A New Clonal Propagation Protocol Develops Quality Root Systems in Chestnut
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Culture Conditions
2.2. Elongation
2.3. Rooting and Acclimatization
2.4. Statistical Analysis
3. Results
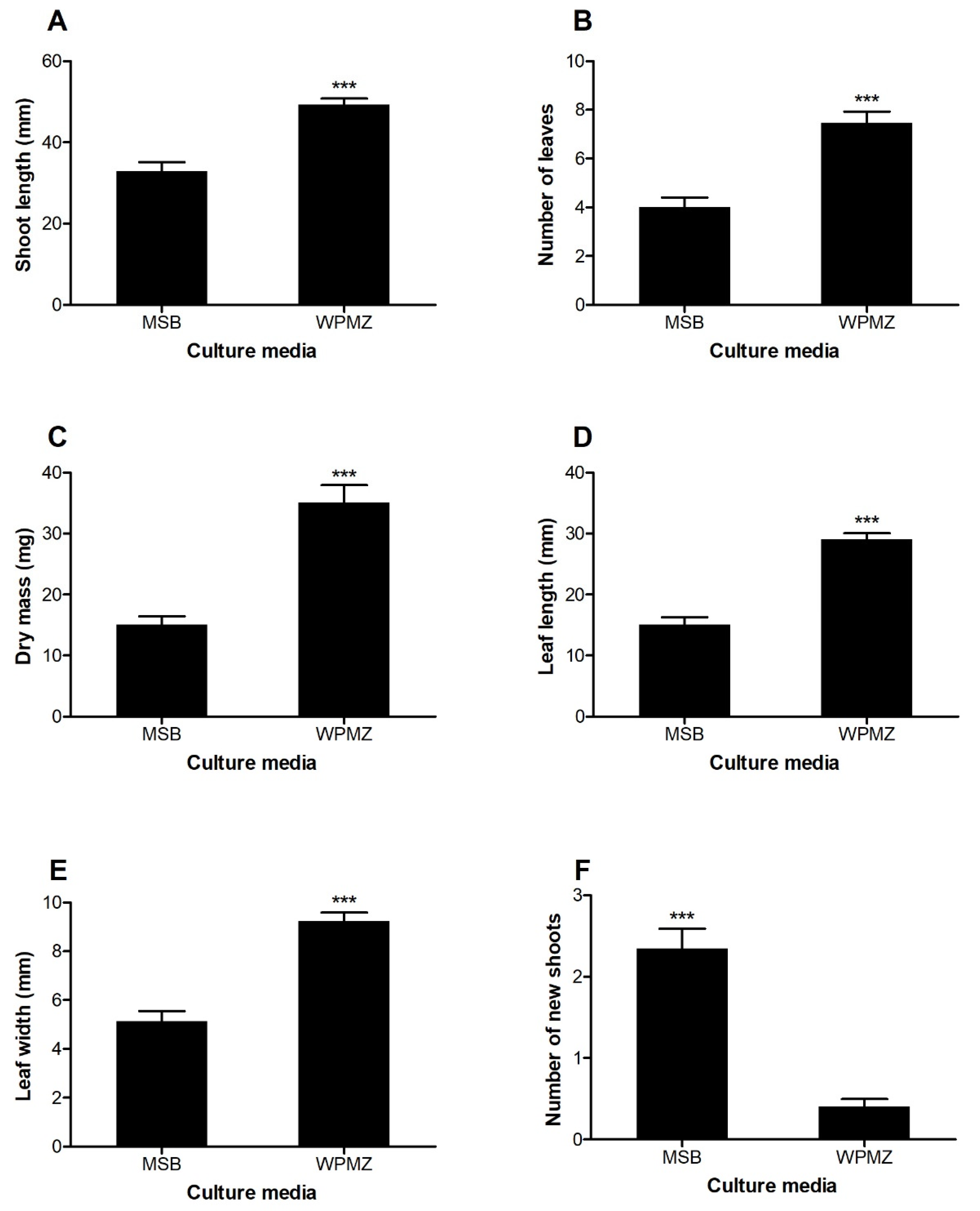
3.1. WPMZ Medium Leads to Improved Shoot Development
3.2. MMN Liquid Medium Induces High Rooting Rates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vieitez, J.; Kingston, D.G.; Ballester, A.; Vieitez, E. Identification of two compounds correlated with lack of rooting capacity of chestnut cuttings. Tree Physiol. 1987, 3, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Vieitez, E. The Lack of Rootability of Chestnut Cuttings. In Proceedings of the Proceedings International Chestnut Conference; Double, M.L., MacDonald, W.L., Eds.; West Virginia University Press: Morgantown, WV, USA, 1992; pp. 82–88. [Google Scholar]
- Pereira-Lorenzo, S.; Fernandez-Lopez, J. Propagation of chestnut cultivars by grafting: Methods, rootstocks and plant quality. J. Hortic. Sci. Biotechnol. 1997, 72, 731–739. [Google Scholar] [CrossRef]
- Hardham, A.R. Phytophthora cinnamomi. Mol. Plant Pathol. 2005, 6, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.C.; Diogo, G.; Amâncio, S. In vitro propagation of chestnut (Castanea sativa × C. crenata): Effects of rooting treatments on plant survival, peroxidase activity and anatomical changes during adventitious root formation. Sci. Hortic. (Amsterdam) 1998, 72, 265–275. [Google Scholar] [CrossRef]
- Breisch, H.; Boutitie, A.; Reyne, J.; Salesses, G.; Vaysse, P. Châtaignes et Marrons; FRA: Washington, DC, USA; CTIFL Centre Technique des Fruits et Légumes: Paris, France, 1995.
- Burgess, T.I.; Scott, J.K.; Mcdougall, K.L.; Stukely, M.J.C.; Crane, C.; Dunstan, W.A.; Brigg, F.; Andjic, V.; White, D.; Rudman, T.; et al. Current and projected global distribution of Phytophthora cinnamomi, one of the world’s worst plant pathogens. Glob. Chang. Biol. 2017, 23, 1661–1674. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Santos, C.; Tavares, F.; Machado, H.; Gomes-Laranjo, J.; Kubisiak, T.; Nelson, C. Mapping and transcriptomic approches implemented for understanding disease resistance to Phytophthora cinammomi in Castanea sp. BMC Proc. 2011, 5, O18. [Google Scholar] [CrossRef]
- Santos, C.; Machado, H.; Correia, I.; Gomes, F.; Gomes-Laranjo, J.; Costa, R. Phenotyping Castanea hybrids for Phytophthora cinnamomi resistance. Plant Pathol. 2015, 64, 901–910. [Google Scholar] [CrossRef]
