Chitosan Oligosaccharide Addition to Buddhist Pine (Podocarpus macrophyllus (Thunb) Sweet) under Drought: Reponses in Ecophysiology and δ13C Abundance
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
2.2. Drought and Re-Water Treatments
2.3. Experimental Design and Seedling Sampling
2.4. Sampled Seedling Measurement and Determination
2.5. Statistic Analysis
3. Results
3.1. Shoot Growth
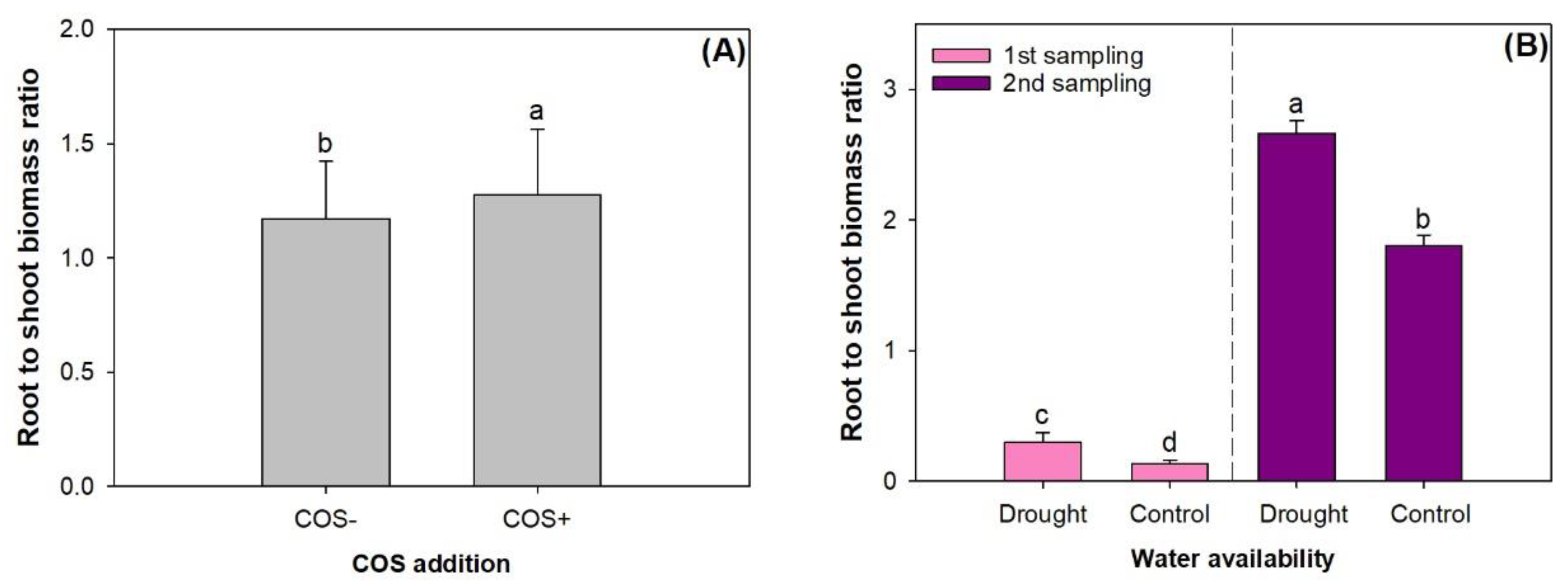
3.2. Biomass Accumulation
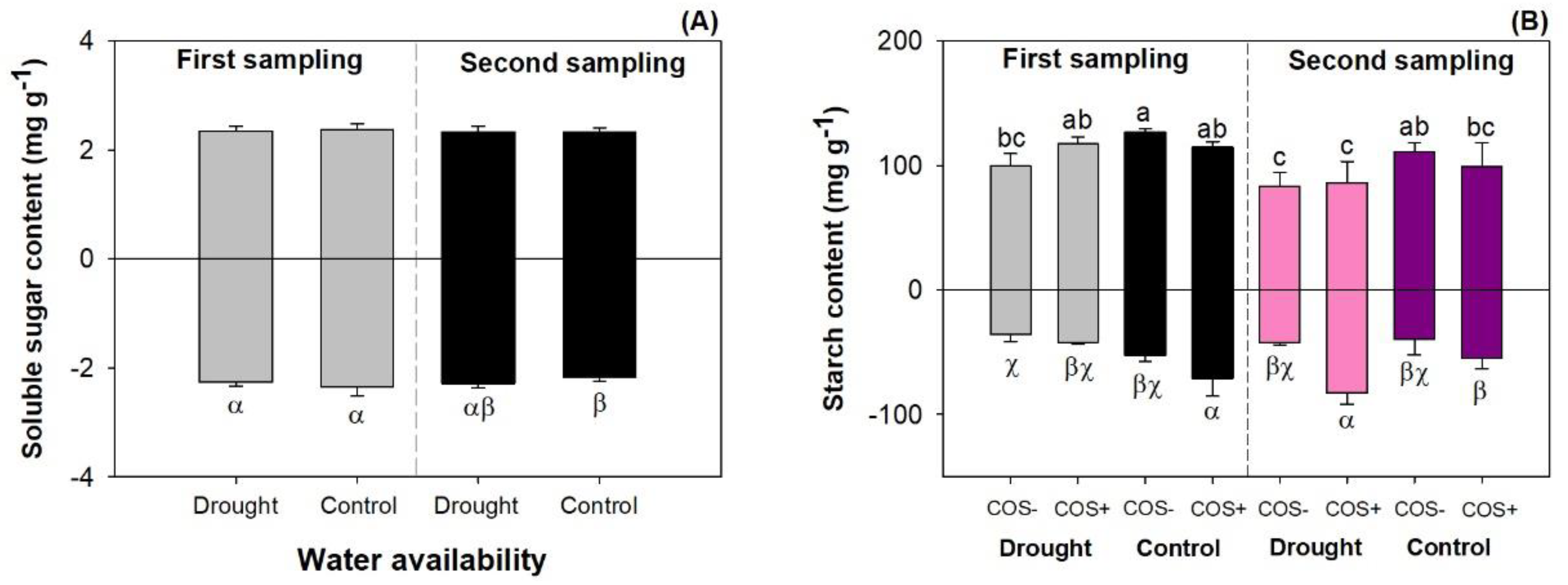
3.3. Carbohydrate Metabolism
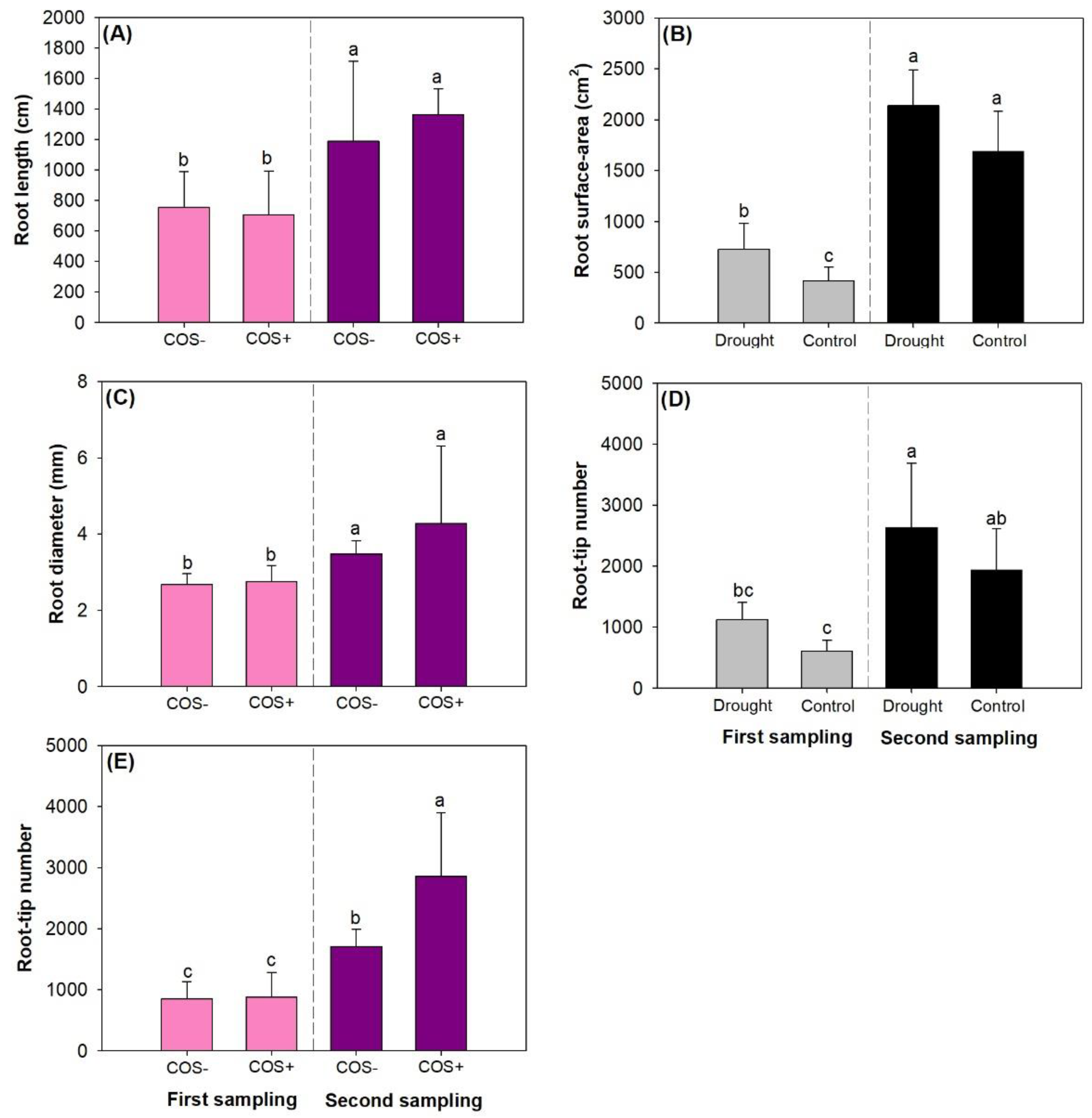
3.4. Fine Root Morphology
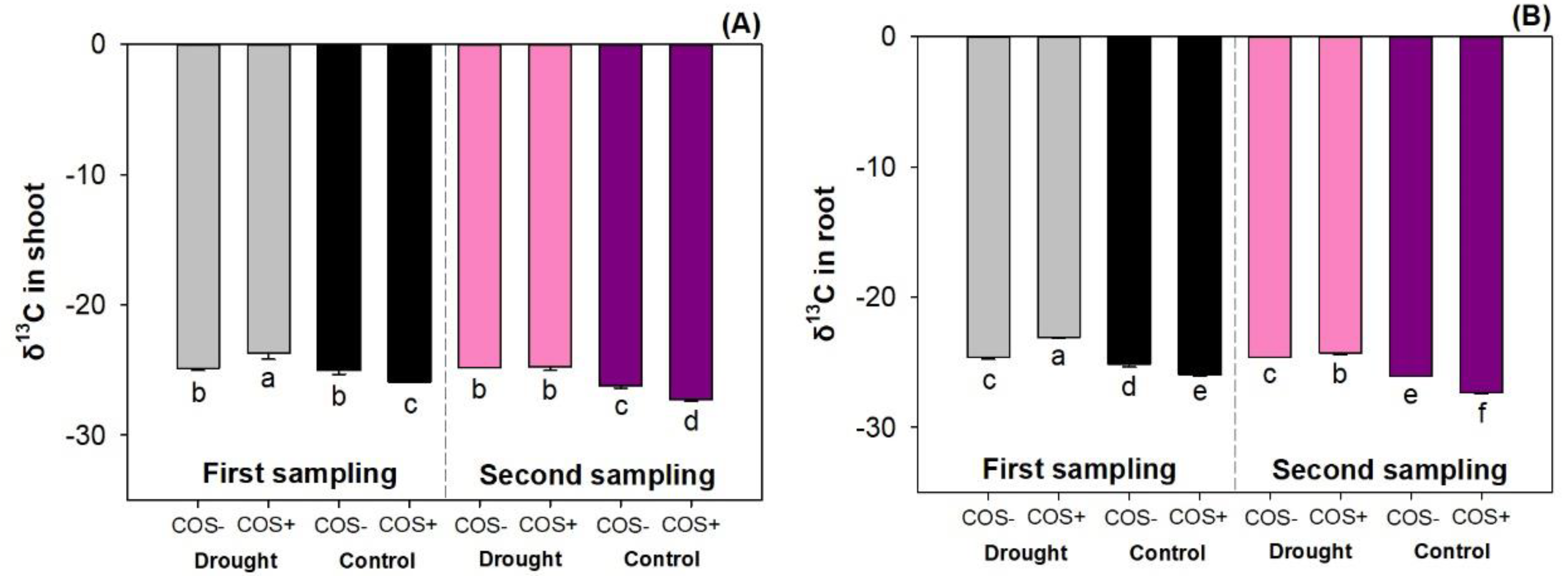
3.5. The δ13C Abundance
3.6. The Antioxidant Enzyme Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aubry-Kientz, M.; Rossi, V.; Wagner, F.; Herault, B. Identifying climatic drivers of tropical forest dynamics. Biogeosciences 2015, 12, 5583–5596. [Google Scholar] [CrossRef]
- Corlett, R.T. The Impacts of Droughts in Tropical Forests. Trends Plant Sci. 2016, 21, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.A.; Kueppers, L.M.; Chambers, J.Q. Novel tropical forests: Response to global change. New Phytol. 2017, 213, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Phillips, O.L.; van der Heijden, G.; Lewis, S.L.; Lopez-Gonzalez, G.; Aragao, L.; Lloyd, J.; Malhi, Y.; Monteagudo, A.; Almeida, S.; Davila, E.A.; et al. Drought-mortality relationships for tropical forests. New Phytol. 2010, 187, 631–646. [Google Scholar] [CrossRef]
- Bottero, A.; D’Amato, A.W.; Palik, B.J.; Bradford, J.B.; Fraver, S.; Battaglia, M.A.; Asherin, L.A. Density-dependent vulnerability of forest ecosystems to drought. J. Appl. Ecol. 2017, 54, 1605–1614. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; Sanchez-Salguero, R.; Rodriguez, C.; Lazo, J.D.; Moreno-Rojas, J.M.; Palacios-Rodriguez, G.; Camarero, J.J. Is thinning an alternative when trees could die in response to drought? The case of planted Pinus nigra and P-Sylvestris stands in southern Spain. For. Ecol. Manag. 2019, 433, 313–324. [Google Scholar] [CrossRef]
