Regular Biochar and Bacteria-Inoculated Biochar Alter the Composition of the Microbial Community in the Soil of a Chinese Fir Plantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Design
2.2. Soil Sampling
2.3. Analyses of Soil Properties
2.4. Soil Microbial Community
2.5. Statistical Analysis
3. Results
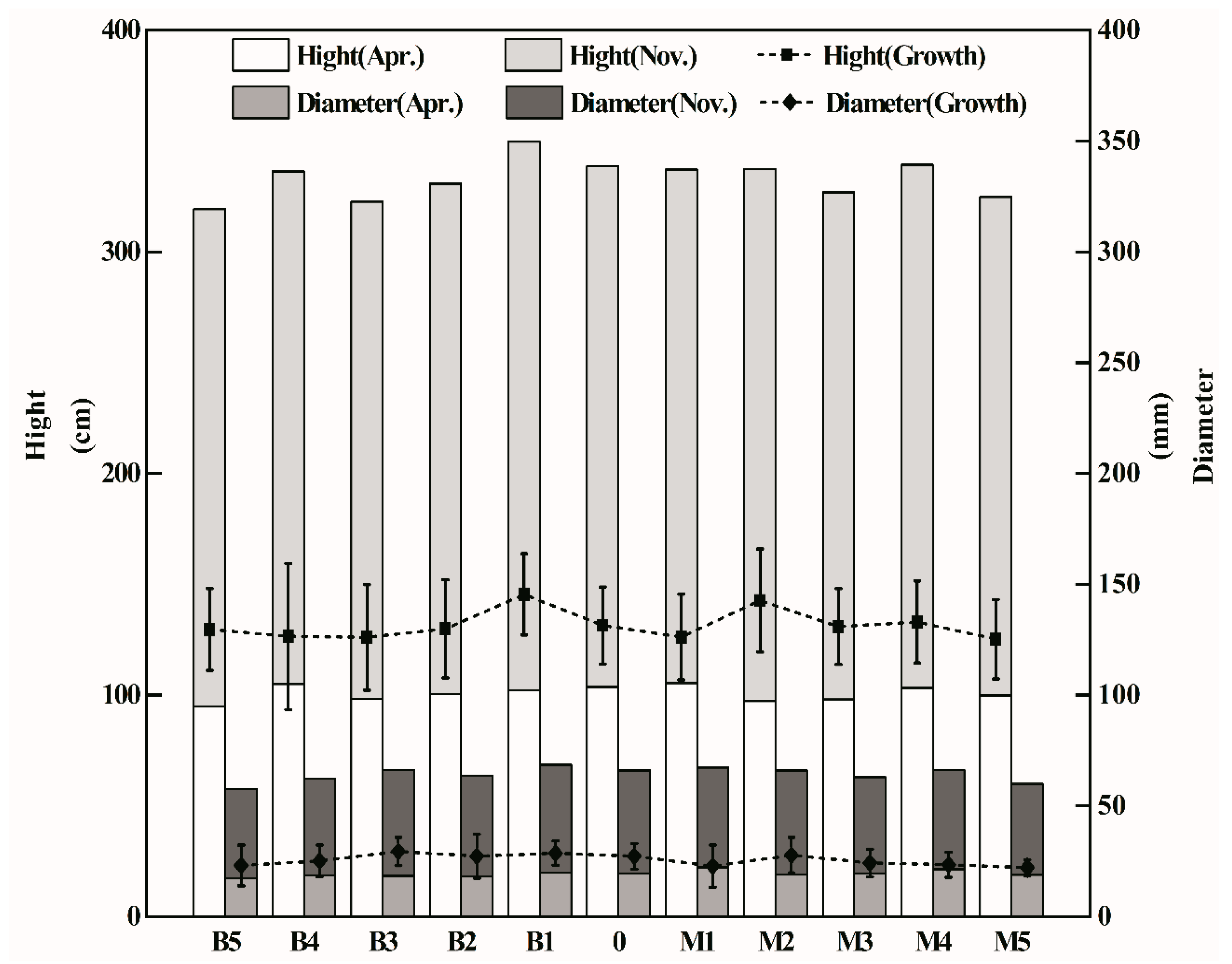
3.1. Soil Properties and Plant Growth
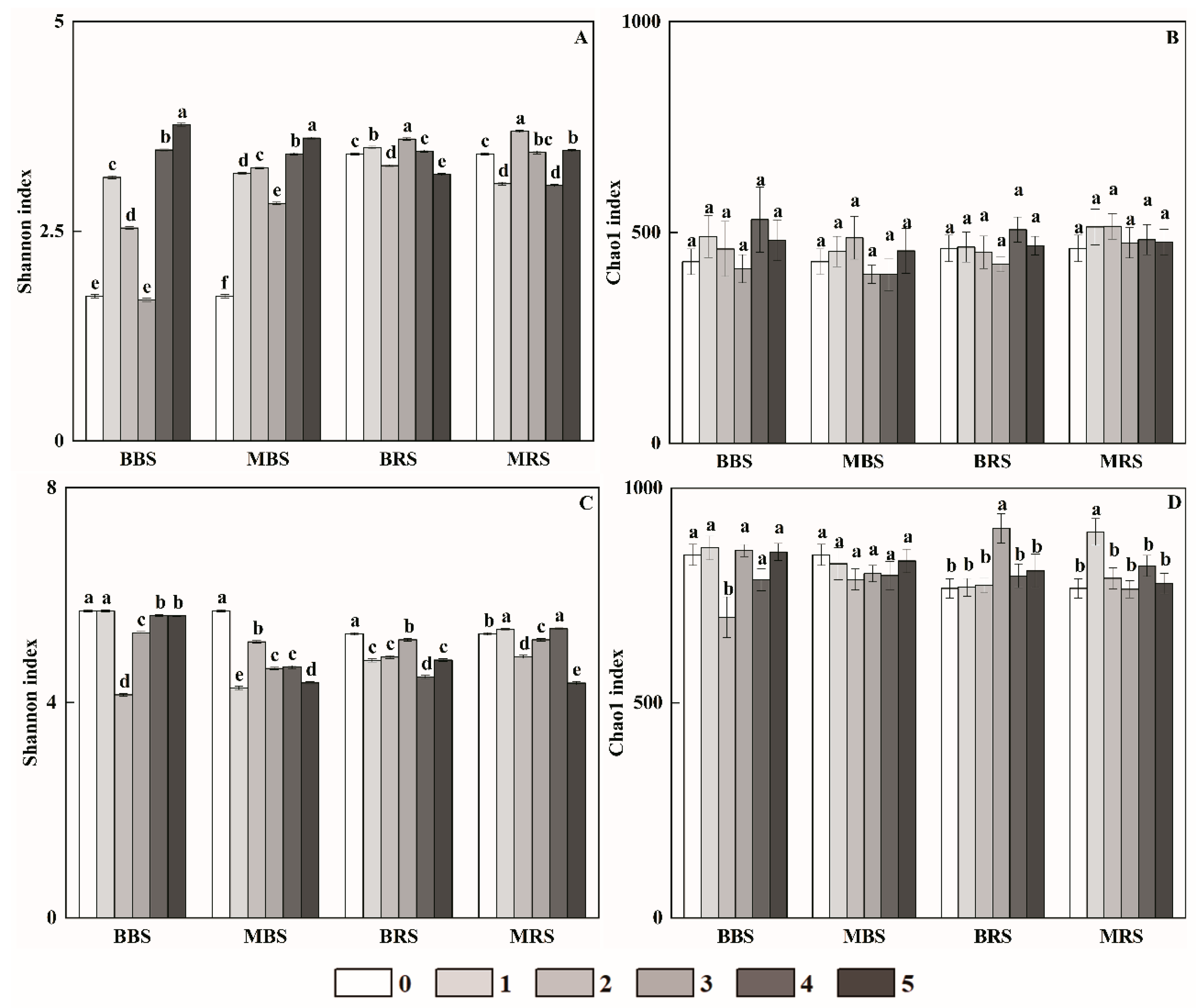
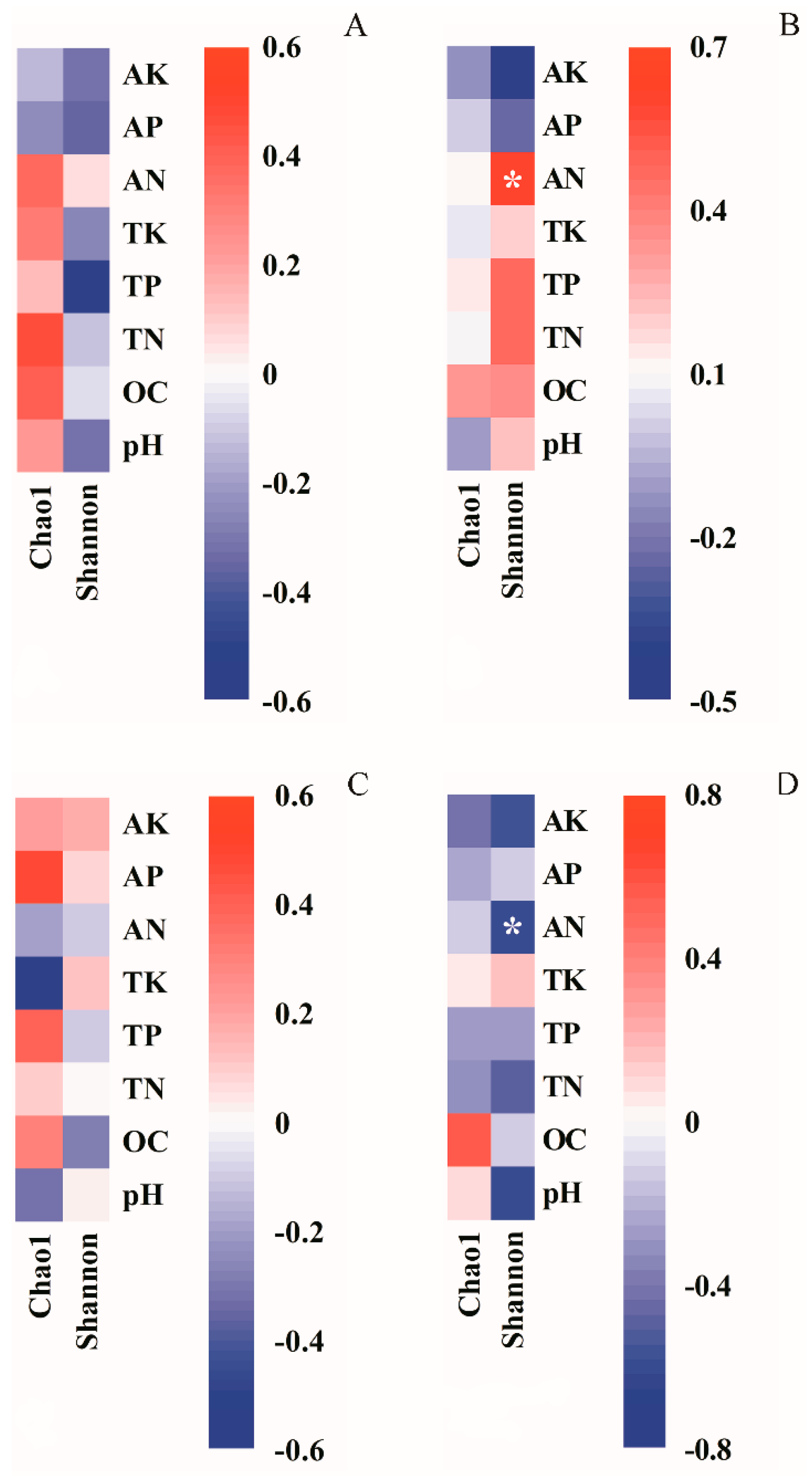
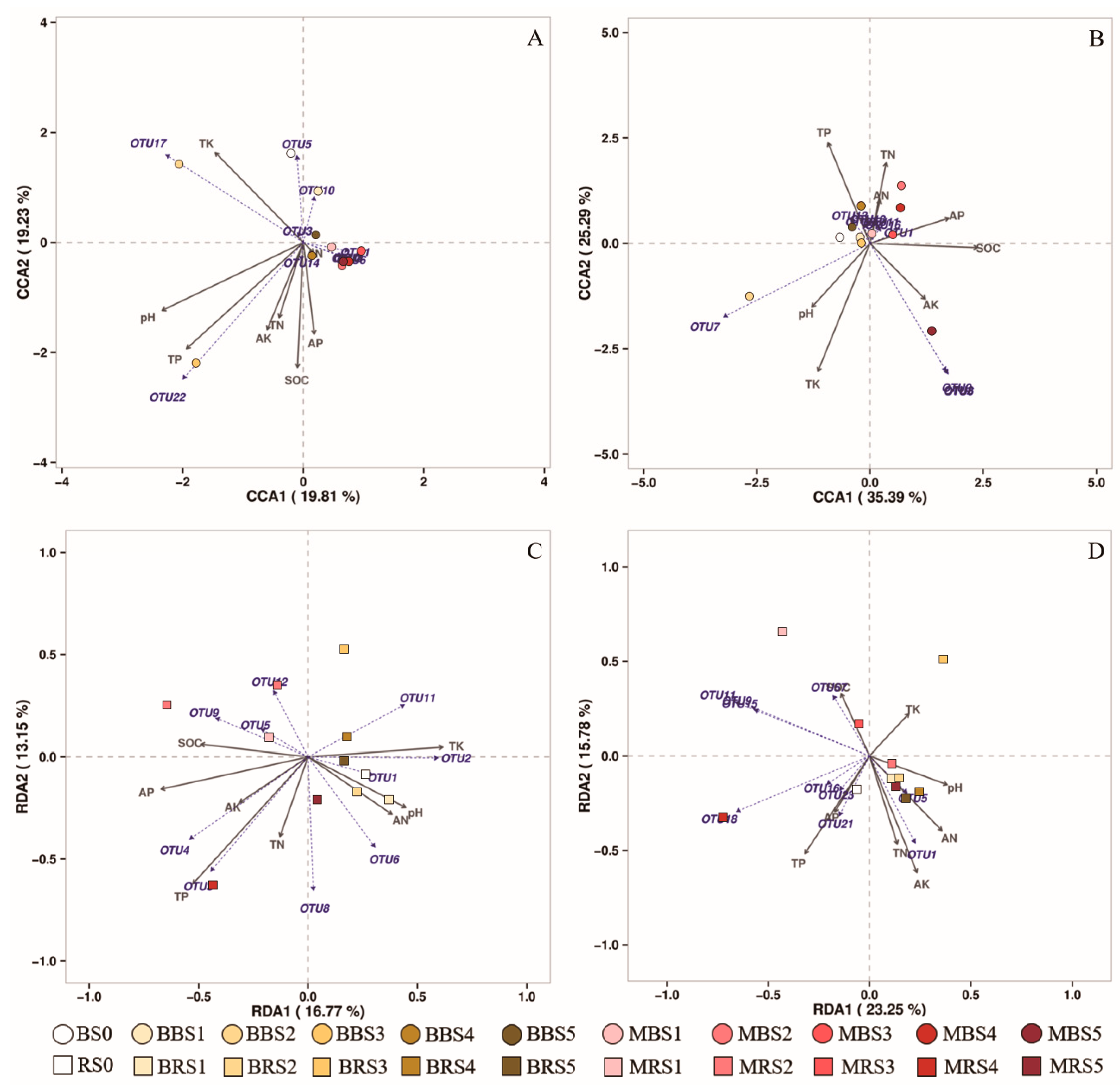
3.2. Soil Microbial Diversity and Its Relationships with Soil Properties
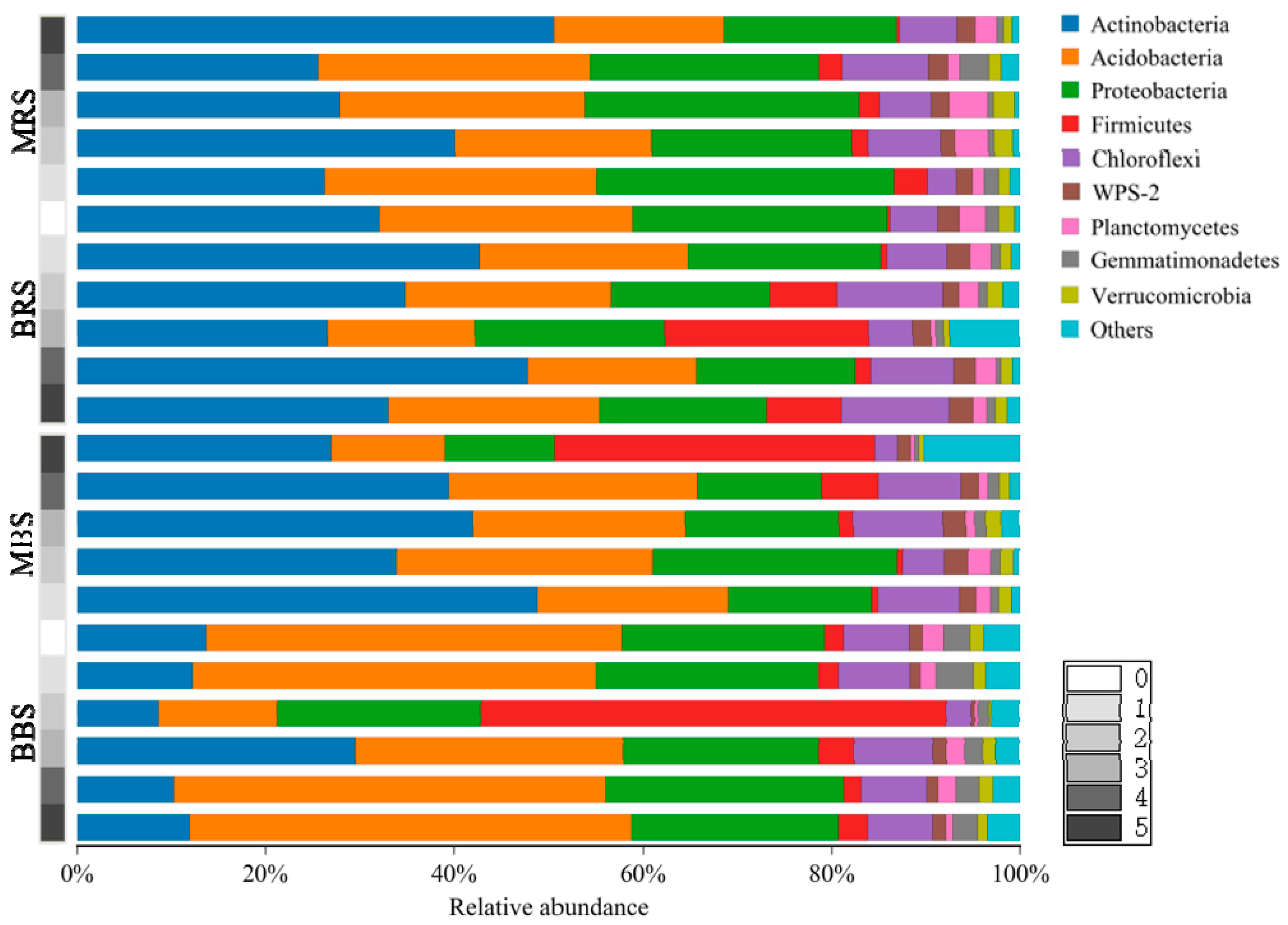
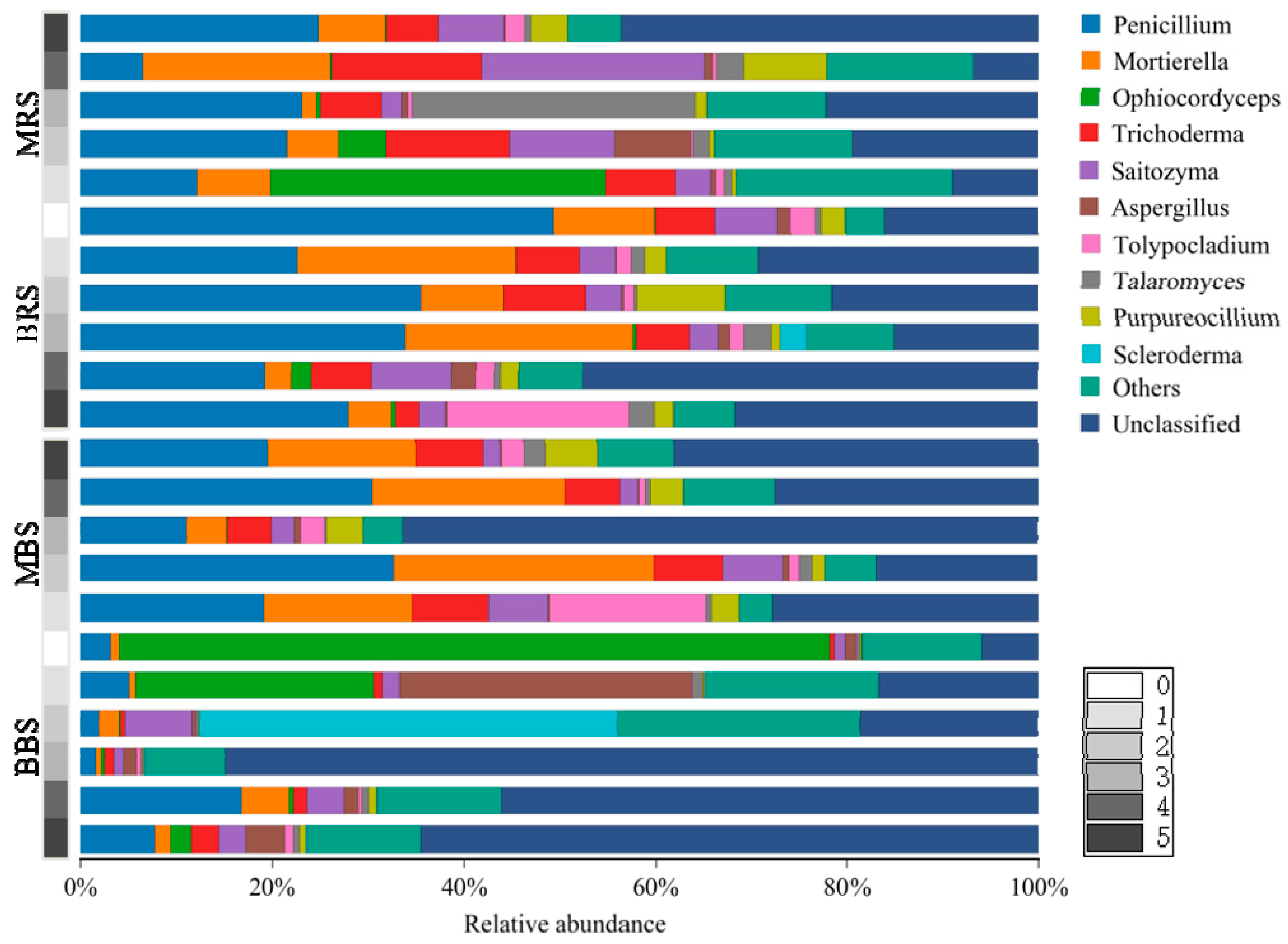
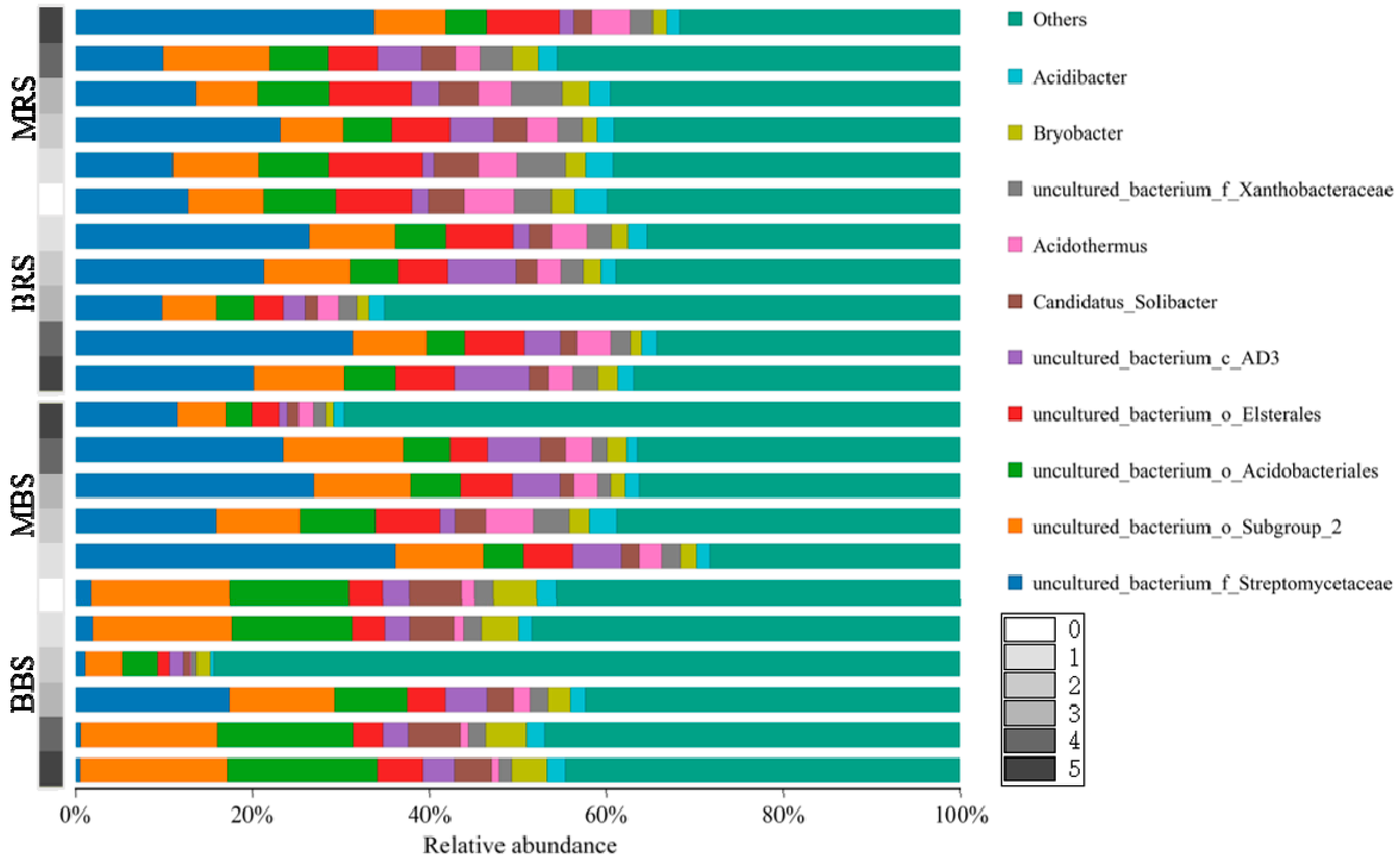
3.3. Taxonomic Classification and Relative Abundances of Fungi and Bacteria
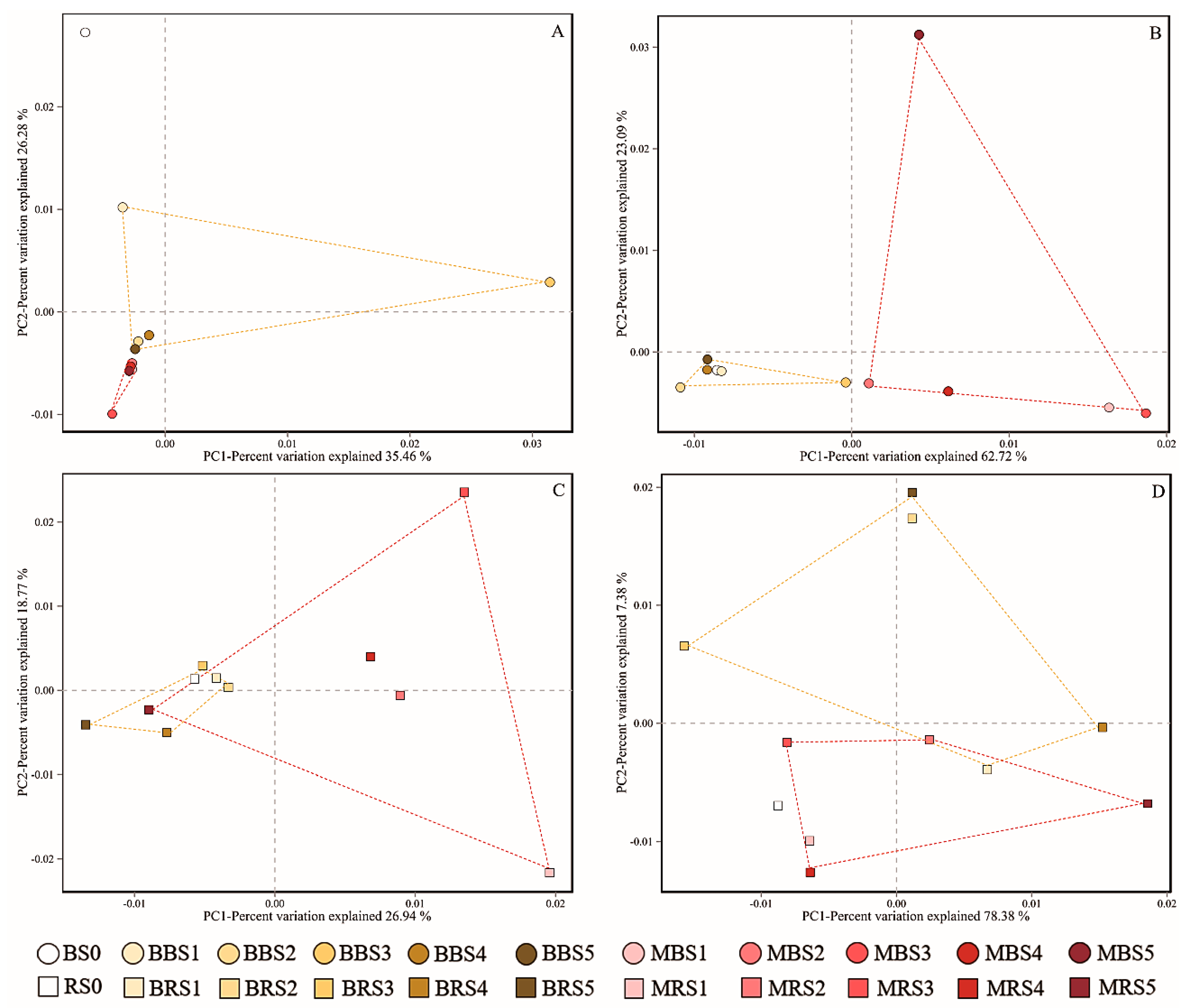
3.4. Soil Microbial Community Structure
4. Discussion
4.1. Effects of Biochar on Plant Growth and Soil Properties
4.2. Effects of Biochar on Soil Microbial Diversity
4.3. Effects of Biochar on Soil Fungal and Bacterial Community Structures
4.4. Differences Between Rhizosphere and Bulk Soils
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doran, J.W. Soil health and global sustainability: Translating science into practice. Agric. Ecosyst. Environ. 2002, 88, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.; Wang, B.; Liu, X.; Callaham, M.A.; Feng, G. Recovery of collembola in Pinus tabulaeformis plantations. Pedosphere 2017, 27, 129–137. [Google Scholar] [CrossRef]
- Tonks, A.J.; Aplin, P.; Beriro, D.J.; Cooper, H.V.; Evers, S.; Vane, C.H.; Sjogersten, S. Impacts of conversion of tropical peat swamp forest to oil palm plantation on peat organic chemistry, physical properties and carbon stocks. Geoderma 2017, 289, 36–45. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, S.; Liu, X.; Xiong, D.; Xu, C.; Arthur, M.A.; Mcculley, R.L.; Shi, S.; Yang, Y. Loss of soil organic carbon following natural forest conversion to Chinese fir plantation. For. Ecol. Manag. 2019, 449, 117476. [Google Scholar] [CrossRef]
- Burton, J.M.; Chen, C.; Xu, Z.; Ghadiri, H. Soil microbial biomass, activity and community composition in adjacent native and plantation forests of subtropical Australia. J. Soils Sediments 2010, 10, 1267–1277. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.E.; Masiello, C.A.; Hockaday, W.C.; Crowley, D.E. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Wang, C.; Xue, L.; Dong, Y.; Wei, Y.; Jiao, R. Unravelling the functional diversity of the soil microbial community of Chinese fir plantations of different densities. Forests 2018, 9, 532. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Hou, L.; Li, Z.; Zhao, D.; Song, L.; Shao, G.; Ai, J.; Sun, Q. Leguminous supplementation increases the resilience of soil microbial community and nutrients in Chinese fir plantations. Sci. Total Environ. 2020, 703, 134917. [Google Scholar] [CrossRef]
