Discrimination and Determination of Extractive Content of Ebony (Diospyros celebica Bakh.) from Celebes Island by Near-Infrared Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
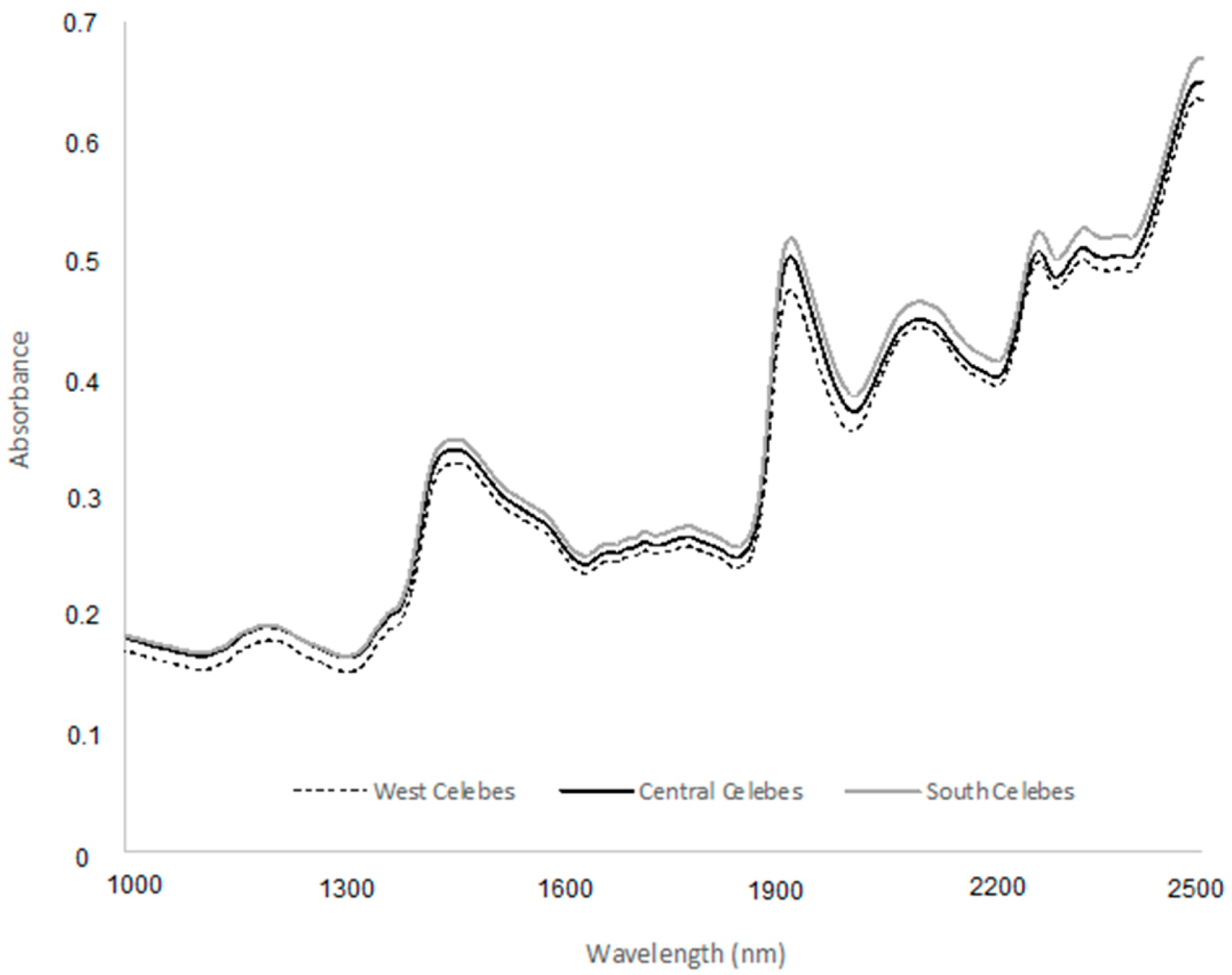
2.2. NIR Spectra Acquisition
2.3. Wet Chemical Laboratory Methods
2.4. Data Analysis
3. Results
3.1. Spectroscopic Characterization
3.2. Wet Chemical Analysis
3.3. Chemometric Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Noda, E.; Aoki, T.; Minato, K. Physical and chemical characteristics of the blackened portion of Japanese persimmon (Diospyros kaki). J. Wood Sci. 2002, 48, 245–249. [Google Scholar] [CrossRef]
- Whitmore, T.C.; Tantra, I.G.M.; Sutisna, U. Tree Flora of Indonesia: Check List for Sulawesi; Ministry of Forestry, Agency for Forestry Research and Development: Jakarta, Indonesia; Forest Research and Development Centre: Jakarta, Indonesia, 1989. [Google Scholar]
- Lemmens, R.H.M.J.; Soerianegara, I.; Wong, W.C. (Eds.) Plant Resources of South-East Asia. No. 5(2): Timber Trees: Minor Commercial Timbers; Backhuys: Leiden, The Netherlands, 1995; ISBN 978-90-73348-44-8. [Google Scholar]
- Martawijaya, A.; Kartasujana, I.; Kadir, K.; Prawira, S.A. Atlas Kayu Indonesia Jilid I; Pusat Penelitian dan Pengembangan Hasil Hutan: Bogor, Indonesia, 2015. [Google Scholar]
- Asdar, M.; Prayitno, T.A.; Lukmandaru, G.; Faridah, E. Chemical properties of Diospyros celebica Bakh. in different streaks pattern and radial direction. J. Ilmu Teknol. Kayu Tropis 2016, 14, 165–174. [Google Scholar]
- Prajadinata, S.; Murniati; Effendi, R. Review of Management and Conservation Status of Ulin (Eusideroxylon Zwageri Teijsm & Binn.), Ebony (Diospyros celebica Bakh.), and Cempaka (Michelia champaca Linn.) in Indonesia; ITTO Project PD 539/09 Rev. 1 (F) in Cooperation with Center for Conservation and Rehabilitation Research and Development, Forestry Research and Development Agency; Ministry of Forestry: Bogor, Indonesia, 2011; ISBN 978-602-8964-18-0.
- Centre, W.C.M. IUCN Red List of Threatened Species: Diospyros celebica. Available online: https://www.iucnredlist.org/en (accessed on 2 December 2020).
- Rahayu, Y.C. Radial Variation Durability of Eboni (Diospyros celebica Bakh.) Wood against Drywood Termites and Fungal Decay Schizophyllum commune. Bachelor’s Thesis, Institut Pertanian Bogor (IPB University), Bogor, Indonesia, 2018. [Google Scholar]
- Sharma, V. Brief review on the genus Diospyros: A rich source of Naphthoquinones. Asian J. Adv. Basic Sci. 2017, 5, 34–53. [Google Scholar]
- Maiti, B.C.; Musgrave, O.C. Ebenaceae extractives. Part 9. New naphthoquinones and binaphthylquinones from macassar ebony. J. Chem. Soc. Perkin Trans. 1986, 1, 675–681. [Google Scholar] [CrossRef]
- Li, Y.; Altaner, C.M. Effects of variable selection and processing of NIR and ATR-IR spectra on the prediction of extractive content in Eucalyptus bosistoana heartwood. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Snel, F.A.; Braga, J.W.B.; da Silva, D.; Wiedenhoeft, A.C.; Costa, A.; Soares, R.; Coradin, V.T.R.; Pastore, T.C.M. Potential field-deployable NIRS identification of seven Dalbergia species listed by CITES. Wood Sci. Technol. 2018, 52, 1411–1427. [Google Scholar] [CrossRef] [Green Version]