- Sánchez, M.; Ballester, A.; Vieitez, A. Reinvigoration treatments for the micropropagation of mature chestnut trees. Ann. Sci. For. 1997, 54, 359–370. [Google Scholar] [CrossRef]
- Giovannelli, A.; Giannini, R. Reinvigoration of mature chestnut (Castanea sativa) by repeated graftings and micropropagation. Tree Physiol. 2000, 20, 1243–1248. [Google Scholar] [CrossRef]
- Ballester, A.; Bourrain, L.; Corredoira, E.; Gonçalves, J.C.; Lê, C.-L.; Miranda-Fontaíña, E.; San-josé, M.C.; Sauer, U.; Vieitez, A.M.; Wilhelm, E. Improving chestnut micropropagation through axillary shoot development and somatic embryogenesis. For. Snow Landsc. Res. 2001, 76, 460–467. [Google Scholar]
- Tetsumura, T.; Yamashita, K. Micropropagation of Japanese chestnut (Castanea crenata Sieb. et Zucc.) seedlings. HortScience 2004, 39, 1684–1687. [Google Scholar] [CrossRef]
- Oakes, A.; Desmarais, T.; Powell, W.A.; Maynard, C.A. Improving Rooting and Shoot Tip Survival of Micropropagated Transgenic American Chestnut Shoots. HortScience 2016, 51, 171–176. [Google Scholar] [CrossRef]
- Vieitez, A.M.; Sánchez, C.; San-José, C. Prevention of shoot-tip necrosis in shoot cultures of chestnut and oak. Sci. Hortic. (Amsterdam) 1989, 41, 151–159. [Google Scholar] [CrossRef]
- Gonçalves, J.C.; Amâncio, S.; Pereira, J.S. Rooting and acclimatization of chestnut by in vitro propagation. In Physiology, Growth and Development of Plants in Culture; Lumdsen, P.J., Nicholas, J.R., Davies, W.J., Eds.; Kluwer Acad. Pub.: Dordrecht, The Netherlands, 1994; pp. 303–308. [Google Scholar]
- Vieitez, E.; Vieitez, A. Juvenility factors related to the rootability of chestnut cuttings. Acta Hortic. 1976, 269–274. [Google Scholar] [CrossRef]
- Ballester, A.; San-José, M.C.; Vidal, N.; Fernández-Lorenzo, J.L.; Vieitez, A.M. Anatomical and Biochemical Events during in vitro Rooting of Microcuttings from Juvenile and Mature Phases of Chestnut. Ann. Bot. 1999, 83, 619–629. [Google Scholar] [CrossRef]
- Lê, C.-L. Factors influencing in vitro rooting of chestnut. For. Snow Landsc. Res. 2001, 76, 468–470. [Google Scholar]
- Oakes, A.D.; Powell, W.A.; Maynard, C.A. Doubling Acclimatization Survival of Micropropagated American Chestnuts with Darkness and Shortened Rooting Induction Time. J. Environ. Hort. 2013, 31, 77–83. [Google Scholar]
- Seabra, R.C.; Pais, M.S. Micropropagação de Clones de Castanheiro (Castanea sativa Mill.) Resistentes à doença da tinta. Silva Lusit. 1993, 1, 169–181. [Google Scholar]