- Lafond, V.; Lagarrigues, G.; Cordonnier, T.; Courbaud, B. Uneven-aged management options to promote forest resilience for climate change adaptation: Effects of group selection and harvesting intensity. Ann. For. Sci. 2014, 71, 173–186. [Google Scholar] [CrossRef]
- Ahmed, K.B.M.; Khan, M.M.A.; Siddiqui, H.; Jahan, A. Chitosan and its oligosaccharides, a promising option for sustainable crop production—A review. Carbohydr. Polym. 2020, 227, 17. [Google Scholar]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Tassinary, J.A.F.; Lunardelli, A.; Basso, B.S.; Stulp, S.; Pozzobon, A.; Pedrazza, L.; Bartrons, R.; Ventura, F.; Rosa, J.L.; Melo, D.A.S.; et al. Therapeutic Ultrasound Stimulates MC3T3-E1 Cell Proliferation Through the Activation of NF-kappa B1, p38 alpha, and mTOR. Lasers Surg. Med. 2015, 47, 765–772. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y.; Wei, H.X. Chitosan oligosaccharide addition affects current-year shoot of post-transplant Buddhist pine (Podocarpus macrophyllus) seedlings under contrasting photoperiods. iForest 2017, 10, 715–721. [Google Scholar] [CrossRef]
- Olicon-Hernandez, D.R.; Vazquez-Landaverde, P.A.; Cruz-Camarillo, R.; Rojas-Avelizapa, L.I. Comparison of chito-oligosaccharide production from three different colloidal chitosans using the endochitonsanolytic system of Bacillus thuringiensis. Prep. Biochem. Biotechnol. 2017, 47, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Abdel-Hamid, A.M.E.; Yessoufou, K.; Al-Mana, F.A.; El-Ansary, D.O.; Mahmoud, E.A.; Al-Yafrasi, M.A. Physiological and molecular characterization of water-stressed Chrysanthemum under robinin and chitosan treatment. Acta Physiol. Plant. 2020, 42, 14. [Google Scholar] [CrossRef]