- Tang, Y.L.; Zhang, X.; Li, D.; Wang, H.; Chen, F.; Fu, X.; Fang, X.; Sun, X.; Yu, G. Impacts of nitrogen and phosphorus additions on the abundance and community structure of ammonia oxidizers and denitrifying bacteria in Chinese fir plantations. Soil Biol. Biochem. 2016, 103, 284–293. [Google Scholar] [CrossRef]
- Ma, X.; Heal, K.; Liu, A.; Jarvis, P.G. Nutrient cycling and distribution in different-aged plantations of Chinese fir in southern China. For. Ecol. Manag. 2007, 243, 61–74. [Google Scholar] [CrossRef]
- Gabriel, J.L.; Alonsoayuso, M.; Garciagonzalez, I.; Hontoria, C.; Quemada, M. Nitrogen use efficiency and fertiliser fate in a long-term experiment with winter cover crops. Eur. J. Agron. 2016, 79, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Selvaraj, S.; Duraisamy, V.; Huang, Z.; Guo, F.; Ma, X. Influence of long-term successive rotations and stand age of Chinese fir (Cunninghamia lanceolata) plantations on soil properties. Geoderma 2017, 306, 127–134. [Google Scholar] [CrossRef]
- Mann, C.; Lynch, D.; Fillmore, S.; Mills, A. Relationships between field management, soil health, and microbial community composition. Appl. Soil Ecol. 2019, 144, 12–21. [Google Scholar] [CrossRef]
- Sohi, S.P. Carbon storage with benefits. Science 2012, 338, 1034–1035. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2017, 447, 143–144. [Google Scholar] [CrossRef]
- Luo, Y.; Yu, Z.; Zhang, K.; Xu, J.; Brookes, P.C. The properties and functions of biochars in forest ecosystems. J. Soils Sediments 2016, 16, 2005–2020. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, M.; Nawaz, A.; Alsadi, A.M.; Solaiman, Z.M.; Alghamdi, S.S.; Ammara, U.; Ok, Y.S.; Siddique, K.H.M. Biochar for crop production: Potential benefits and risks. J. Soils Sediments 2017, 17, 685–716. [Google Scholar] [CrossRef]
- Liu, L.; Tan, Z.; Gong, H.; Huang, Q. Migration and Transformation Mechanisms of Nutrient Elements (N, P, K) within Biochar in Straw–Biochar–Soil–Plant Systems: A Review. ACS Sustain. Chem. Eng. 2019, 7, 22–32. [Google Scholar] [CrossRef]
- Ghysels, S.; Acosta, N.; Estrada, A.; Pala, M.; Rabaey, K. Integrating anaerobic digestion and slow pyrolysis improves the product portfolio of a cocoa waste biorefinery. Sustain. Energy Fuels 2020, 4, 3712–3725. [Google Scholar] [CrossRef]