- Tsuchikawa, S.; Kobori, H. A review of recent application of near infrared spectroscopy to wood science and technology. J. Wood Sci. 2015, 61, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Kludt, K.D. Use of Near Infrared Spectroscopy Technology for Predicting Bending Properties of Clear Wood Specimens. Master’s Thesis, Washington State University, Washington, DC, USA, 2003. [Google Scholar]
- Karlinasari, L.; Sabed, M.; Wistara, I.N.J.; Wijayanto, H.; Purwanto, Y.A. Near infrared (NIR) spectroscopy to predict physical properties of acacia mangium at three different age classes. J. Indones. Wood Res. Soc. 2013, 4, 7–12. [Google Scholar]
- Sandak, A.; Sandak, J.; Riggio, M. Assessment of wood structural members degradation by means of infrared spectroscopy: An overview. Struct. Control Health Monit. 2016, 23, 396–408. [Google Scholar] [CrossRef]
- Schimleck, L.R.; Matos, J.L.M.; Trianoski, R.; Prata, J.G. Comparison of methods for estimating mechanical properties of wood by NIR spectroscopy. J. Spectrosc. 2018, 2018, 4823285. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Hu, H. Prediction of hot-water-soluble extractive, pentosan and cellulose content of various wood species using FT-NIR spectroscopy. Bioresour. Technol. 2013, 140, 299–305. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Hu, H. Rapid prediction of different wood species extractives and lignin content using near infrared spectroscopy. J. Wood Chem. Technol. 2013, 33, 52–64. [Google Scholar] [CrossRef]
- Da Silva, A.R.; Pastore, T.C.M.; Braga, J.W.B.; Davrieux, F.; Okino, E.Y.A.; Coradin, V.T.R.; Camargos, J.A.A.; do Prado, A.G.S. Assessment of total phenols and extractives of mahogany wood by near infrared spectroscopy (NIRS). Holzforschung 2013, 67, 1–8. [Google Scholar] [CrossRef]
- Karlinasari, L.; Sabed, M.; Wistara, I.N.J.; Purwanto, Y.A. Near infrared (NIR) spectroscopy for estimating the chemical composition of (Acacia mangium Willd.) wood. J. Indian Acad. Wood Sci. 2014, 11, 162–167. [Google Scholar] [CrossRef]
- Zhou, C.; Jiang, W.; Via, B.K.; Fasina, O.; Han, G. Prediction of mixed hardwood lignin and carbohydrate content using ATR-FTIR and FT-NIR. Carbohydr. Polym. 2015, 121, 336–341. [Google Scholar] [CrossRef] [Green Version]
- Bergo, M.C.J.; Pastore, T.C.M.; Coradin, V.T.R.; Wiedenhoeft, A.C.; Braga, J.W.B. NIRS identification of Swietenia macrophylla is robust across specimens from 27 countries. IAWA J. 2016, 37, 420–430. [Google Scholar] [CrossRef] [Green Version]
- Sierra, V.; de Luque, M.; Millán, Á.-S.; Pérez, S.; García, E.; Luque, L. NIRWOOD: An EU innovation project to determine species and geographical origin of timber using NIR spectrometry. In Proceedings of the 28th International Conference on Wood Science and Technology, Zagreb, Croatia, 7–8 December 2017. [Google Scholar]