- Muley, A.B.; Shingote, P.R.; Patil, A.P.; Dalvi, S.G.; Suprasanna, P. Gamma radiation degradation of chitosan for application in growth promotion and induction of stress tolerance in potato (Solanum tuberosum L.). Carbohydr. Polym. 2019, 210, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Soares, B.; Goufo, P.; Castro, I.; Cosme, F.; Pinto-Sintra, A.L.; Ines, A.; Oliveira, A.A.; Falco, V. Chitosan upregulates the genes of the ROS pathway and enhances the antioxidant potential of grape (Vitis vinifera L. ‘Touriga Franca’ and ‘Tinto Cao’) tissues. Antioxidants 2019, 8, 525. [Google Scholar] [CrossRef]
- Li, X.W.; Chen, Q.X.; Lei, H.Q.; Wang, J.W.; Yang, S.; Wei, H.X. Nutrient uptake and utilization by Fragrant rosewood (Dalbergia odorifera) seedlings cultured with oligosaccharide addition under different lighting spectra. Forests 2018, 9, 29. [Google Scholar] [CrossRef]
- Nguyen, A.D.; Vo, T.P.K.; Tran, T.D. Research on impact of chitosan oligomers on biophysical characteristics, growth, development and drought resistance of coffee. Carbohydr. Polym. 2011, 84, 751–755. [Google Scholar]
- Elansary, H.O.; Abdel-Hamid, A.M.E.; Mahmoud, E.A.; Al-Mana, F.A.; El-Ansary, D.O.; El-Abedin, T.K.Z. Heuchera Creme Brulee and Mahogany Medicinal Value under Water Stress and Oligosaccharide (COS) Treatment. Evid.-Based Complement. Altern. Med. 2019. Article ID 4242359. [Google Scholar] [CrossRef]
- Adams, H.D.; Germino, M.J.; Breshears, D.D.; Barron-Gafford, G.A.; Guardiola-Claramonte, M.; Zou, C.B.; Huxman, T.E. Nonstructural leaf carbohydrate dynamics of Pinus edulis during drought-induced tree mortality reveal role for carbon metabolism in mortality mechanism. New Phytol. 2013, 197, 1142–1151. [Google Scholar] [CrossRef]
- Escobar-Gutierrez, A.J.; Zipperlin, B.; Carbonne, F.; Moing, A.; Gaudillere, J.P. Photosynthesis, carbon partitioning and metabolite content during drought stress in peach seedlings. Funct. Plant Biol. 1998, 25, 197–205. [Google Scholar] [CrossRef]
- Wei, H.X.; Guo, P. Carbohydrate metabolism during new root growth in transplanted Larix olgensis seedlings: Post-transplant response to nursery-applied inorganic fertilizer and organic amendment. iForest 2017, 10, 15–22. [Google Scholar] [CrossRef]