- Li, Y.; Hu, S.; Chen, J.; Muller, K.; Li, Y.; Fu, W.; Lin, Z.; Wang, H. Effects of biochar application in forest ecosystems on soil properties and greenhouse gas emissions: A review. J. Soils Sediments 2018, 18, 546–563. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M.; Kammann, C.; Muller, C. A review of biochar and soil nitrogen dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, A.K.; Frenkel, O.; Elad, Y.; Lew, B.; Graber, E.R. Non-monotonic influence of biochar dose on bean seedling growth and susceptibility to Rhizoctonia solani: The “Shifted Rmax-Effect”. Plant Soil 2015, 395, 125–140. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Spokas, K.A.; Novak, J.M.; Lentz, R.D.; Cantrell, K.B. Biochar elemental composition and factors influencing nutrient retention. In Biochar for Envrionmental Management: Science, Technolody and Implementation, 2nd ed.; Lehmann, J., Joseph, S., Eds.; Routledge: Abingdon-on-Thames, UK, 2015; pp. 137–161. ISBN 978-0-415-70415-1. [Google Scholar]
- Wang, D.; Jiang, P.; Zhang, H.; Yuan, W. Biochar production and applications in agro and forestry systems: A review. Sci. Total Environ. 2020, 723, 137775. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.J.; Simpson, A.J.; Soong, R.; Simpson, M.J. Shifts in microbial community and water-extractable organic matter composition with biochar amendment in a temperate forest soil. Soil Biol. Biochem. 2015, 81, 244–254. [Google Scholar] [CrossRef]
- Mitchell, P.J.; Simpson, A.J.; Soong, R.; Schurman, J.S.; Thomas, S.C.; Simpson, M.J. Biochar amendment and phosphorus fertilization altered forest soil microbial community and native soil organic matter molecular composition. Biogeochemistry 2016, 130, 227–245. [Google Scholar] [CrossRef]
- Noyce, G.L.; Basiliko, N.; Fulthorpe, R.R.; Sackett, T.E.; Thomas, S.C. Soil microbial responses over 2 years following biochar addition to a north temperate forest. Biol. Fertil. Soils 2015, 51, 649–659. [Google Scholar] [CrossRef]
- Steinbeiss, S.; Gleixner, G.; Antonietti, M. Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol. Biochem. 2009, 41, 1301–1310. [Google Scholar] [CrossRef]
- Wu, B.; Wang, Z.; Zhao, Y.; Gu, Y.; Wang, Y.; Yu, J.; Xu, H. The performance of biochar-microbe multiple biochemical material on bioremediation and soil micro-ecology in the cadmium aged soil. Sci. Total Environ. 2019, 686, 719–728. [Google Scholar] [CrossRef]
- Maestrini, B.; Herrmann, A.M.; Nannipieri, P.; Schmidt, M.; Abiven, S. Ryegrass-derived pyrogenic organic matter changes organic carbon and nitrogen mineralization in a temperate forest soil. Soil Biol. Biochem. 2014, 69, 291–301. [Google Scholar] [CrossRef]
