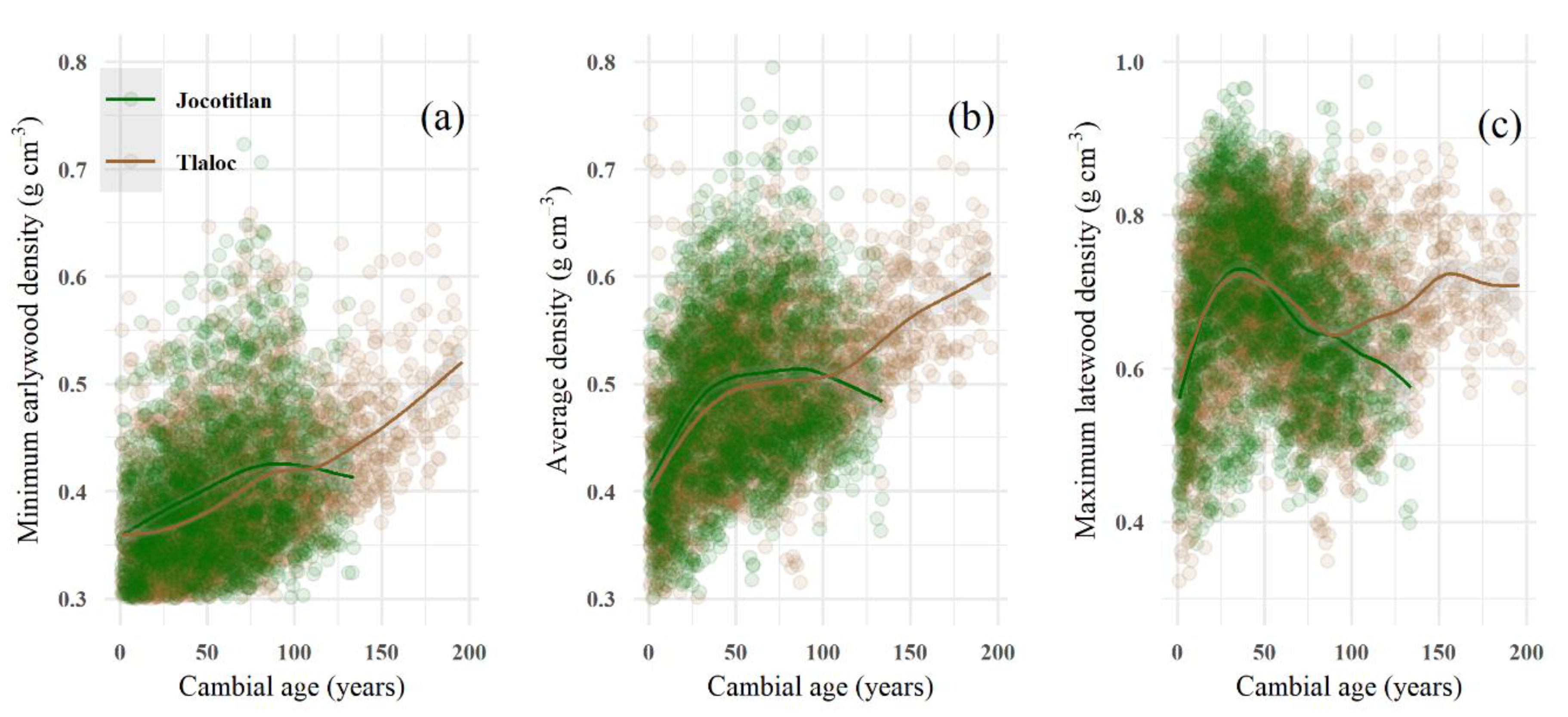
3.1. Tree-Ring Density Statistics and the Cambial Age Effect
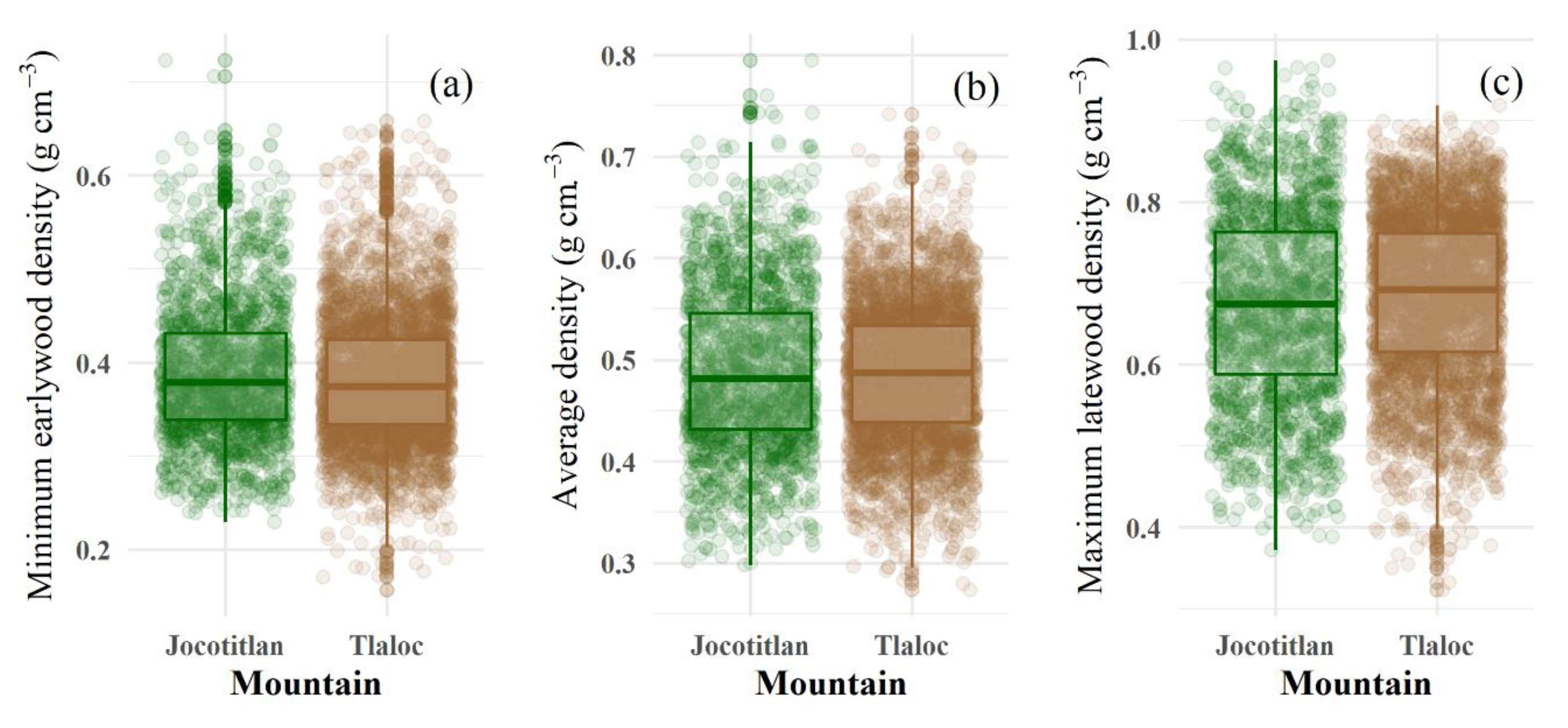
3.2. Elevation and Aspect Variations for Tree-Ring Wood Density
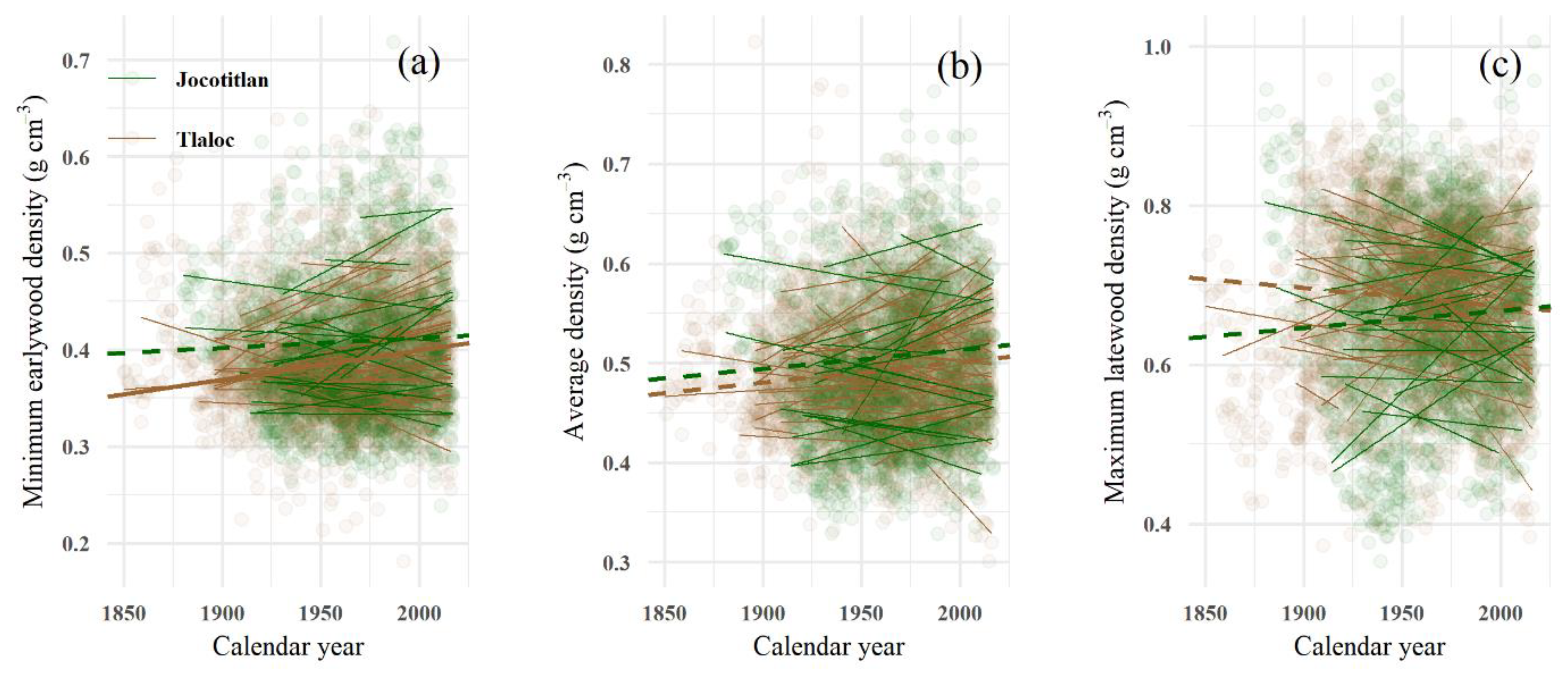
3.3. Trends and Stability in Adjusted Tree-Ring Wood Density Profiles
3.4. Responses of Wood Density to Climate
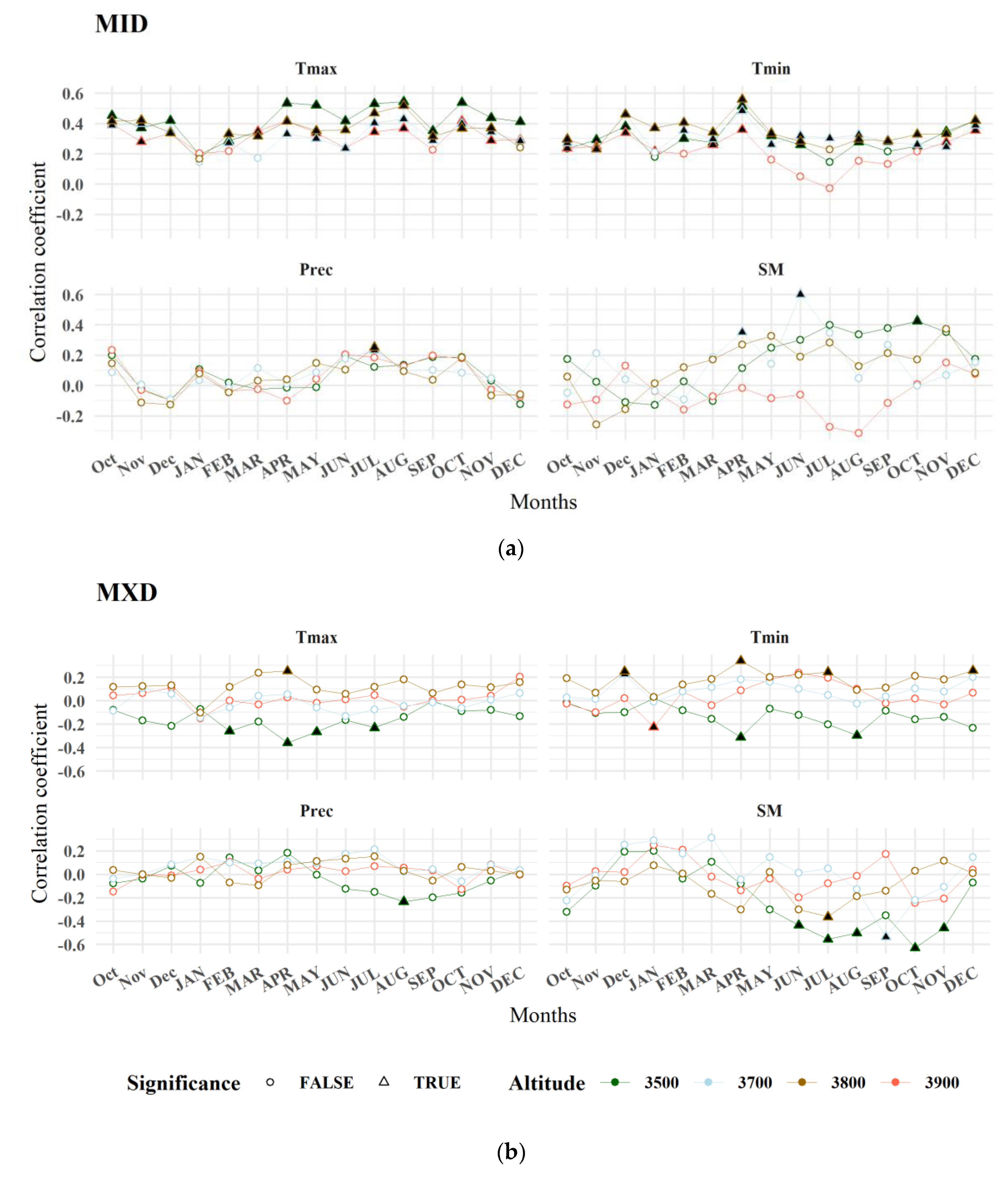
3.5. The Connection between Remotely Sensed Information and Wood Density Traits
4. Discussion
4.1. Hypothesis Testing Summary
4.2. Average Wood Density Profiles of Pinus hartwegii
4.3. Tree-Ring Wood Density Patterns across Mountains, Elevations, and Aspects
4.4. The Temporal Stability of Tree-Ring Wood Density at Two High-Elevation Forests
4.5. Climate—Wood Density Responses
4.6. Mountain Dependent Associations between Wood Density and MODIS Variables
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McDowell, N.G.; Allen, C.D. Darcy’s law predicts widespread forest mortality under climate warming. Nat. Clim. Chang. 2015, 5, 669. [Google Scholar] [CrossRef]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Granda, E.; Camarero, J.J.; Galván, J.D.; Sangüesa-Barreda, G.; Alla, A.Q.; Gutierrez, E.; Dorado-Liñán, I.; Andreu-Hayles, L.; Labuhn, I.; Grudd, H.; et al. Aged but withstanding: Maintenance of growth rates in old pines is not related to enhanced water-use efficiency. Agric. For. Meteorol. 2017, 243, 43–54. [Google Scholar] [CrossRef]
- Babst, F.; Bouriaud, O.; Papale, D.; Gielen, B.; Janssens, I.A.; Nikinmaa, E.; Ibrom, A.; Wu, J.; Bernhofer, C.; Köstner, B.; et al. Above-ground woody carbon sequestration measured from tree rings is coherent with net ecosystem productivity at five eddy-covariance sites. New Phytol. 2014, 201, 1289–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hacke, U.G.; Sperry, J.S.; Pockman, W.T.; Davis, S.D.; McCulloh, K.A. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 2001, 126, 457–461. [Google Scholar] [CrossRef]
- Martinez-Meier, A.; Fernández, M.E.; Dalla-Salda, G.; Gyenge, J.; Licata, J.; Rozenberg, P. Ecophysiological basis of wood formation in ponderosa pine: Linking water flux patterns with wood microdensity variables. For. Ecol. Manag. 2015, 346, 31–40. [Google Scholar] [CrossRef]