- Arndt, S.K.; Wanek, W.; Clifford, S.C.; Popp, M. Contrasting adaptations to drought stress in field-grown Ziziphus mauritiana and Prunus persica trees: Water relations, osmotic adjustment and carbon isotope composition. Funct. Plant Boil. 2000, 27, 985–996. [Google Scholar] [CrossRef]
- Galvez, D.A.; Landhausser, S.M.; Tyree, M.T. Low root reserve accumulation during drought may lead to winter mortality in poplar seedlings. New Phytol. 2013, 198, 139–148. [Google Scholar] [CrossRef]
- Meier, I.C.; Leuschner, C. Belowground drought response of European beech: Fine root biomass and carbon partitioning in 14 mature stands across a precipitation gradient. Glob. Chang. Biol. 2008, 14, 2081–2095. [Google Scholar] [CrossRef]
- Churakova, O.V.; Lehmann, M.M.; Siegvvolf, R.T.W.; Saurer, M.; Fonti, M.V.; Schmid, L.; Timofeeva, G.; Rinne-Garmston, K.T.; Bigler, C. Compound-specific carbon isotope patterns in needles of conifer tree species from the Swiss National Park under recent climate change. Plant Physiol. Biochem. 2019, 139, 264–272. [Google Scholar] [CrossRef]
- Cao, X.; Shen, Q.; Liu, L.; Cheng, J. Relationships of growth, stable carbon isotope composition and anatomical properties of leaf and xylem in seven mulberry cultivars: A hint towards drought tolerance. Plant Biol. 2020, 22, 287–297. [Google Scholar] [CrossRef]
- Hong, K.Q.; Xie, J.H.; Zhang, L.B.; Sun, D.Q.; Gong, D.Q. Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage. Sci. Hortic. 2012, 144, 172–178. [Google Scholar] [CrossRef]
- Pasquariello, M.S.; Di Patre, D.; Mastrobuoni, F.; Zampella, L.; Scortichini, M.; Petriccione, M. Influence of postharvest chitosan treatment on enzymatic browning and antioxidant enzyme activity in sweet cherry fruit. Postharvest Biol. Technol. 2015, 109, 45–56. [Google Scholar] [CrossRef]
- Farjon, A. Podocarpus Macrophyllus; The IUCN Red List of Threatened Species 2013: e.T42517A2984343. Available online: http://dx.doi.org/10.2305/IUCN.UK.2013-1.RLTS.T42517A2984343.en (accessed on 12 November 2019).