- Wang, S.; Ai, S.; Nzediegwu, C.; Kwak, J.-H.; Islam, M.S.; Li, Y.; Chang, S.X. Carboxyl and hydroxyl groups enhance ammonium adsorption capacity of iron (III) chloride and hydrochloric acid modified biochars. Bioresour. Technol. 2020, 309, 123390. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; Tang, L.; Su, M.; Tian, D.; Zhang, L.; Li, Z.; Hu, S. Enhanced Pb immobilization via the combination of biochar and phosphate solubilizing bacteria. Environ. Int. 2019, 127, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.; Diazalcantara, C.; Urbano, B.; Gonzalezandres, F. Inoculation with native Bradyrhizobium strains formulated with biochar as carrier improves the performance of pigeonpea (Cajanus cajan L.). Eur. J. Agron. 2020, 113, 125985. [Google Scholar] [CrossRef]
- Scheer, C.; Grace, P.; Rowlings, D.W.; Kimber, S.; van Zwieten, L. Effect of biochar amendment on the soil-atmosphere exchange of greenhouse gases from an intensive subtropical pasture in northern New South Wales, Australia. Plant Soil 2011, 345, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Slavich, P.G.; Sinclair, K.; Morris, S.; Kimber, S.; Downie, A.; van Zwieten, L. Contrasting effects of manure and green waste biochars on the properties of an acidic ferralsol and productivity of a subtropical pasture. Plant Soil 2013, 366, 213–227. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Ok, Y.S.; Awad, Y.M.; Lee, S.S.; Sung, J.K.; Koutsospyros, A.; Moon, D.H. Impacts of biochar application on upland agriculture: A review. J. Environ. Manag. 2019, 234, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Elmageed, T.A.A.; Rady, M.M.; Taha, R.S.; Azeam, S.A.E.; Simpson, C.R.; Semida, W.M. Effects of integrated use of residual sulfur-enhanced biochar with effective microorganisms on soil properties, plant growth and short-term productivity of Capsicum annuum under salt stress. Sci. Hortic. 2020, 261, 108930. [Google Scholar] [CrossRef]
- Lefroy, R.D.; Blair, G.J.; Strong, W.M. Changes in soil organic matter with cropping as measured by organic carbon fractions and 13C natural isotope abundance. Plant Soil 1993, 155, 399–402. [Google Scholar] [CrossRef]
- Kjeldahl, J. Neue methode zur bestimmung des stickstoffs in organischen Körpern. Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef] [Green Version]
- Institute of Soil Science, Chinese Academy Science (ISSCAS). Physical and Chemical Analysis Methods of Soils; Shanghai Science Technology Press: Shanghai, China, 1978; pp. 7–59. [Google Scholar]
- Wang, C.; Xue, L.; Dong, Y.; Hou, L.; Wei, Y.; Chen, J.; Jiao, R. Contrasting effects of Chinese fir plantations of different stand ages on soil enzyme activities and microbial communities. Forests 2018, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Menšík, L.; Hlisnikovský, L.; Pospíšilová, L.; Kunzová, E. The effect of application of organic manures and mineral fertilizers on the state of soil organic matter and nutrients in the long-term field experiment. J. Soils Sediments 2018, 18, 2813–2822. [Google Scholar] [CrossRef]