- Cerrato, R.; Salvatore, M.C.; Gunnarson, B.E.; Linderholm, H.W.; Carturan, L.; Brunetti, M.; De Blasi, F.; Baroni, C. A Pinus cembra L. tree-ring record for late spring to late summer temperature in the Rhaetian Alps, Italy. Dendrochronologia 2019, 53, 22–31. [Google Scholar] [CrossRef]
- Chen, F.; Yuan, Y.; Wei, W.; Yu, S.; Fan, Z.; Zhang, R.; Zhang, T.; Li, Q.; Shang, H. Temperature reconstruction from tree-ring maximum latewood density of Qinghai spruce in middle Hexi Corridor, China. Theor. Appl. Climatol. 2012, 107, 633–643. [Google Scholar] [CrossRef]
- Briffa, K.R.; Osborn, T.J.; Schweingruber, F.H. Large-scale temperature inferences from tree rings: A review. Glob. Planet. Chang. 2004, 40, 11–26. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gutiérrez, E. Wood density of silver fir reflects drought and cold stress across climatic and biogeographic gradients. Dendrochronologia 2017, 45, 101–112. [Google Scholar] [CrossRef]
- Camarero, J.J.; Rozas, V.; Olano, J.M.; Fernández-Palacios, J.M. Minimum wood density of Juniperus thurifera is a robust proxy of spring water availability in a continental Mediterranean climate. J. Biogeogr. 2014, 41, 1105–1114. [Google Scholar] [CrossRef]
- Wang, L.; Payette, S.; Bégin, Y. Relationships between anatomical and densitometric characteristics of black spruce and summer temperature at tree line in northern Quebec. Can. J. For. Res. 2002, 32, 477–486. [Google Scholar] [CrossRef]
- Wu, P.; Wang, L.; Shao, X. Reconstruction of summer temperature variation from maximum density of alpine pine during 1917-2002 for west Sichuan Plateau, China. J. Geogr. Sci. 2008, 18, 201–210. [Google Scholar] [CrossRef]
- Blunden, J.; Arndt, D.S. State of the Climate in 2017. Bull. Am. Meteorol. Soc. 2018, 99, 332. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Summary for Policymakers; Cambridge University Press: London, UK, 2013; p. 231. [Google Scholar]
- Körner, C. Alpine Treelines; Springer: Basel, Switzerland, 2012; p. 220. [Google Scholar] [CrossRef]
- Holtmeier, F.-K. Mountain Timberlines: Ecology, Patchiness, and Dynamics; Springer Science & Business Media: Berlin, Germany, 2009; Volume 36, p. 421. [Google Scholar]
- Körner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef]
