The Climatic Response of Tree Ring Width Components of Ash (Fraxinus excelsior L.) and Common Oak (Quercus robur L.) from Eastern Europe
Abstract
:1. Introduction
2. Materials and Methods
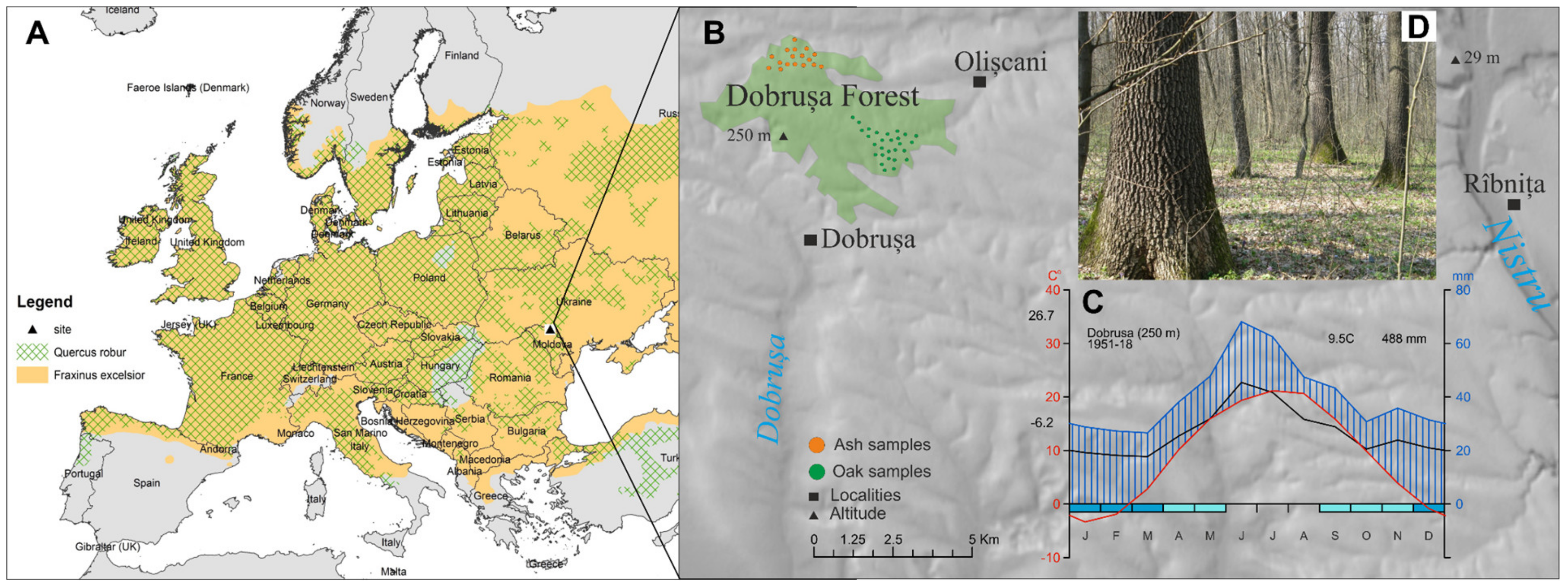
2.1. Site Description
2.2. Chronology Development
2.3. Climate–Growth Relationship
2.4. Stability Maps
3. Results and Discussion
3.1. Chronologies Characteristics
3.2. Climate–Growth Relationship
3.2.1. Correlation with Temperature and Precipitation
3.2.2. Correlation with the Drought Index
3.2.3. Temporal Stability of the Dendroclimatic Response
3.3. Stability Maps
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5°C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Alexandrov, V.; Gajdusek, M.; Knight, C.; Yotova, A. Global Environmental Change: Challenges to Science and Society in Southeastern Europe: Selected Papers presented in the International Conference, Sofia Bulgaria, 19–21 May 2008; Springer Netherlands: Dorleck, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Di Filippo, A.; Alessandrini, A.; Biondi, F.; Blasi, S.; Portoghesi, L.; Piovesan, G. Climate change and oak growth decline: Dendroecology and stand productivity of a Turkey oak (Quercus cerris L.) old stored coppice in Central Italy. Ann. Forest Sci. 2010, 67, 706–706. [Google Scholar] [CrossRef] [Green Version]
- Gentilesca, T.; Camarero, J.; Colangelo, M.; Nole, A.; Ripullone, F. Drought-induced oak decline in the western Mediterranean region: An overview on current evidences, mechanisms and management options to improve forest resilience. iForest Biogeosci. For. 2017, 10, 796–806. [Google Scholar] [CrossRef] [Green Version]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Helama, S.; Sohar, K.; Läänelaid, A.; Mäkelä, H.M.; Raisio, J. Oak Decline as Illustrated Through Plant–Climate Interactions Near the Northern Edge of Species Range. Bot. Rev. 2016, 82, 1–23. [Google Scholar] [CrossRef]
- Mette, T.; Dolos, K.; Meinardus, C.; Bräuning, A.; Reineking, B.; Blaschke, M.; Pretzsch, H.; Beierkuhnlein, C.; Gohlke, A.; Wellstein, C. Climatic turning point for beech and oak under climate change in Central Europe. Ecosphere 2013, 4, art145. [Google Scholar] [CrossRef]
- Führer, E. Oak Decline in Central Europe: A Synopsis of Hypotheses. In Proceedings of the Population Dynamics, Impacts, and Integrated Management of Forest Defoliating Insects, BanskA Stiavnica, Slovak Republic, 18–23 August 1996; USDA Forest Service General Technical Report NE-247. USDA Forest Service: Newtown Square, PA, USA, 1998. [Google Scholar]