- Myrvang, M.B.; Hillersoy, M.H.; Heim, M.; Bleken, M.A.; Gjengedal, E. Uptake of macro nutrients, barium, and strontium by vegetation from mineral soils on carbonatite and pyroxenite bedrock at the Lillebukt Alkaline Complex on Stjernøy, Northern Norway. J. Plant Nutr. Soil Sci. 2016, 179, 705–716. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; Desantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Liu, J.; Yu, Z.; Li, Y.; Jin, J.; Liu, X.; Wang, G. Three years of biochar amendment alters soil physiochemical properties and fungal community composition in a black soil of northeast China. Soil Biol. Biochem. 2017, 110, 56–67. [Google Scholar] [CrossRef]
- Lehmann, J.; Silva Junior, J.; Rondon, M.; Silva, C.; Greenwood, J.; Nehls, T.; Steiner, C.; Glaser, B. Slash and Char: A feasible alternative for soil fertility management in the Central Amazon? In Proceedings of the 17th World Congress of Soil Science, Bangkok, Thailand, 14–21 August 2002; pp. 1–12. [Google Scholar]
- Al-Wabel, M.I.; Hussain, Q.; Usman, A.R.A.; Ahmad, M.; Abduljabbar, A.; Sallam, A.S.; Ok, Y.S. Impact of biochar properties on soil conditions and agricultural sustainability: A review. Land Degrad. Dev. 2018, 29, 2124–2161. [Google Scholar] [CrossRef]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.A.; Lima, I.M.; Lamb, M.C.; McAloon, A.J.; et al. Biochar: A synthesis of its agronomic impact beyond carbon sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef]
- Prendergast miller, M.T.; Duvall, M.; Sohi, S. Biochar—Root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur. J. Soil Sci. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Wang, S.; Xing, G. Successive straw biochar application as a strategy to sequester carbon and improve fertility: A pot experiment with two rice/wheat rotations in paddy soil. Plant Soil 2014, 378, 279–294. [Google Scholar] [CrossRef]
- Kloss, S.; Zehetner, F.; Wimmer, B.; Buecker, J.; Rempt, F.; Soja, G. Biochar application to temperate soils: Effects on soil fertility and crop growth under greenhouse conditions. J. Plant Nutr. Soil Sci. 2014, 177, 3–15. [Google Scholar] [CrossRef]
- Nutman, P.S. Host factors influencing infection and nodule development in leguminous plants. Proceedings of the Royal Society of London. Ser. B Biol. Sci. 1952, 139, 176–185. [Google Scholar] [CrossRef]
- Makoto, K.; Tamai, Y.; Kim, Y.S.; Koike, T. Buried charcoal layer and ectomycorrhizae cooperatively promote the growth of Larix gmelinii seedlings. Plant Soil 2010, 327, 143–152. [Google Scholar] [CrossRef]