- Pepin, N.; Bradley, R.S.; Diaz, H.F.; Baraer, M.; Caceres, E.B.; Forsythe, N.; Fowler, H.; Greenwood, G.; Hashmi, M.Z.; Liu, X.D.; et al. Elevation-dependent warming in mountain regions of the world. Nat. Clim. Chang. 2015, 5, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Mayr, S.; Schwienbacher, F.; Bauer, H. Winter at the alpine timberline. Why does embolism occur in Norway spruce but not in stone pine? Plant Physiol. 2003, 131, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Andreu-Hayles, L.; D’Arrigo, R.; Anchukaitis, K.J.; Beck, P.S.A.; Frank, D.; Goetz, S. Varying boreal forest response to Arctic environmental change at the Firth River, Alaska. Environ. Res. Lett. 2011, 6. [Google Scholar] [CrossRef]
- Briffa, K.R.; Schweingruber, F.H.; Jones, P.D.; Osborn, T.J.; Shiyatov, S.G.; Vaganov, E.A. Reduced sensitivity of recent tree-growth to temperature at high northern latitudes. Nature 1998, 391, 678–682. [Google Scholar] [CrossRef]
- Franceschini, T.; Bontemps, J.-D.; Leban, J.-M. Transient historical decrease in earlywood and latewood density and unstable sensitivity to summer temperature for Norway spruce in northeastern France. Can. J. For. Res. 2012, 42, 219–226. [Google Scholar] [CrossRef]
- González-Cásares, M.; Yerena-Yamallel, J.I.; Pompa-García, M. Measuring temporal wood density variation improves carbon capture estimates in Mexican forests. Acta Univ. 2016, 26, 11–14. [Google Scholar] [CrossRef]
- Morgado-González, G.; Gómez-Guerrero, A.; Villanueva-Díaz, J.; Terrazas, T.; Ramírez-Herrera, C.; de la Rosa, P.H. Wood density of Pinus hartwegii Lind. At two altitude and exposition levels. Agrociencia 2019, 53, 645–660. [Google Scholar]
- Pompa-García, M.; Venegas-González, A. Temporal Variation of Wood Density and Carbon in Two Elevational Sites of Pinus cooperi in Relation to Climate Response in Northern Mexico. PLoS ONE 2016, 11, e0156782. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Díaz, J.; Cerano Paredes, J.; Vázquez Selem, L.; Stahle, D.W.; Fulé, P.Z.; Yocom, L.L.; Franco Ramos, O.; Ariel Ruiz Corral, J. Red dendrocronológica del pino de altura (Pinus hartwegii Lindl.) para estudios dendroclimáticos en el noreste y centro de México. Investig. Geográficasboletín Del Inst. De Geogr. 2015, 2015, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Yocom, L.L.; Fulé, P.Z.; Brando, P. Human and climate influences on frequent fire in a high-elevation tropical forest. J. Appl. Ecol. 2012, 49, 1356–1364. [Google Scholar] [CrossRef]