- Colangelo, M.; Camarero, J.; Ripullone, F.; Gazol, A.; Sanchez-Salguero, R.; Oliva, J.; Redondo, M. Drought Decreases Growth and Increases Mortality of Coexisting Native and Introduced Tree Species in a Temperate Floodplain Forest. Forests 2018, 9, 205. [Google Scholar] [CrossRef] [Green Version]
- ICAS. Raport Privind Starea Fondului Forestier şi Rezultatele Activităţii Agenţiei Moldsilva în Perioada Anilor 2010–2015; ICAS: Chișinau, Moldova, 2016. [Google Scholar]
- Thomas, F.M.; BLANK, R.; Hartmann, G. Abiotic and biotic factors and their interactions as causes of oak decline in Central Europe. For. Pathol. 2002, 32, 277–307. [Google Scholar] [CrossRef]
- Sohar, K.; Helama, S.; Läänelaid, A.; Raisio, J.; Tuomenvirta, H. Oak decline in a southern Finnish forest as affected by a drought sequence. Geochron 2014, 41, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Stojanović, D.B.; Levanič, T.; Matović, B.; Orlović, S. Growth decrease and mortality of oak floodplain forests as a response to change of water regime and climate. Eur. J. For. Res. 2015, 134, 555–567. [Google Scholar] [CrossRef]
- Cufar, K.; Grabner, M.; Morgos, A.; Martinez del Castillo, E.; Merela, M.; De Luis, M. Common climatic signals affecting oak tree-ring growth in SE Central Europe. Trees 2014, 28, 1267–1277. [Google Scholar] [CrossRef]
- Starkey, D.A.; Oliveria, F.; Mangini, A.; Mielke, M. Oak decline and red oak borer in the interior highlands of Arkansas and Missouri: Natural phenomena, severe occurrences. In Upland Oak Ecology Symposium: History, Current Conditions, and Sustainability; Southern Research Station: Asheville, NC, USA, 2004; pp. 217–222. [Google Scholar]
- Catton, H.; St., George, S.; Remphrey, W. An Evaluation of Bur Oak (Quercus macrocarpa) Decline in the Urban Forest of Winnipeg, Manitoba, Canada. Arboric. Urban For. 2007, 33, 22. [Google Scholar]
- Elliott, K.J.; Swank, W.T. Impacts of drought on tree mortality and growth in a mixed hardwood forest. J. Veg. Sci. 1994, 5, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, H.; Ishida, M. Decline of Quercus crispula in abandoned coppice forests caused by secondary succession and Japanese oak wilt disease: Stand dynamics over twenty years. For. Ecol. Manag. 2014, 334, 18–27. [Google Scholar] [CrossRef]
- Čufar, K.; De Luis, M.; Zupančič, M.; Eckstein, D. A 548-Year Tree-Ring Chronology of Oak (Quercus spp.) for Southeast Slovenia and its Significance as a Dating Tool and Climate Archive. Tree-Ring Res. 2008, 64, 3–15. [Google Scholar] [CrossRef]
- Kern, Z.; Grynaeus, A.; Morgos, A. Reconstructed precipitation for southern Bakony Mountains (Transdanubia, Hungary) back to 1746 AD based on ring width of oak trees. Időjárás 2009, 113, 299–314. [Google Scholar]
- Popa, I.; Caisîn, V. Comparative response of beech and oak to drought in Codrii Natural Reserve (R. Moldova). Bucov. For. 2015, 15, 45–53. [Google Scholar]