- Noguera, D.; Rondón, M.; Laossi, K.-R.; Hoyos, V.; Lavelle, P.; Cruz de Carvalho, M.H.; Barot, S. Contrasted effect of biochar and earthworms on rice growth and resource allocation in different soils. Soil Biol. Biochem. 2010, 42, 1017–1027. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, J.; da Silva, J.P.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Joseph, S.D.; Camps-Arbestain, M.; Lin, Y.; Munroe, P.; Chia, C.H.; Hook, J.; van Zwieten, L.; Kimber, S.; Cowie, A.; Singh, B.P.; et al. An investigation into the reactions of biochar in soil. Soil Res. 2010, 48, 501–515. [Google Scholar] [CrossRef]
- D’Hose, T.; Debode, J.; Tender, C.; Ruysschaert, G.; Vandecasteele, B. Has compost with biochar applied during the process added value over biochar or compost for increasing soil quality in an arable cropping system? Appl. Soil Ecol. 2020, 156, 103706. [Google Scholar] [CrossRef]
- Ameloot, N.; De Neve, S.; Jegajeevagan, K.; Yildiz, G.; Buchan, D.; Funkuin, Y.N.; Prins, W.; Bouckaert, L.; Sleutel, S. Short-term CO2 and N2O emissions and microbial properties of biochar amended sandy loam soils. Soil Biol. Biochem. 2013, 57, 401–410. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Zheng, J.; Zhang, B.; Lu, H.; Chi, Z.; Pan, G.; Li, L.; Zheng, J.; Zhang, X. Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl. Soil Ecol. 2013, 71, 33–44. [Google Scholar] [CrossRef]
- Lucheta, A.R.; Cannavan, F.d.S.; Roesch, L.F.W.; Tsai, S.M.; Kuramae, E.E. Fungal Community Assembly in the Amazonian Dark Earth. Microb. Ecol. 2016, 71, 962–973. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, Z.; Yang, K.; Wang, P.; Wang, H.; Guo, L.; Zhu, S.; Zhu, Y.; He, X. Biochar Application Alleviated Negative Plant-Soil Feedback by Modifying Soil Microbiome. Front. Microbiol. 2020, 11, 799. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Chang, S.X.; Liang, X.; Qin, H.; Chen, J.; Xu, Q. Linking soil fungal community structure and function to soil organic carbon chemical composition in intensively managed subtropical bamboo forests. Soil Biol. Biochem. 2017, 107, 19–31. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.; Liang, C.; Xu, Q.; Li, Y.; Qin, H.; Fuhrmann, J.J. Response of microbial community structure and function to short-term biochar amendment in an intensively managed bamboo (Phyllostachys praecox) plantation soil: Effect of particle size and addition rate. Sci. Total Environ. 2017, 574, 24–33. [Google Scholar] [CrossRef]