- Silva, D.C.; Pastore, T.C.M.; Soares, L.F.; de Barros, F.A.S.; Bergo, M.C.J.; Coradin, V.T.H.; Gontijo, A.B.; Sosa, M.H.; Chacón, C.B.; Braga, J.W.B. Determination of the country of origin of true mahogany (Swietenia macrophylla King) wood in five Latin American countries using handheld NIR devices and multivariate data analysis. Holzforschung 2018, 72, 521–530. [Google Scholar] [CrossRef]
- Dormontt, E.E.; Boner, M.; Braun, B.; Breulmann, G.; Degen, B.; Espinoza, E.; Gardner, S.; Guillery, P.; Hermanson, J.C.; Koch, G.; et al. Forensic timber identification: It’s time to integrate disciplines to combat illegal logging. Biol. Conserv. 2015, 191, 790–798. [Google Scholar] [CrossRef]
- Beeckman, H.; Blanc-Jolivet, C.; Boeschoten, L.E.; Braga, J.W.B.; Cabezas, J.A.; Chaix, G.; Crameri, S.; Degen, B.; Deklerck, V.; Dormontt, E.; et al. Overview of Current Practices in Data Analysis for Wood Identification. A Guide for the Different Timber Tracking Methods; Schmitz, N., Ed.; Global Timber Tracking Network: Grosshansdorf, Germany; European Forest Institute: Joensuu, Finland; Thünen Institute: Grosshansdorf, Germany, 2020. [Google Scholar]
- Technical Association of the Pulp and Paper Industry (TAPPI). Sampling and Preparing Wood for Analysis; T 257 cm-85; Technical Association of the Pulp and Paper Industry (TAPPI): Atlanta, GA, USA, 1996. [Google Scholar]
- Technical Association of the Pulp and Paper Industry (TAPPI). Preparation of Wood for Chemical Analysis; T 264 cm-97; Technical Association of the Pulp and Paper Industry (TAPPI): Atlanta, GA, USA, 1997; p. 3. [Google Scholar]
- Technical Association of the Pulp and Paper Industry (TAPPI). Solvent Extractives of Wood and Pulp; T 204 cm-97; Technical Association of the Pulp and Paper Industry (TAPPI): Atlanta, GA, USA, 1997; p. 4. [Google Scholar]
- Technical Association of the Pulp and Paper Industry (TAPPI). Water Solubility of Wood and Pulp; T 207 cm-99; Technical Association of the Pulp and Paper Industry (TAPPI): Atlanta, GA, USA, 1999; p. 3. [Google Scholar]
- Santos, J.; Vieira, H.; Silva, E.; Muñiz, G.I.B.; Soffiatti, P.; Nisgoski, S. Near infrared spectroscopy for separation of tauari wood in Brazilian amazon native forest. JTFS 2020, 32, 227–236. [Google Scholar] [CrossRef]
- Rodrigues, J.; Alves, A.; Pereira, H.; Perez, D.d.S.; Chantre, G.; Schwanninger, M. NIR PLSR results obtained by calibration with noisy, low-precision reference values: Are the results acceptable? Holzforschung 2006, 60, 402–408. [Google Scholar] [CrossRef]
- Via, B.K.; Zhou, C.; Acquah, G.; Jiang, W.; Eckhardt, L. Near Infrared spectroscopy calibration for wood chemistry: Which chemometric technique is best for prediction and interpretation? Sensors 2014, 14, 13532–13547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baillères, H.; Davrieux, F.; Ham-Pichavant, F. Near infrared analysis as a tool for rapid screening of some major wood characteristics in a eucalyptus breeding program. Ann. For. Sci. 2002, 59, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Bokobza, L. Near infrared spectroscopy. J. Near Infrared Spectrosc. 2017, 6, 3–17. [Google Scholar] [CrossRef]