- Astudillo-Sánchez, C.C.; Villanueva-Díaz, J.; Endara-Agramont, A.R.; Nava-Bernal, G.E.; Gómez-Albores, M.A. Climatic variability at the treeline of Monte Tlaloc, Mexico: A dendrochronological approach. Trees 2017, 31, 441–453. [Google Scholar] [CrossRef]
- Cerano-Paredes, J.; Villanueva-Diaz, J.; Vazquez-Selem, L.; Cervantes-Martinez, R.; Esquivel-Arriaga, G.; Guerra-de la Cruz, V.; Fule, P.Z. Historical fire regime and its relationship with climate in a forest of Pinus hartwegii to the north of Puebla State, Mexico. Bosque 2016, 37, 389–399. [Google Scholar] [CrossRef] [Green Version]
- Ricker, M.; Gutierrez-Garcia, G.; Daly, D.C. Modeling long-term tree growth curves in response to warming climate: Test cases from a subtropical mountain forest and a tropical rainforest in Mexico. Can. J. For. Res. 2007, 37, 977–989. [Google Scholar] [CrossRef]
- Gómez-Mendoza, L.; Arriaga, L. Modeling the Effect of Climate Change on the Distribution of Oak and Pine Species of Mexico. Conserv. Biol. 2007, 21, 1545–1555. [Google Scholar] [CrossRef]
- Rehfeldt, G.E.; Crookston, N.L.; Sáenz-Romero, C.; Campbell, E.M. North American vegetation model for land-use planning in a changing climate: A solution to large classification problems. Ecol. Appl. 2012, 22, 119–141. [Google Scholar] [CrossRef]
- Correa-Díaz, A.; Silva, L.C.R.; Horwath, W.R.; Gómez-Guerrero, A.; Vargas-Hernández, J.; Villanueva-Díaz, J.; Velázquez-Martínez, A.; Suárez-Espinoza, J. Linking Remote Sensing and Dendrochronology to Quantify Climate-Induced Shifts in High-Elevation Forests Over Space and Time. J. Geophys. Res. Biogeosci. 2019, 124, 166–183. [Google Scholar] [CrossRef]
- Berner, L.T.; Beck, P.S.A.; Bunn, A.G.; Lloyd, A.H.; Goetz, S.J. High-latitude tree growth and satellite vegetation indices: Correlations and trends in Russia and Canada (1982–2008). J. Geophys. Res. Biogeosci. 2011, 116, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bunn, A.G.; Hughes, M.K.; Kirdyanov, A.V.; Losleben, M.; Shishov, V.V.; Berner, L.T.; Oltchev, A.; Vaganov, E.A. Comparing forest measurements from tree rings and a space-based index of vegetation activity in Siberia. Environ. Res. Lett. 2013, 8. [Google Scholar] [CrossRef]