- Yuan, W.P.; Cai, W.W.; Chen, Y.; Liu, S.G.; Dong, W.J.; Zhang, H.C.; Yu, G.R.; Chen, Z.Q.; He, H.L.; Guo, W.D.; et al. Severe summer heatwave and drought strongly reduced carbon uptake in Southern China. Sci. Rep. 2016, 6, 12. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, L.Y.; Jia, Z.K.; Wei, H.X.; Duan, J. Interactive effects of irrigation and exponential fertilization on nutritional characteristics in Populus x euramericana cv. ‘74/76’ cuttings in an open-air nursery in Beijing, China. J. For. Res. 2016, 27, 569–582. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X.; Zhang, D.H.; Wei, H.X.; Guo, J. Using morphological attributes for the fast assessment of nutritional responses of Buddhist pine (Podocarpus macrophyllus Thunb. D. Don) seedlings to exponential fertilization. PLoS ONE 2019, 14, 14. [Google Scholar] [CrossRef]
- Apostol, K.G.; Dumroese, R.K.; Pinto, J.R.; Davis, A.S. Response of conifer species from three latitudinal populations to light spectra generated by light-emitting diodes and high-pressure sodium lamps. Can. J. For. Res. 2015, 45, 1711–1719. [Google Scholar] [CrossRef]
- Wei, H.X.; Hauer, R.J.; Chen, G.S.; Chen, X.; He, X.Y. Growth, nutrient assimilation, and carbohydrate metabolism in Korean pine (Pinus koraiensis) seedlings in response to light spectra. Forests 2020, 11, 44. [Google Scholar] [CrossRef]
- Wei, H.X.; Chen, X.; Chen, G.S.; Zhao, H.T. Foliar nutrient and carbohydrate in Aralia elata can be modified by understory light quality in forests with different structures at Northeast China. Ann. For. Res. 2019, 62, 125–137. [Google Scholar] [CrossRef]
- Wei, H.X.; Zhao, H.T.; Chen, X. Foliar N:P stoichiometry in Aralia elata distributed on different slope degrees. Not. Bot. Horti Agrobot. Cluj- Napoca 2019, 47, 887–895. [Google Scholar] [CrossRef]
- Wei, H.X.; Xu, C.Y.; Ma, L.Y.; Duan, J.; Jiang, L.N.; Ren, J. Effect of late-season fertilization on nutrient reserves and carbohydrate accumulation in bareroot Larix olgensis seedlings. J. Plant Nutr. 2014, 37, 279–293. [Google Scholar] [CrossRef]