- Osborne, B.G.; Fearn, T.; Hindle, P.H.; Hindle, P.T. Practical NIR Spectroscopy with Applications in Food and Beverage Analysis; Longman Scientific & Technical: London, UK, 1993; ISBN 978-0-582-09946-3. [Google Scholar]
- Workman, J., Jr.; Weyer, L. Practical Guide to Interpretive Near-Infrared Spectroscopy; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-1-4200-1831-8. [Google Scholar]
- Schwanninger, M.; Rodrigues, J.C.; Fackler, K. A Review of band assignments in near infrared spectra of wood and wood components. J. Near Infrared Spectrosc. 2011, 19, 287–308. [Google Scholar] [CrossRef]
- Weyer, L.G.; Lo, S.-C. Spectra-structure correlations in the Near-Infrared. In Handbook of Vibrational Spectroscopy; Chalmers, J.M., Griffiths, P.R., Eds.; John Wiley & Sons: Chichester, UK, 2002; Volume 3, pp. 1–21. ISBN 978-0-470-02732-5. [Google Scholar]





| Site | n | MC (%) | Hot Water Solubility (%) | Ethanol-Benzene Solubility | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Max | Min | Mean | S.Dev | Max | Min | Mean | S.Dev | |||
| West Celebes | 15 | 7.14 (0.65) | 13.840 | 8.320 | 10.846 a | 1.505 | 13.639 | 7.941 | 11.395 a | 1.687 |
| Central Celebes | 17 | 8.14 (1.23) | 12.484 | 8.798 | 10.540 a | 1.126 | 14.058 | 8.230 | 10.879 ab | 1.468 |
| South Celebes | 13 | 8.33 (1.42) | 13.095 | 6.144 | 9.720 a | 2.225 | 13.717 | 6.417 | 9.920 b | 2.116 |
| Total | 45 | 7.84 | 13.840 | 6.144 | 10.408 | 1.655 | 14.058 | 6.417 | 10.774 | 1.806 |
| Preprocessing | LV | Calibration | Validation | RPD | ||||
|---|---|---|---|---|---|---|---|---|
| N | R2cal | RMSEC | N | R2val | RMSEP | |||
| Hot water-soluble extractive | ||||||||
| Raw spectra | 7 | 90 | 0.684 | 1.003 | 42 | 0.598 | 0.788 | 3.627 |
| Ethanol-benzene soluble extractive | ||||||||
| 2ndDvt21pts, MSC | 2 | 90 | 0.609 | 1.109 | 45 | 0.704 | 0.973 | 3.569 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karlinasari, L.; Noviyanti, N.; Purwanto, Y.A.; Majiidu, M.; Dwiyanti, F.G.; Rafi, M.; Damayanti, R.; Harnelly, E.; Siregar, I.Z. Discrimination and Determination of Extractive Content of Ebony (Diospyros celebica Bakh.) from Celebes Island by Near-Infrared Spectroscopy. Forests 2021, 12, 6. https://doi.org/10.3390/f12010006
Karlinasari L, Noviyanti N, Purwanto YA, Majiidu M, Dwiyanti FG, Rafi M, Damayanti R, Harnelly E, Siregar IZ. Discrimination and Determination of Extractive Content of Ebony (Diospyros celebica Bakh.) from Celebes Island by Near-Infrared Spectroscopy. Forests. 2021; 12(1):6. https://doi.org/10.3390/f12010006
Chicago/Turabian StyleKarlinasari, Lina, Noviyanti Noviyanti, Y. Aris Purwanto, Muhammad Majiidu, Fifi G. Dwiyanti, Mohamad Rafi, Ratih Damayanti, Essy Harnelly, and Iskandar Z. Siregar. 2021. "Discrimination and Determination of Extractive Content of Ebony (Diospyros celebica Bakh.) from Celebes Island by Near-Infrared Spectroscopy" Forests 12, no. 1: 6. https://doi.org/10.3390/f12010006