- Kaufmann, R.K.; D’Arrigo, R.D.; Paletta, L.F.; Tian, H.Q.; Jolly, W.M.; Myneni, R.B. Identifying Climatic Controls on Ring Width: The Timing of Correlations between Tree Rings and NDVI. Earth Interact. 2008, 12, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Vicente-Serrano, S.M.; Camarero, J.J.; Olano, J.M.; Martin-Hernandez, N.; Pena-Gallardo, M.; Tomas-Burguera, M.; Gazol, A.; Azorin-Molina, C.; Bhuyan, U.; El-Kenawy, A. Diverse relationships between forest growth and the Normalized Difference Vegetation Index at a global scale. Remote Sens. Environ. 2016, 187, 14–29. [Google Scholar] [CrossRef] [Green Version]
- Correa-Díaz, A.; Silva, L.C.R.; Horwath, W.R.; Gómez-Guerrero, A.; Vargas-Hernández, J.; Villanueva-Díaz, J.; Suárez-Espinoza, J.; Velázquez-Martínez, A. From Trees to Ecosystems: Spatiotemporal Scaling of Climatic Impacts on Montane Landscapes Using Dendrochronological, Isotopic, and Remotely Sensed Data. Glob. Biogeochem. Cycles 2020, 34, e2019GB006325. [Google Scholar] [CrossRef]
- D’Arrigo, R.D.; Jacoby, G.C.; Bunker, D.E.; Malmstrom, C.M.; Los, S.O. Correlation between maximum latewood density of annual tree rings and NDVI based estimates of forest productivity. Int. J. Remote Sens. 2000, 21, 2329–2336. [Google Scholar] [CrossRef]
- Beck, P.S.A.; Andreu-Hayles, L.; D’Arrigo, R.; Anchukaitis, K.J.; Tucker, C.J.; Pinzon, J.E.; Goetz, S.J. A large-scale coherent signal of canopy status in maximum latewood density of tree rings at arctic treeline in North America. Glob. Planet. Chang. 2013, 100, 109–118. [Google Scholar] [CrossRef]
- Miyeni, R.B.; Hall, F.G.; Sellers, P.J.; Marshak, A.L. The interpretation of spectral vegetation indexes. IEEE Trans. Geosci. Remote Sens. 1995, 33, 481–486. [Google Scholar] [CrossRef]
- Perry, J.P. The Pines of Mexico and Central America; Timber Press: Portland, OR, USA, 1991; p. 231. [Google Scholar]
- Wang, T.; Hamann, A.; Spittlehouse, D.; Carroll, C. Locally Downscaled and Spatially Customizable Climate Data for Historical and Future Periods for North America. PLoS ONE 2016, 11, e0156720. [Google Scholar] [CrossRef]
- García, E. Modificaciones al Sistema de Clasificación Climática de Koppen, 5th ed.; Universidad Nacional Autónoma de México: Ciudad de México, CDMX, Mexico, 2004; p. 91. [Google Scholar]