- He, C.X.; Zhang, J.S.; Meng, P.; Gao, J. Seasonal dynamics of foliar C-13 and nutrient concentration of Chinese chastetree and spine jujube in foothill rock outcrop habitat of the southern Taihang Mountains, Central China. J. For. Res. 2019, 30, 45–56. [Google Scholar] [CrossRef]
- Man, R.Z.; Greenway, K.J. Effects of soil moisture and species composition on growth and productivity of trembling aspen and white spruce in planted mixtures: 5-year results. New For. 2013, 44, 23–38. [Google Scholar] [CrossRef]
- Turcsán, A.; Steppe, K.; Sárközi, E.; Erdélyi, É.; Missoorten, M.; Mees, G.; Mijnsbrugge, K.V. Early summer drought stress during the first growing year stimulates extra shoot growth in oak seedlings (Quercus petraea). Front. Plant Sci. 2016, 7, 193. [Google Scholar] [CrossRef]
- Vander Mijnsbrugge, K.; Turcsán, A.; Maes, J.; Duchêne, N.; Meeus, S.; Steppe, K.; Steenackers, M. Repeated summer drought and re-watering during the first growing year of oak (Quercus petraea) delay autumn senescence and bud burst in the following spring. Front. Plant Sci. 2016, 7, 419. [Google Scholar] [CrossRef]
- Pirbalouti, A.G.; Malekpoor, F.; Salimi, A.; Golparvar, A. Exogenous application of chitosan on biochemical and physiological characteristics, phenolic content and antioxidant activity of two species of basil (Ocimum ciliatum and Ocimum basilicum) under reduced irrigation. Sci. Hortic. 2017, 217, 114–122. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wei, H.X.; Ge, L.L.; Sun, N.W.; Wang, T.; Zhang, Q.C.; Han, L.B.; Ge, X.Y.; Jin, G.Y. Chitosan oligosaccharide addition modifies nutrient utilization in highly-valued ornamental tree seedlings. For. Environ. Sci. 2018, 34, 136–144. [Google Scholar]