- Fontaine, S.; Henault, C.; Aamor, A.; Bdioui, N.; Bloor, J.M.G.; Maire, V.; Mary, B.; Revaillot, S.; Maron, P. Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol. Biochem. 2011, 43, 86–96. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, Q.; Durenkamp, M.; Dungait, A.J.; Brookes, P.C. Soil priming effects following substrates addition to biochar-treated soils after 431 days of pre-incubation. Biol. Fertil. Soils 2017, 53, 315–326. [Google Scholar] [CrossRef]
- Borrell, A.N.; Shi, Y.; Gan, Y.; Bainard, L.D.; Germida, J.J.; Hamel, C. Fungal diversity associated with pulses and its influence on the subsequent wheat crop in the Canadian prairies. Plant Soil 2017, 414, 13–31. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Bååth, E. Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef] [Green Version]

| Sample | pH | TC (%) | TN (g·kg−1) | TP (g·kg−1) | TK (g·kg−1) | AN (mg·kg−1) | AP (mg·kg−1) | AK (mg·kg−1) |
|---|---|---|---|---|---|---|---|---|
| B | 9.69 ± 0.02 | 82.10 ± 1.01 | 6.15 ± 0.06 | 1.23 ± 0.00 | 14.10 ± 0.01 | 0.00 ± 0.00 | 133.13 ± 1.78 | 8656.00 ± 11.00 |
| M | 8.21 ± 0.01 | 78.70 ± 0.26 | 9.14 ± 0.18 | 3.67 ± 0.00 | 12.72 ± 0.00 | 715.58 ± 5.12 | 108.43 ± 0.12 | 5623.33 ± 24.50 |
| Sample | pH | OC (g·kg−1) | TN (g·kg−1) | TP (g·kg−1) | TK (g·kg−1) | AN (mg·kg−1) | AP (mg·kg−1) | AK (mg·kg−1) | |
|---|---|---|---|---|---|---|---|---|---|
| B B S | 0 | 4.82 ± 0.35a | 29.49 ± 9.48a | 1.75 ± 0.16b | 0.19 ± 0.01ab | 20.43 ± 0.93a | 131.83 ± 41.46a | 13.72 ± 1.55a | 153.52 ± 39.48ab |
| 1 | 4.71 ± 0.23a | 33.79 ± 3.75a | 1.85 ± 0.15ab | 0.19 ± 0.01ab | 21.05 ± 1.81a | 119.00 ± 43.71a | 13.11 ± 2.95a | 159.03 ± 25.05ab | |
| 2 | 4.91 ± 0.30a | 27.27 ± 3.95a | 1.57 ± 0.34b | 0.18 ± 0.02ab | 20.07 ± 2.63a | 112.00 ± 29.90a | 12.18 ± 4.16a | 168.80 ± 60.90ab | |
| 3 | 5.14 ± 0.38a | 36.06 ± 6.91a | 1.95 ± 0.32ab | 0.22 ± 0.03a | 19.22 ± 2.43a | 122.85 ± 42.14a | 15.19 ± 7.63a | 199.07 ± 108.78a | |
| 4 | 5.24 ± 0.60a | 35.95 ± 4.63a | 2.28 ± 0.38a | 0.20 ± 0.05ab | 19.63 ± 3.59a | 157.50 ± 52.96a | 13.61 ± 5.02a | 171.13 ± 45.19ab | |
| 5 | 4.70 ± 0.09a | 27.90 ± 0.87a | 1.49 ± 0.05b | 0.17 ± 0.00b | 17.78 ± 2.23a | 127.17 ± 14.57a | 8.72 ± 1.26a | 94.03 ± 32.49b | |
| M B S | 0 | 4.82 ± 0.35a | 29.49 ± 9.48a | 1.75 ± 0.16a | 0.19 ± 0.01a | 20.43 ± 0.93a | 131.83 ± 41.46a | 13.72 ± 1.55a | 153.52 ± 39.48a |
| 1 | 4.64 ± 0.23a | 31.04 ± 7.78a | 1.74 ± 0.48a | 0.18 ± 0.04a | 18.66 ± 1.18a | 102.67 ± 49.04a | 15.70 ± 8.31a | 210.40 ± 111.75a | |
| 2 | 4.63 ± 0.18a | 33.94 ± 4.14a | 1.81 ± 0.30a | 0.20 ± 0.03a | 18.56 ± 1.66a | 114.33 ± 31.76a | 14.78 ± 4.80a | 177.99 ± 55.08a | |
| 3 | 4.73 ± 0.36a | 29.46 ± 4.15a | 1.63 ± 0.24a | 0.17 ± 0.01a | 17.30 ± 2.20a | 121.33 ± 10.69a | 13.24 ± 3.38a | 172.47 ± 75.62a | |
| 4 | 4.64 ± 0.31a | 27.74 ± 3.67a | 1.63 ± 0.06a | 0.18 ± 0.00a | 18.38 ± 2.57a | 120.17 ± 21.10a | 14.10 ± 6.02a | 148.63 ± 25.61a | |