- Dubroeucq, D.; Geissert, D.; Barois, I.; Ledru, M.-P. Biological and mineralogical features of Andisols in the Mexican volcanic higlands. Catena 2002, 49, 183–202. [Google Scholar] [CrossRef]
- Polge, H. Fifteen years of wood radiation densitometry. Wood Sci. Technol. 1978, 12, 187–196. [Google Scholar] [CrossRef]
- Mothe, F.; Duchanois, G.; Zannier, B.; Leban, J.-M. Microdensitometric analysis of wood samples: Data computation method used at Inra-ERQB (CERD program). Ann. Des Sci. For. 1998, 55, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Schweingruber, F.H. Tree Rings and Environment Dendroecology; Paul Haupt: Berne, Switzerland, 1996; p. 609. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models; R Package Version 3.1131.1; R Foundation: Vienna, Austria, 2018. [Google Scholar]
- Hochberg, Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 1988, 75, 800–802. [Google Scholar] [CrossRef]
- Esper, J.; Cook, E.R.; Krusic, P.J.; Peters, K.; Schweingruber, F.H. Tests of the RCS method for preserving low-frequency variability in long tree-ring chronologies. Tree-Ring Res. 2003, 59, 81–98. [Google Scholar]
- Zang, C.; Biondi, F. treeclim: An R package for the numerical calibration of proxy-climate relationships. Ecography 2015, 38, 431–436. [Google Scholar] [CrossRef]
- Wang, T.; Hamann, A.; Spittlehouse, D.L.; Murdock, T.Q. ClimateWNA—High-Resolution Spatial Climate Data for Western North America. J. Appl. Meteorol. Climatol. 2012, 51, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Didan, K. MOD13Q1 MODIS/Terra Vegetation Indices 16-Day L3 Global 250m SIN Grid V006; NASA EOSDIS Land Processes DAAC: Sioux Falls, SD, USA, 2015.
- Myneni, R.; Knyazikhin, Y.; Park, T. MOD15A2H MODIS/Terra Leaf Area Index/FPAR 8-Day L4 Global 500m SIN Grid V006; NASA EOSDIS Land Processes DAAC: Sioux Falls, SD, USA, 2015.
- AppEEARS, T. Application for Extracting and Exploring Analysis Ready Samples (AppEEARS). Ver. 2.27; NASA EOSDIS Land Processes Distributed Active Archive Center (LP DAAC), USGS/Earth Resources Observation and Science (EROS) Center: Sioux Falls, SD, USA, 2019.
- Eastman, J. TerrSet Manual; Clark University: Worcester, MA, USA, 2016; p. 390. [Google Scholar]
- Jenkerson, C.; Maiersperger, T.; Schmidt, G. eMODIS: A User-friendly Data Source; Open-File Report 2010-1055; U.S. Geological Survey: Reston, VA, USA, 2010; p. 10.