- Behboudi, F.; Tahmasebi-Sarvestani, Z.; Kassaee, M.Z.; Modarres-Sanavy, S.A.M.; Sorooshzadeh, A.; Mokhtassi-Bidgoli, A. Evaluation of chitosan nanoparticles effects with two application methods on wheat under drought stress. J. Plant Nutr. 2019, 42, 1439–1451. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Siadat, S.A.; Bakhshandeh, A.; Pirbalouti, A.G.; Hashemi, M. Morpho-physiological and phytochemical traits of (Thymus daenensis Celak.) in response to deficit irrigation and chitosan application. Acta Physiol. Plant. 2017, 39, 13. [Google Scholar] [CrossRef]
- Silveira, N.M.; Seabra, A.B.; Marcos, F.C.C.; Pelegrino, M.T.; Machado, E.C.; Ribeiro, R.V. Encapsulation of S-nitrosoglutathione into chitosan nanoparticles improves drought tolerance of sugarcane plants. Nitric Oxide-Biol. Chem. 2019, 84, 38–44. [Google Scholar] [CrossRef]
- Brugnoli, E.; Hubick, K.T.; von Caemmerer, S.; Wong, S.C.; Farquhar, G.D. Correlation between the carbon isotope discrimination in leaf starch and sugars of C3 plants and the ratio of intercellular and atmospheric partial pressures of carbon dioxide. Plant Physiol. 1988, 88, 1418–1424. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Iriti, M.; Picchi, V.; Rossoni, M.; Gomarasca, S.; Ludwig, N.; Gargano, M.; Faoro, F. Chitosan antitranspirant activity is due to abscisic acid-dependent stomatal closure. Environ. Exp. Bot. 2009, 66, 493–500. [Google Scholar] [CrossRef]
- Ludwig, N.; Cabrini, R.; Faoro, F.; Gargano, M.; Gomarasca, S.; Iriti, M.; Picchi, V.; Soave, C. Reduction of evaporative flux in bean leaves due to chitosan treatment assessed by infrared thermography. Infrared Phys. Technol. 2010, 53, 65–70. [Google Scholar] [CrossRef]


| Parameter | W | O | T | W × O | O × T | W × T | W × O × T |
|---|---|---|---|---|---|---|---|
| Height | <0.0001 1 | 0.0021 | <0.0001 | 0.9691 | 0.1576 | 0.1089 | 0.0075 |
| RCD 2 | <0.0001 | 0.0033 | <0.0001 | 0.5998 | 0.0454 | 0.8406 | 0.0162 |
| Shoot biomass | <0.0001 | 0.0021 | <0.0001 | 0.6508 | 0.1170 | 0.0357 | 0.1745 |
| Root biomass | <0.0001 | 0.0010 | <0.0001 | 0.6727 | 0.0017 | <0.0001 | 0.5005 |
| R/S 3 | <0.0001 | 0.0011 | <0.0001 | 0.6295 | 0.4677 | <0.0001 | 0.5413 |
| Shoot sugar | 0.6865 | 0.3667 | 0.4590 | 0.6865 | 0.6009 | 0.6800 | 0.4377 |