- Prokop, O.; Kolar, T.; Büntgen, U.; Kyncl, J.; Kyncl, T.; Bošeľa, M.; Choma, M.; Barta, P.; Rybníček, M. On the palaeoclimatic potential of a millennium-long oak ring width chronology from Slovakia. Dendrochronologia 2016, 40, 93–101. [Google Scholar] [CrossRef]
- Nechita, C.; Popa, I.; Eggertsson, Ó. Climate response of oak (Quercus spp.), an evidence of a bioclimatic boundary induced by the Carpathians. Sci. Total Environ. 2017, 599–600, 1598–1607. [Google Scholar] [CrossRef]
- Netsvetov, M.; Sergeyev, M.; Nikulina, V.; Korniyenko, V.; Prokopuk, Y. The climate to growth relationships of pedunculate oak in steppe. Dendrochronologia 2017, 44, 31–38. [Google Scholar] [CrossRef]
- Arvai, M.; Morgos, A.; Kern, Z. Growth-climate relations and the enhancement of drought signals in pedunculate oak (Quercus robur L.) tree-ring chronology in Eastern Hungary. iForest Biogeosciences For. 2018, 11, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Mikac, S.; Žmegač, A.; Trlin, D.; Paulić, V.; Oršanić, M.; Anić, I. Drought-induced shift in tree response to climate in floodplain forests of Southeastern Europe. Sci. Rep. 2018, 8, 16495. [Google Scholar] [CrossRef] [PubMed]
- Nagavciuc, V.; Ionita, M.; Perșoiu, A.; Popa, I.; Loader, N.J.; McCarroll, D. Stable oxygen isotopes in Romanian oak tree rings record summer droughts and associated large-scale circulation patterns over Europe. Clim. Dyn. 2019, 52, 6557–6568. [Google Scholar] [CrossRef] [Green Version]
- Coker, T.L.R.; Rozsypálek, J.; Edwards, A.; Harwood, T.P.; Butfoy, L.; Buggs, R.J.A. Estimating mortality rates of European ash (Fraxinus excelsior) under the ash dieback (Hymenoscyphus fraxineus) epidemic. Plants, People, Planet 2019, 1, 48–58. [Google Scholar] [CrossRef] [Green Version]
- Dobrowolska, D.; Hein, S.; Oosterbaan, A.; Wagner, S.; Clark, J.; Skovsgaard, J.P. A review of European ash (Fraxinus excelsior L.): Implications for silviculture. Forestry 2011, 84, 133–148. [Google Scholar] [CrossRef] [Green Version]
- Kerr, G.; Cahalan, C. A review of site factors affecting the early growth of ash (Fraxinus excelsior L.). For. Ecol. Manag. 2004, 188, 225–234. [Google Scholar] [CrossRef]
- Lygis, V.; Vasiliauskas, R.; Larsson, K.-H.; Stenlid, J. Wood-inhabiting fungi in stems of Fraxinus excelsior in declining ash stands of northern Lithuania, with particular reference to Armillaria cepistipes. Scand. J. For. Res. 2005, 20, 337–346. [Google Scholar] [CrossRef]
- Bochenek, G.M.; Eriksen, B. Annual growth of male and female individuals of the Common Ash (Fraxinus excelsior L.). Plant Ecol. Divers. 2010, 3, 47–57. [Google Scholar] [CrossRef]