- Santos, C.; Duarte, S.; Tedesco, S.; Fevereiro, P.; Costa, R.L. Expression profiling of Castanea genes during resistant and susceptible interactions with the oomycete pathogen Phytophthora cinnamomi reveal possible mechanisms of immunity. Front. Plant Sci. 2017, 8, 515. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially feasible micropropagation of mountain laurel, (Kalmia latifolia) by use of shoot tip culture. Comb. Proc. Int. Plant Propagators’ Soc. 1980, 30, 421–427. [Google Scholar]
- Marx, D.H. The influence of ectotrophic mycorrhizal fungi on the resistance of pine roots to pathogenic infections. II. Production, identification, and biological activity of antibiotics produced by Leucopaxillus cerealis var. piceina. Phytopathology 1969, 59, 411–417. [Google Scholar] [PubMed]
- Feijó, J.A.; Pais, M.S.S. Rejuvenation of Adult Specimens of Castanea Sativa Mill: Through In Vitro Micropropagation. In Plant Aging: Basic and Applied Approaches.; Rodrigues, R., Sánchez Tamés, R., Durzan, D., Eds.; Plenum Press: New York, NY, USA, 1990; pp. 383–387. [Google Scholar]
- Ballester, A.; Sanchez, M.C.; Vieitez, A.M. Etiolation as a pretreatment for in vitro establishment and multiplication of mature chestnut. Physiol. Plant. 1989, 77, 395–400. [Google Scholar] [CrossRef]
- Thomas, T.D. The role of activated charcoal in plant tissue culture. Biotechnol. Adv. 2008, 26, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Beyl, C.A. Getting Started with Tissue Culture: Media Preparation, Sterile Technique, and Laboratory Equipment. In Plant Propagation Concepts and Laboratory Exercises; Beyl, C.A., Trigiano, R.N., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 371–384. [Google Scholar]
- Woodward, S.; Thomson, R.J. Micropropagation of the silk tassel bush, Garrya elliptica Dougl. Plant Cell. Tissue Organ Cult. 1996, 44, 31–35. [Google Scholar] [CrossRef]
- Makunga, N.P.; Van Staden, J. An efficient system for the production of clonal plantlets of the medicinally important aromatic plant: Salvia africana-lutea L. Plant Cell. Tissue Organ Cult. 2008, 92, 63–72. [Google Scholar] [CrossRef]
- Sanchez, M.C.; San-jose, M.C.; Ferro, E.; Ballester, A.; Vieitez, A.M. Improving micropropagation conditions for adult- phase shoots of chestnut. J. Hortic. Sci. 1997, 72, 433–443. [Google Scholar] [CrossRef]
- Xing, Z.; Satchwell, M.F.; Powell, W.A.; Maynard, C.A. Micropropagation of american chestnut: Increasing rooting rate and preventing shoot—Tip necrosis. Vitr. Cell. Dev. Biol.-Plant 1997, 33, 43–48. [Google Scholar] [CrossRef]