- Rojas Garcia, F.; Villers Ruiz, L. Comparison of two methods for estimating the wood density of Pinus hartwegii Lindl. from La Malinche volcano. Madera Y Bosques 2005, 11, 63–71. [Google Scholar]
- Rojas-Garcia, F.; Villers-Ruiz, L. Estimation of the forest biomass of Malinche National Park, Tlaxcala-Puebla. Cienc. For. En Mex. 2008, 33, 59–86. [Google Scholar]
- Zobel, B.J. Variation in specific gravity and tracheid length for several species of Mexican Pine. Silvae Genet. 1965, 14, 1–12. [Google Scholar]
- Ivković, M.; Gapare, W.; Wu, H.; Espinoza, S.; Rozenberg, P. Influence of cambial age and climate on ring width and wood density in Pinus radiata families. Ann. For. Sci. 2013, 70, 525–534. [Google Scholar] [CrossRef] [Green Version]
- Zubizarreta-Gerendiain, A.; Gort-Oromi, J.; Mehtätalo, L.; Peltola, H.; Venäläinen, A.; Pulkkinen, P. Effects of cambial age, clone and climatic factors on ring width and ring density in Norway spruce (Picea abies) in southeastern Finland. For. Ecol. Manag. 2012, 263, 9–16. [Google Scholar] [CrossRef]
- Björklund, J.; von Arx, G.; Nievergelt, D.; Wilson, R.; Van den Bulcke, J.; Günther, B.; Loader, N.J.; Rydval, M.; Fonti, P.; Scharnweber, T.; et al. Scientific Merits and Analytical Challenges of Tree-Ring Densitometry. Rev. Geophys. 2019, 57, 1–41. [Google Scholar] [CrossRef] [Green Version]
- Kiaei, M. Effect of site and elevation on wood density and shrinkage and their relationships in Carpinus betulus. For. Stud. China 2012, 14, 229–234. [Google Scholar] [CrossRef]
- Govorčin, S.; Sinković, T.; Trajković, J. Some physical and mechanical properties of beech wood grown in Croatia. Wood Res. 2003, 48, 39–52. [Google Scholar]
- Fajardo, A. Insights into intraspecific wood density variation and its relationship to growth, height and elevation in a treeline species. Plant Biol. 2018, 20, 456–464. [Google Scholar] [CrossRef]
- Topaloğlu, E.; Ay, N.; Altun, L.; Serdar, B. Effect of altitude and aspect on various wood properties ofOriental beech (Fagus orientalis Lipsky) wood. Turk. J. Agric. For. 2016, 40, 397–406. [Google Scholar] [CrossRef]
- Franceschini, T.; Bontemps, J.-D.; Gelhaye, P.; Rittie, D.; Herve, J.-C.; Gegout, J.-C.; Leban, J.-M. Decreasing trend and fluctuations in the mean ring density of Norway spruce through the twentieth century. Ann. For. Sci. 2010, 67, 816. [Google Scholar] [CrossRef] [Green Version]
- Sáenz-Romero, C.; Lamy, J.-B.; Loya-Rebollar, E.; Plaza-Aguilar, A.; Burlett, R.; Lobit, P.; Delzon, S. Genetic variation of drought-induced cavitation resistance among Pinus hartwegii populations from an altitudinal gradient. Acta Physiol. Plant. 2013, 35, 2905–2913. [Google Scholar] [CrossRef]
- Viveros-Viveros, H.; Sáenz-Romero, C.; Vargas-Hernández, J.J.; López-Upton, J.; Ramírez-Valverde, G.; Santacruz-Varela, A. Altitudinal genetic variation in Pinus hartwegii Lindl. I: Height growth, shoot phenology, and frost damage in seedlings. For. Ecol. Manag. 2009, 257, 836–842. [Google Scholar] [CrossRef]
- Chen, F.; Yuan, Y.J.; Wei, W.S.; Fan, Z.A.; Zhang, T.W.; Shang, H.M.; Zhang, R.B.; Yu, S.L.; Ji, C.R.; Qin, L. Climatic response of ring width and maximum latewood density of Larix sibirica in the Altay Mountains, reveals recent warming trends. Ann. For. Sci. 2012, 69, 723–733. [Google Scholar] [CrossRef]
- D’Arrigo, R.D.; Jacoby, G.C.; Free, R.M. Tree-ring width and maximum latewood density at the North American tree line: Parameters of climatic change. Can. J. For. Res. 1992, 22, 1290–1296. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Carraro, V. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia 2007, 152, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bouriaud, O.; Leban, J.M.; Bert, D.; Deleuze, C. Intra-annual variations in climate influence growth and wood density of Norway spruce. Tree Physiol. 2005, 25, 651–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Wilpert, K. Intraannual variation of radial tracheid diameters as monitor of site specific water stress. Dendrochronologia 1991, 9, 95–113. [Google Scholar]
- Liang, E.; Balducci, L.; Ren, P.; Rossi, S. Chapter 3—Xylogenesis and Moisture Stress A2—Kim, Yoon Soo. In Secondary Xylem Biology; Funada, R., Singh, A.P., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 45–58. [Google Scholar] [CrossRef]