- Pušpure, I.; Gerra-Inohosa, L.; Matisons, R.; Laiviņš, M. Tree-ring width of European ash differing by crown condition and its relationship with climatic factors in Latvia. Balt. For. 2017, 23, 244–252. [Google Scholar]
- Matisons, R.; Inohosa, L.G.; Laiviņš, M. Pointer Years in Tree-Ring Width of European Ash with Different Crown Condition and Their Relationships with Climatic Factors in Latvia. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2016, 70, 116. [Google Scholar] [CrossRef]
- Giagli, K.; Baar, J.; Fajstavr, M.; Gryc, V.; Vavrčík, H. Tree-ring width and Variation of Wood Density in Fraxinus excelsior L. and Quercus robur L. Growing in Floodplain Forests. Bioresourse 2017, 13, 804–819. [Google Scholar] [CrossRef]
- Okoński, B. Radial growth of pedunculate oak and European ash on active river terraces. Hydrologic and climatic controls. Infrastrukt. Ekol. Teren. Wiej. 2017, III/1, 1075–1091. [Google Scholar]
- Heklau, H.; Jetschke, G.; Bruelheide, H.; Seidler, G.; Haider, S. Species-specific responses of wood growth to flooding and climate in floodplain forests in Central Germany. iForest Biogeosci. For. 2019, 12, 226–236. [Google Scholar] [CrossRef]
- Bolte, A.; Ammer, C.; Löf, M.; Nabuurs, G.-J.; Schall, P.; Spathelf, P. Adaptive Forest Management: A Prerequisite for Sustainable Forestry in the Face of Climate Change. Plant-Fire Interactions 2010. [Google Scholar] [CrossRef]
- Yousefpour, R.; Temperli, C.; Jacobsen, J.; Thorsen, B.; Meilby, H.; Lexer, M.; Lindner, M.; Bugmann, H.; Borges, J.; Palma, J.; et al. A framework for modeling adaptive forest management and decision making under climate change. Ecol. Soc. 2017, 22. [Google Scholar] [CrossRef]
- Metzger, M.J.; Bunce, R.G.H.; Jongman, R.H.G.; Mücher, C.A.; Watkins, J.W. A climatic stratification of the environment of Europe. Glob. Ecol. Biogeogr. 2005, 14, 549–563. [Google Scholar] [CrossRef]
- Caudullo, G.; Welk, E.; San-Miguel-Ayanz, J. Chorological maps for the main European woody species. Data Brief 2017, 12, 662–666. [Google Scholar] [CrossRef]
- Ellenberg, H. Vegetation Ecology of Central Europe; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Sfeclă, V. Tipuri de pădure din Rezervaţia peisagistică “Dobruşa”. Lucrări ştiinţifice, UASM (partea a II-Horticultură, Viticultură şi Vinificație, Silvicultură şi Grădini Publice. Protecţia plantelor 2013, 36, 158–162. [Google Scholar]
- Gärtner, H.; Nievergelt, D. The core-microtome: A new tool for surface preparation on cores and time series analysis of varying cell parameters. Dendrochronologia 2010, 28, 85–92. [Google Scholar] [CrossRef]
- Cybis Elektronik. CDendro and CooRecorder. 2010. Available online: http://www.cybis.se/forfun/dendro/index.htm.