- Cuenca, B.; Sánchez, C.; Aldrey, A.; Bogo, B.; Blanco, B.; Correa, B.; Vidal, N. Micropropagation of axillary shoots of hybrid chestnut (Castanea sativa × C. crenata) in liquid medium in a continuous immersion system. Plant Cell Tissue Organ Cult. 2017, 131, 307–320. [Google Scholar] [CrossRef]
- Jay-Allemand, C.; Capelli, P.; Cornu, D. Root development of in vitro hybrid walnut microcuttings in a vermiculite-containing gelrite medium. Sci. Hortic. (Amsterdam) 1992, 51, 335–342. [Google Scholar] [CrossRef]
- Newell, C.; Growns, D.; McComb, J. The influence of medium aeration on in vitro rooting of Australian plant microcuttings. Plant Cell. Tissue Organ Cult. 2003, 75, 131–142. [Google Scholar] [CrossRef]
- Corredoira, E.; Martínez, M.; Cernadas, M.; San José, M. Application of Biotechnology in the Conservation of the Genus Castanea. Forests 2017, 8, 394. [Google Scholar] [CrossRef]
- Vieitez, A.M.; Sänchez, M.C.; García-Nimo, M.L.; Ballester, A. Protocol for Micropropagation of Castanea sativa. In Protocols for Micropropagation of Woody Trees and Fruits; Springer: Dordrecht, The Netherlands, 2007; pp. 299–312. [Google Scholar]
- Carvalho, L.; Amâncio, S. Effect of ex vitro conditions on growth and acquisition of autotrophic behaviour during the acclimatisation of chestnut regenerated in vitro. Sci. Hortic. (Amsterdam) 2002, 95, 151–164. [Google Scholar] [CrossRef]
- Sáez, P.L.; Bravo, L.A.; Latsague, M.I.; Toneatti, M.J.; Coopman, R.E.; Álvarez, C.E.; Sánchez-Olate, M.; Ríos, D.G. Influence of in vitro growth conditions on the photosynthesis and survival of Castanea sativa plantlets during ex vitro transfer. Plant Growth Regul. 2015, 75, 625–639. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, P.; Tedesco, S.; Vieira da Silva, I.; Santos, C.; Machado, H.; Lourenço Costa, R. A New Clonal Propagation Protocol Develops Quality Root Systems in Chestnut. Forests 2020, 11, 826. https://doi.org/10.3390/f11080826
Fernandes P, Tedesco S, Vieira da Silva I, Santos C, Machado H, Lourenço Costa R. A New Clonal Propagation Protocol Develops Quality Root Systems in Chestnut. Forests. 2020; 11(8):826. https://doi.org/10.3390/f11080826
Chicago/Turabian StyleFernandes, Patrícia, Sara Tedesco, Inês Vieira da Silva, Carmen Santos, Helena Machado, and Rita Lourenço Costa. 2020. "A New Clonal Propagation Protocol Develops Quality Root Systems in Chestnut" Forests 11, no. 8: 826. https://doi.org/10.3390/f11080826
APA StyleFernandes, P., Tedesco, S., Vieira da Silva, I., Santos, C., Machado, H., & Lourenço Costa, R. (2020). A New Clonal Propagation Protocol Develops Quality Root Systems in Chestnut. Forests, 11(8), 826. https://doi.org/10.3390/f11080826