- Cuny, H.E.; Fonti, P.; Rathgeber, C.B.K.; von Arx, G.; Peters, R.L.; Frank, D.C. Couplings in cell differentiation kinetics mitigate air temperature influence on conifer wood anatomy. Plant Cell Environ. 2019, 42, 1222–1232. [Google Scholar] [CrossRef]
- Cuny, H.E.; Rathgeber, C.B.K.; Frank, D.; Fonti, P.; Fournier, M. Kinetics of tracheid development explain conifer tree-ring structure. New Phytol. 2014, 203, 1231–1241. [Google Scholar] [CrossRef]
- Lapenis, A.G.; Lawrence, G.B.; Heim, A.; Zheng, C.Y.; Shortle, W. Climate warming shifts carbon allocation from stemwood to roots in calcium-depleted spruce forests. Glob. Biogeochem. Cycles 2013, 27, 101–107. [Google Scholar] [CrossRef]
- Mäkinen, H.; Saranpää, P.; Linder, S. Wood-density variation of Norway spruce in relation to nutrient optimization and fibre dimensions. Can. J. For. Res. 2002, 32, 185–194. [Google Scholar] [CrossRef]
- Kaufmann, R.K.; D’Arrigo, R.D.; Laskowski, C.; Myneni, R.B.; Zhou, L.; Davi, N.K. The effect of growing season and summer greenness on northern forests. Geophys. Res. Lett. 2004, 31. [Google Scholar] [CrossRef]



| Variable | Site | |
|---|---|---|
| Tláloc Mt (TLA) | Jocotitlán Mt (JOC) | |
| Summit (m asl) | 4125 | 3910 |
| Average treeline position | 4000 | Not reached for this site |
| Mean annual maximum temperature (°C) | 14.83 ± 0.7 | 14.23 ± 0.4 |
| Mean annual average temperature (°C) | 7.82 ± 0.5 | 7.74 ± 0.3 |
| Mean annual minimum temperature (°C) | 0.81 ± 0.4 | 1.24 ± 0.3 |
| Mean annual precipitation (mm) | 949.77 ± 65.5 | 819.59 ± 71.4 |
| Average soil moisture (m3 m−3) | 0.21 ± 0.03 | 0.18 ± 0.02 |
| Soil type | Andisol (Ustivitrands/Haplustands) | Andisol (Ustivitrands/Haplustands) |
| Variable | Fixed Effect | DF | F-Value | p-Value |
|---|---|---|---|---|
| MID | Elevation | 55 | 4.030 | 0.012 * |
| Aspect | 55 | 5.018 | 0.029 * | |
| Elevation × Aspect | 55 | 2.50 | 0.069 † | |
| Cambial age | 5508 | 187.76 | <0.001 ** | |
| AVE | Elevation | 55 | 3.642 | 0.018* |
| Aspect | 55 | 3.380 | 0.071 † | |
| Elevation × Aspect | 55 | 1.493 | 0.227 | |
| Cambial age | 5508 | 167.06 | <0.001 ** | |
| MXD | Elevation | 55 | 3.592 | 0.019 * |
| Aspect | 55 | 1.412 | 0.240 | |
| Elevation × Aspect | 55 | 2.370 | 0.080 † | |
| Cambial age | 5508 | 39.581 | <0.001 ** |
| Tree-Ring Wood Density Variable | Tláloc (TLA) <1950 vs. >1950 | Jocotitlán (JOC) <1950 vs. >1950 | ||
|---|---|---|---|---|
| Minimum earlywood density | Null hypothesis | p-value | Null hypothesis | p-value |
| Equal 1 | Rejected | <0.001 | Rejected | 0.005 |
| Larger 2 | Not rejected | 0.11 | Not rejected | 0.07 |
| Smaller 3 | Rejected | <0.001 | Rejected | 0.002 |
| Average density | ||||
| Equal 1 | Rejected | <0.001 | Rejected | 0.01 |
| Larger 2 | Not rejected | 0.71 | Not rejected | 0.81 |
| Smaller 3 | Rejected | <0.001 | Rejected | 0.006 |
| Maximum latewood density | ||||
| Equal 1 | Rejected | <0.001 | Rejected | <0.001 |
| Larger 2 | Rejected | <0.001 | Not rejected | 0.22 |
| Smaller 3 | Not rejected | 0.24 | Rejected | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correa-Díaz, A.; Gómez-Guerrero, A.; Vargas-Hernández, J.J.; Rozenberg, P.; Horwath, W.R. Long-Term Wood Micro-Density Variation in Alpine Forests at Central México and Their Spatial Links with Remotely Sensed Information. Forests 2020, 11, 452. https://doi.org/10.3390/f11040452
Correa-Díaz A, Gómez-Guerrero A, Vargas-Hernández JJ, Rozenberg P, Horwath WR. Long-Term Wood Micro-Density Variation in Alpine Forests at Central México and Their Spatial Links with Remotely Sensed Information. Forests. 2020; 11(4):452. https://doi.org/10.3390/f11040452
Chicago/Turabian StyleCorrea-Díaz, A., A. Gómez-Guerrero, J. J. Vargas-Hernández, P. Rozenberg, and W. R. Horwath. 2020. "Long-Term Wood Micro-Density Variation in Alpine Forests at Central México and Their Spatial Links with Remotely Sensed Information" Forests 11, no. 4: 452. https://doi.org/10.3390/f11040452
APA StyleCorrea-Díaz, A., Gómez-Guerrero, A., Vargas-Hernández, J. J., Rozenberg, P., & Horwath, W. R. (2020). Long-Term Wood Micro-Density Variation in Alpine Forests at Central México and Their Spatial Links with Remotely Sensed Information. Forests, 11(4), 452. https://doi.org/10.3390/f11040452