- Wheeler, E.A.; Baas, P.; Gasson, P.E. IAWA List of Microscopic Features for Hardwood Identification with an Appendix on Non-anatomical Information; National Herbarium of the Netherlands: Leiden, The Netherlands, 1989; pp. 219–332. [Google Scholar]
- Rinn, F. TSAP-Win User Reference; Rinntech: Heidelberg, Germany, 2003. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree Ring Bulletin 1983, 43, 69–75. [Google Scholar]
- Grissino-Mayer, H.D. Evaluating Crossdating Accuracy: A Manual and Tutorial for the Computer Program COFECHA. Tree-Ring Res. 2001, 57, 205–221. [Google Scholar]
- Eckstein, D.; Bauch, J. Beitrag zur Rationalisierung eines dendrochronologischen Verfahrens und zur Analyse seiner Aussagesicherheit. Forstwiss. Cent. 1969, 88, 230–250. [Google Scholar] [CrossRef]
- Baillie, M.G.L.; Pilcher, J.R. A Simple Crossdating Program for Tree-Ring Research; Tree-Ring Bulletin: Loveland, CO, USA, 1973. [Google Scholar]
- Melvin, T.M.; Briffa, K.R.; Nicolussi, K.; Grabner, M. Time-varying-response smoothing. Dendrochronologia 2007, 25, 65–69. [Google Scholar] [CrossRef]
- Cook, E.R.; Krusic, P.J. ARSTAN4.1b_XP. Available online: http://www.ldeo.columbia.edu (accessed on 23 May 2020).
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK, 1976. [Google Scholar]
- Popa, I. Fundamente Metodologice şi Aplicaţii de Dendrocronologie; Editura Tehnică Silvică: Bucureşti, Romania, 2004. [Google Scholar]
- Briffa, K.; Jones, P. Basic chronology statistics and assessment. In Methods of Dendrochronology:Applications in the Environmental Sciences; Cook, E., Kairiukstis, L., Eds.; Kluwer Academic Publishers: Dordrecht, Germany, 1990; pp. 137–152. [Google Scholar]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the Average Value of Correlated Time Series, with Applications in Dendroclimatology and Hydrometeorology. J. Clim. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Harris, I.; Jones, P.D.; Osborn, T.J.; Lister, D.H. Updated high-resolution grids of monthly climatic observations–the CRU TS3.10 Dataset. Int. J. Climatol. 2014, 34, 623–642. [Google Scholar] [CrossRef] [Green Version]
- Van Oldenborgh, G.J.; Drijfhout, S.; van Ulden, A.; Haarsma, R.; Sterl, A.; Severijns, C.; Hazeleger, W.; Dijkstra, H. Western Europe is warming much faster than expected. Clim. Past 2009, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I.; Angulo, M.; Kenawy, A.E. A New Global 0.5° Gridded Dataset (1901–2006) of a Multiscalar Drought Index: Comparison with Current Drought Index Datasets Based on the Palmer Drought Severity Index. J. Hydrometeorol. 2010, 11, 1033–1043. [Google Scholar] [CrossRef] [Green Version]
- R Development Core Team R: A Language an Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2010.
- Zang, C.; Biondi, F. Dendroclimatic calibration in R: The bootRes package for response and correlation function analysis. Dendrochronologia 2012, 31, 68–74. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Ionita, M.; Dima, M.; Lohmann, G.; Scholz, P.; Rimbu, N. Predicting the June 2013 European Flooding Based on Precipitation, Soil Moisture, and Sea Level Pressure. J. Hydrometeorol. 2015, 16, 598–614. [Google Scholar] [CrossRef] [Green Version]
- Ionita, M.; Grosfeld, K.; Scholz, P.; Treffeisen, R.; Lohmann, G. September Arctic sea ice minimum prediction- A skillful new statistical approach. Earth Syst. Dyn. 2019, 10, 189–203. [Google Scholar] [CrossRef] [Green Version]
- Ionita, M. Mid Range Forecasting of the German Waterways Streamflow Based on Hydrologic, Atmospheric and Oceanic Data. Rep. Polar Marine Res. 2017. [Google Scholar] [CrossRef]