| 5 | 4.88 ± 0.28a | 33.52 ± 9.37a | 1.65 ± 0.43a | 0.17 ± 0.02a | 20.29 ± 4.34a | 122.50 ± 53.88a | 13.26 ± 1.33a | 182.23 ± 79.20a | |
| B R S | 0 | 4.70 ± 0.17a | 24.01 ± 9.80a | 1.67 ± 0.41a | 0.18 ± 0.02a | 19.94 ± 2.47a | 148.28 ± 62.05a | 12.63 ± 2.94a | 138.03 ± 9.23a |
| 1 | 5.07 ± 0.23a | 25.09 ± 10.45a | 1.54 ± 0.22a | 0.18 ± 0.02a | 20.52 ± 1.97a | 136.50 ± 18.52a | 10.15 ± 0.58a | 121.23 ± 19.66a | |
| 2 | 5.09 ± 0.57a | 32.59 ± 3.25a | 1.89 ± 0.40a | 0.19 ± 0.03a | 20.89 ± 2.31a | 183.17 ± 93.02a | 11.96 ± 2.49a | 181.88 ± 87.78a | |
| 3 | 5.02 ± 0.38a | 33.24 ± 4.66a | 1.55 ± 0.19a | 0.17 ± 0.01a | 20.20 ± 3.05a | 151.67 ± 40.57a | 10.48 ± 1.10a | 132.07 ± 10.10a | |
| 4 | 5.02 ± 0.32a | 30.90 ± 1.60a | 1.63 ± 0.25a | 0.18 ± 0.02a | 19.24 ± 3.83a | 172.67 ± 33.63a | 11.18 ± 3.48a | 167.40 ± 50.08a | |
| 5 | 4.92 ± 0.07a | 33.16 ± 6.14a | 1.72 ± 0.16a | 0.18 ± 0.01a | 17.60 ± 4.11a | 161.00 ± 21.29a | 12.37 ± 2.53a | 170.43 ± 35.31a | |
| M R S | 0 | 4.70 ± 0.17b | 24.01 ± 9.80a | 1.67 ± 0.41a | 0.18 ± 0.02a | 19.94 ± 2.47a | 148.28 ± 62.05a | 12.63 ± 2.94a | 138.03 ± 9.23ab |
| 1 | 4.84 ± 0.16ab | 36.77 ± 15.27a | 1.58 ± 0.25a | 0.18 ± 0.01a | 19.19 ± 1.05a | 138.83 ± 10.69a | 11.94 ± 0.74a | 110.50 ± 7.25b | |
| 2 | 4.80 ± 0.29ab | 33.91 ± 6.79a | 1.82 ± 0.25a | 0.20 ± 0.02a | 17.85 ± 0.63a | 138.83 ± 59.64a | 15.23 ± 2.04a | 196.57 ± 99.58a | |
| 3 | 4.71 ± 0.19b | 28.78 ± 9.78a | 1.45 ± 0.43a | 0.17 ± 0.03a | 18.93 ± 1.45a | 129.50 ± 71.47a | 11.55 ± 3.76a | 132.43 ± 16.49ab | |
| 4 | 4.91 ± 0.10ab | 33.21 ± 8.17a | 1.68 ± 0.45a | 0.20 ± 0.03a | 18.49 ± 1.60a | 149.33 ± 28.07a | 12.69 ± 2.52a | 163.50 ± 49.51ab | |
| 5 | 5.05 ± 0.16a | 35.74 ± 5.49a | 1.92 ± 0.52a | 0.20 ± 0.02a | 18.20 ± 2.36a | 175.00 ± 35.52a | 13.87 ± 0.58a | 182.83 ± 34.08ab | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Hou, L.; Zhang, Y.; Li, Z.; Wang, W.; Sun, Q. Regular Biochar and Bacteria-Inoculated Biochar Alter the Composition of the Microbial Community in the Soil of a Chinese Fir Plantation. Forests 2020, 11, 951. https://doi.org/10.3390/f11090951
Song L, Hou L, Zhang Y, Li Z, Wang W, Sun Q. Regular Biochar and Bacteria-Inoculated Biochar Alter the Composition of the Microbial Community in the Soil of a Chinese Fir Plantation. Forests. 2020; 11(9):951. https://doi.org/10.3390/f11090951
Chicago/Turabian StyleSong, Liguo, Lingyu Hou, Yongqiang Zhang, Zhichao Li, Wenzheng Wang, and Qiwu Sun. 2020. "Regular Biochar and Bacteria-Inoculated Biochar Alter the Composition of the Microbial Community in the Soil of a Chinese Fir Plantation" Forests 11, no. 9: 951. https://doi.org/10.3390/f11090951