| Root sugar | 0.7205 | 0.9142 | 0.0417 | 0.8296 | 0.1842 | 0.0183 | 0.2975 |
| Shoot starch | 0.0016 | 0.8723 | 0.0018 | 0.0133 | 0.4039 | 0.3682 | 0.4008 |
| Root starch | 0.2817 | 0.0004 | 0.0957 | 0.3724 | 0.0118 | <0.0001 | 0.0040 |
| Water Availability | Oligosaccharide Addition | Sampling Time | POD 1 (∆470 g−1 FW min−1) | SOD 2 (U g−1 FW) | CAT 3 (U g−1 FW) |
|---|---|---|---|---|---|
| Drought | No | First | 11.54 ± 3.79 ab 4 | 5.64 ± 0.60 ab | 2.10 ± 0.00 g |
| Yes | 7.34 ± 0.49 bcd | 6.38 ± 1.07 ab | 2.38 ± 0.01 b | ||
| Irrigated control | No | 7.01 ± 1.67 cd | 4.93 ± 0.76 b | 2.31 ± 0.00 c | |
| Yes | 6.47 ± 2.14 d | 3.64 ± 1.07 b | 2.25 ± 0.01 e | ||
| Drought | No | Second | 10.98 ± 0.67 abc | 5.58 ± 0.92 ab | 2.28 ± 0.02 d |
| Yes | 14.35 ± 2.74 a | 8.26 ± 1.85 a | 2.50 ± 0.00 a | ||
| Irrigated control | No | 10.60 ± 1.30 abcd | 4.08 ± 1.53 b | 2.30 ± 0.00 c | |
| Yes | 12.16 ± 2.23 a | 4.42 ± 2.56 b | 2.17 ± 0.01 f |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, C.; Zhao, Y.; Zhang, J.; Gao, J. Chitosan Oligosaccharide Addition to Buddhist Pine (Podocarpus macrophyllus (Thunb) Sweet) under Drought: Reponses in Ecophysiology and δ13C Abundance. Forests 2020, 11, 526. https://doi.org/10.3390/f11050526
He C, Zhao Y, Zhang J, Gao J. Chitosan Oligosaccharide Addition to Buddhist Pine (Podocarpus macrophyllus (Thunb) Sweet) under Drought: Reponses in Ecophysiology and δ13C Abundance. Forests. 2020; 11(5):526. https://doi.org/10.3390/f11050526
Chicago/Turabian StyleHe, Chunxia, Yan Zhao, Jinsong Zhang, and Jun Gao. 2020. "Chitosan Oligosaccharide Addition to Buddhist Pine (Podocarpus macrophyllus (Thunb) Sweet) under Drought: Reponses in Ecophysiology and δ13C Abundance" Forests 11, no. 5: 526. https://doi.org/10.3390/f11050526
APA StyleHe, C., Zhao, Y., Zhang, J., & Gao, J. (2020). Chitosan Oligosaccharide Addition to Buddhist Pine (Podocarpus macrophyllus (Thunb) Sweet) under Drought: Reponses in Ecophysiology and δ13C Abundance. Forests, 11(5), 526. https://doi.org/10.3390/f11050526