- Nagavciuc, V.; Roibu, C.-C.; Ionita, M.; Mursa, A.; Cotos, M.-G.; Popa, I. Different climate response of three tree ring proxies of Pinus sylvestris from the Eastern Carpathians, Romania. Dendrochronologia 2019, 54, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Kern, Z.; Patkó, M.; Kázmér, M.; Fekete, J.; Kele, S.; Pályi, Z. Multiple tree-ring proxies (earlywood width, latewood width and δ13C) from pedunculate oak (Quercus robur L.), Hungary. Quat. Int. 2013, 293, 257–267. [Google Scholar] [CrossRef]
- Fonti, P.; García-González, I. Earlywood vessel size of oak as a potential proxy for spring precipitation in mesic sites. J. Biogeogr. 2008, 35, 2249–2257. [Google Scholar] [CrossRef]
- Methods of dendrochronology. Applications in the Enviromnental Science; Cook, E.R., Kairiukstis, L.A., Eds.; Kluwer: South Holland, The Netherlands, 1990. [Google Scholar]
- González, I.G.; Eckstein, D. Climatic signal of earlywood vessels of oak on a maritime site. Tree Physiol. 2003, 23, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Lebourgeois, F.; Cousseau, G.; Ducos, Y. Climate-tree-growth relationships of Quercus petraea Mill. stand in the Forest of Bercé (“Futaie des Clos”, Sarthe, France). Ann. For. Sci. 2013, 61, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Pallardy, G.S. Physiology of Woody Plants, 3rd ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Bréda, N.; Granier, A. Intra- and interannual variations of transpiration, leaf area index and radial growth of a sessile oak stand (Quercus petraea). Ann. For. Sci. 1996, 53, 521–536. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Midmore, D.J. Modelling plant resource allocation and growth partitioning in response to environmental heterogeneity. Ecol. Model. 2005, 181, 59–77. [Google Scholar] [CrossRef]
- Vincent-Barbaroux, C.; Breda, N. Contrasting distribution and seasonal dynamics of carbohydrate reserves in stem wood of adult ring-porous sessile oak and diffuse-porous beech trees. Tree Physiol. 2003, 22, 1201–1210. [Google Scholar] [CrossRef]
- Fonti, P.; Eilmann, B.; García-González, I.; von Arx, G. Expeditious building of ring-porous earlywood vessel chronologies without losing signal information. Trees 2009, 23, 665–671. [Google Scholar] [CrossRef] [Green Version]
- García-González, I.; Fonti, P. Selecting earlywood vessels to maximize their environmental signal. Tree Physiol. 2006, 26, 1289–1296. [Google Scholar] [CrossRef] [Green Version]
- Hacke, U.; Sauter, J.J. Xylem dysfunction during winter and recovery of hydraulic conductivity in diffuse-porous and ring-porous trees. Oecologia 1996, 105, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Weber, P.; Bugmann, H.; Rigling, A. Radial growth responses to drought of Pinus sylvestris and Quercus pubescens in an inner-Alpine dry valley. J. Veg. Sci. 2007, 18, 777–792. [Google Scholar] [CrossRef]
- Koprowski, M.; Okoński, B.; Gričar, J.; Puchałka, R. Streamflow as an ecological factor influencing radial growth of European ash (Fraxinus excelsior (L.)). Ecol. Indic. 2018, 85, 390–399. [Google Scholar] [CrossRef]
- Song, B.; Niu, S.; Wan, S. Precipitation regulates plant gas exchange and its long-term response to climate change in a temperate grassland. J. Plant Ecol. 2016, 9, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Kozlowski, T.T.; Pallardy, G. Growth Control in Woody Plants; Elsevier: Amsterdam, The Netherlands, 1997; p. 641. [Google Scholar]
- Popa, I.; Leca, S.; Craciunescu, A.; Sidor, C.; Badea, O.N. Dendroclimatic Response Variability of Quercus species in the Romanian Intensive Forest Monitoring Network. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41. [Google Scholar] [CrossRef] [Green Version]
- Nechita, C.; Chiriloaei, F. Interpreting the effect of regional climate fluctuations on Quercus robur L. trees under a temperate continental climate (southern Romania). Dendrobiology 2018, 79, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Rust, S.; Savill, P.S. The root systems of Fraxinus excelsior and Fagus sylvatica and their competitive relationships. Forestry 2000, 73, 499–503. [Google Scholar] [CrossRef]
- Marigo, G.; Peltier, J.-P.; Girel, J.; Pautou, G. Success in the demographic expansion of Fraxinus excelsior L. Trees 2000, 15, 1–13. [Google Scholar] [CrossRef]
- Allen, M.F. How oaks respond to water limitation. In Proceedings of the seventh California Oak Symposium: Managing oak Woodlands in a Dynamic World, Berkeley, CA, USA, 10 December 2015; Department of Agriculture, Forest Service, Pacific Southwest Research Station: Berkeley, CA, USA, 2015. [Google Scholar]
- Kuster, T.; Arend, M.; Günthardt-Goerg, M.; Schulin, R. Root growth of different oak provenances in two soils under drought stress and air warming conditions. Plant Soil 2012, 369. [Google Scholar] [CrossRef] [Green Version]
- Tumajer, J.; Treml, V. Response of floodplain pedunculate oak (Quercus robur L.) tree-ring width and vessel anatomy to climatic trends and extreme hydroclimatic events. For. Ecol. Manag. 2016, 379, 185–194. [Google Scholar] [CrossRef]
- Rybníček, M.; Čermák, P.; Žid, T.; Kolar, T. Radial Growth and Health Condition of Norway Spruce (Picea Abies (L.) Karst.) Stands in Relation to Climate (Silesian Beskids, Czech Republic). Geochron 2010, 36. [Google Scholar] [CrossRef] [Green Version]
- Kolar, T.; Giagli, K.; Trnka, M.; Bednářová, E.; Vavrčík, H.; Rybníček, M. Response of the leaf phenology and tree-ring width of European beech to climate variability. Silva Fenn. 2016, 50. article id 1520. [Google Scholar] [CrossRef] [Green Version]
- Friedrichs, D.; Buntgen, U.; Frank, D.; Esper, J.; Neuwirth, B.; Loffler, J. Complex climate controls on 20th century oak growth in Central-West Germany. Tree Physiol. 2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef] [Green Version]
- Oren, R.; Sperry, J.; Katul, G.; Pataki, D.; Ewers, B.; Phillips, N.; Schafer, K. Survey and synthesis of intra- and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant Cell Environ. 1999, 22, 1515–1526. [Google Scholar] [CrossRef] [Green Version]
- Gessler, A.; Schaub, M.; McDowell, N.G. The role of nutrients in drought-induced tree mortality and recovery. New Phytol. 2017, 214, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Dinis, C.; Surovy, P.; Ribeiro, N.; Oliveira, M. The effect of soil compaction at different depths on cork oak seedling growth. New Forests 2014, 46. [Google Scholar] [CrossRef]
- Frank, D.; Büntgen, U.; Böhm, R.; Maugeri, M.; Esper, J. Warmer early instrumental measurements versus colder reconstructed temperatures: Shooting at a moving target. Quat. Sci. Rev. 2007, 26, 3298–3310. [Google Scholar] [CrossRef]
- Esper, J.; Büntgen, U.; Frank, D.C.; Nievergelt, D.; Liebhold, A. 1200 years of regular outbreaks in alpine insects. Proc. R. Soc. B Biol. Sci. 2007, 274, 671–679. [Google Scholar] [CrossRef] [Green Version]
- Rozas, V. Detecting the impact of climate and disturbances on tree-rings of Fagus sylvatica L. and Quercus robur L. in a lowland forest in Cantabria, Northern Spain. Ann. For. Sci. 2000, 58, 237–251. [Google Scholar] [CrossRef] [Green Version]
- Birsan, M.-V.; Dumitrescu, A. Snow variability in Romania in connection to large-scale atmospheric circulation. Int. J. Climatol. 2014, 34, 134–144. [Google Scholar] [CrossRef]
- Vaganov, E.A.; Hughes, M.; Kirdyanov, A.V.; Schweingruber, F.; Silkin, P. Influence of snowfall and melt timing on tree growth in Subarctic Eurasia. Nature 1999, 400, 149–151. [Google Scholar] [CrossRef]
- Nagavciuc, V.; Kern, Z.; Ionita, M.; Hartl, C.; Konter, O.; Esper, J.; Popa, I. Climate signals in carbon and oxygen isotope ratios of Pinus cembra tree-ring cellulose from the Călimani Mountains, Romania. Int. J. Climatol. 2020, 40, 2539–2556. [Google Scholar] [CrossRef]
- Yang, F.; Wang, N.; Shi, F.; Ljungqvist, F.C.; Wang, S.; Fan, Z.; Lu, J. Multi-Proxy Temperature Reconstruction from the West Qinling Mountains, China, for the Past 500 Years. PLoS ONE 2013, 8, e57638. [Google Scholar] [CrossRef] [Green Version]
- McCarroll, D.; Loader, N.J. Stable isotopes in tree rings. Quat. Sci. Rev. 2004, 23, 771–801. [Google Scholar] [CrossRef]
- Coppola, A.; Leonelli, G.; Salvatore, M.C.; Pelfini, M.; Baroni, C. Weakening climatic signal since mid-20th century in European larch tree-ring chronologies at different altitudes from the Adamello-Presanella Massif (Italian Alps). Quat. Res. 2012, 77, 344–354. [Google Scholar] [CrossRef]
- Bale, R.; Robertson, I.; Leavitt, S.; Loader, N.J.; Harlan, T.P.; Gagen, M.; Young, G.H.F.; Csank, A.; Froyd, C.; McCarroll, D. Temporal stability in bristlecone pine tree-ring stable oxygen isotope chronologies over the last two centuries. Holocene 2010, 20, 3–6. [Google Scholar] [CrossRef]







| Species | Ring Type | Mean Age (Max) | AGR ± SD (mm) | MS | AC1 | rbar | EPS | %var | SNR |
|---|---|---|---|---|---|---|---|---|---|
| Ash | EW | 130 ± 17 (147) | 0.77 ± 0.20 | 0.17 | 0.531 | 0.275 | 0.831 | 33.9 | 4.93 |
| LW | 1.24 ± 0.91 | 0.49 | 0.557 | 0.533 | 0.926 | 58.1 | 12.54 | ||
| RW | 2.01 ± 0.98 | 0.31 | 0.578 | 0.572 | 0.946 | 61.1 | 17.41 | ||
| Oak | EW | 109 ± 18 (165) | 1.03 ± 0.37 | 0.21 | 0.590 | 0.159 | 0.773 | 21.8 | 3.41 |
| LW | 1.95 ± 1.39 | 0.39 | 0.706 | 0.415 | 0.923 | 46.7 | 12.06 | ||
| RW | 2.98 ± 1.61 | 0.25 | 0.762 | 0.422 | 0.929 | 47.1 | 13.14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roibu, C.-C.; Sfeclă, V.; Mursa, A.; Ionita, M.; Nagavciuc, V.; Chiriloaei, F.; Leșan, I.; Popa, I. The Climatic Response of Tree Ring Width Components of Ash (Fraxinus excelsior L.) and Common Oak (Quercus robur L.) from Eastern Europe. Forests 2020, 11, 600. https://doi.org/10.3390/f11050600
Roibu C-C, Sfeclă V, Mursa A, Ionita M, Nagavciuc V, Chiriloaei F, Leșan I, Popa I. The Climatic Response of Tree Ring Width Components of Ash (Fraxinus excelsior L.) and Common Oak (Quercus robur L.) from Eastern Europe. Forests. 2020; 11(5):600. https://doi.org/10.3390/f11050600
Chicago/Turabian StyleRoibu, Cătălin-Constantin, Victor Sfeclă, Andrei Mursa, Monica Ionita, Viorica Nagavciuc, Francisca Chiriloaei, Ilarie Leșan, and Ionel Popa. 2020. "The Climatic Response of Tree Ring Width Components of Ash (Fraxinus excelsior L.) and Common Oak (Quercus robur L.) from Eastern Europe" Forests 11, no. 5: 600. https://doi.org/10.3390/f11050600
APA StyleRoibu, C. -C., Sfeclă, V., Mursa, A., Ionita, M., Nagavciuc, V., Chiriloaei, F., Leșan, I., & Popa, I. (2020). The Climatic Response of Tree Ring Width Components of Ash (Fraxinus excelsior L.) and Common Oak (Quercus robur L.) from Eastern Europe. Forests, 11(5), 600. https://doi.org/10.3390/f11050600







