Stem CO2 Efflux as an Indicator of Forests’ Productivity in Relict Juniper Woodlands (Juniperus thurifera L.) of Southern Spain
Abstract
:1. Introduction
2. Materials and Methods
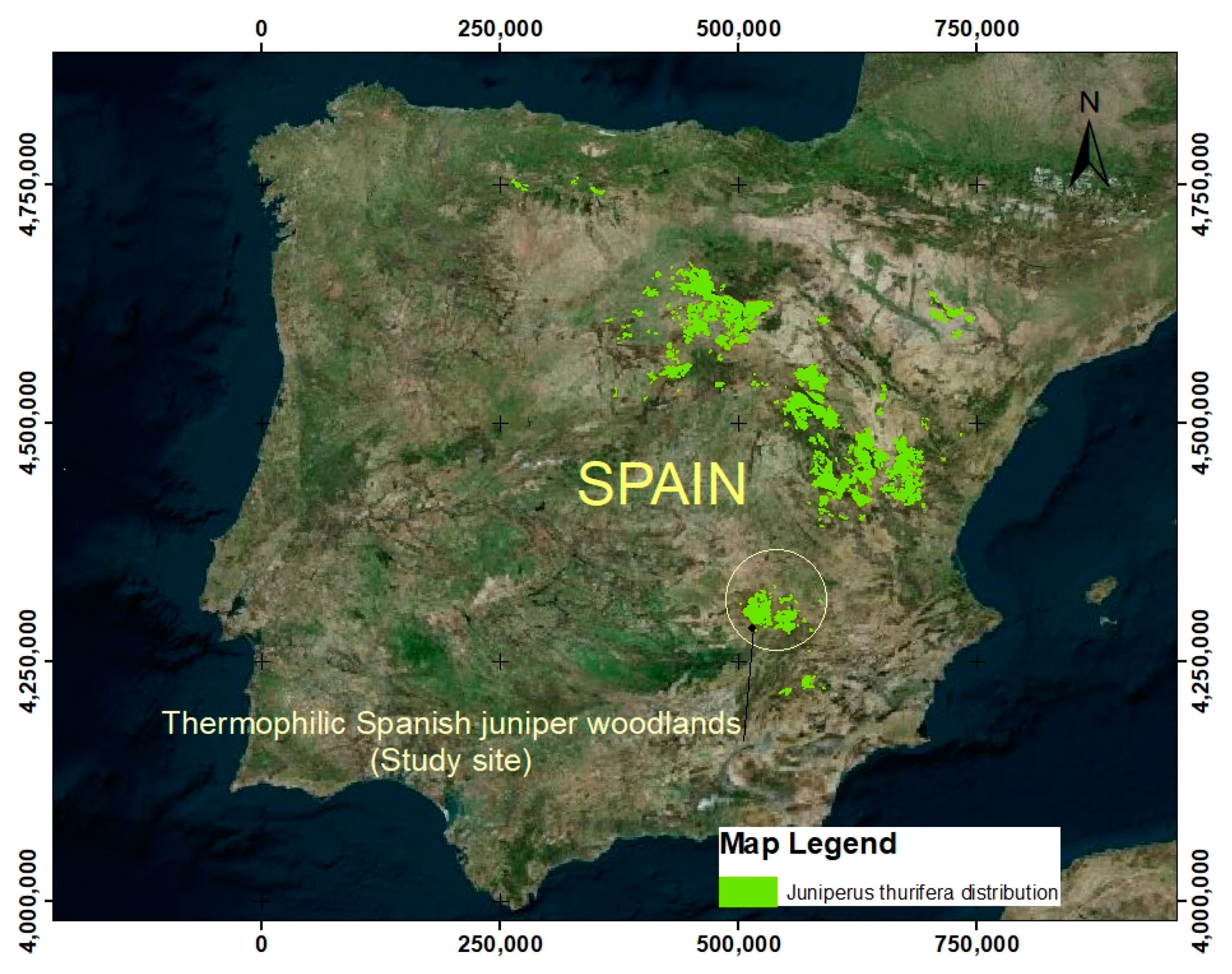
2.1. Study Area
2.2. Measurement of Stem CO2 Efflux and Stem Temperature in Juniper Trees, and Soil Water Content
2.3. Data Analysis
3. Results
3.1. Effects of Juniper Woodland Type on Stem CO2 Efflux
3.2. Seasonal Variation of Stem CO2 Efflux in the Juniper Woodlands
3.3. Climatic Conditions: Stem Temperature (Ts; °C) and Soil Water Content (SWC; %)
3.4. Effects of Stem Temperature on Stem CO2 Efflux
4. Discussion
4.1. Effects of Juniper Woodland Type on Stem CO2 Efflux
4.2. Seasonal Variation of Stem CO2 Efflux in the Two Juniper Woodlands
4.3. Effects of Stem Temperature on Stem CO2 Efflux
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landsberg, J.J.; Gower, S.T. Applications of Physiological Ecology to Forest Management; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Waring, R.; Running, S. Forest Ecosystems. Analysis at Multiple Scales, 3rd ed.; Academic Press: San Diego, CA, USA, 2007; p. 440. [Google Scholar]
- Damesin, C.; Ceschia, E.; Le Goff, N.; Ottorini, J.M.; Dufrêne, E. Stem and branch respiration of beech: From tree measurements to estimations at the stand level. New Phytol. 2002, 153, 159–172. [Google Scholar] [CrossRef]
- Teskey, R.O.; Saveyn, A.; Steppe, K.; McGuire, M.A. Origin, fate and significance of CO2 in tree stems. New Phytol. 2008, 177, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Calcerrada, J.; Martin-StPaul, N.K.; Lempereur, M.; Ourcival, J.-M.; Rey, M.d.C.d.; Joffre, R.; Rambal, S. Stem CO2 efflux and its contribution to ecosystem CO2 efflux decrease with drought in a Mediterranean forest stand. Agric. For. Meteorol. 2014, 195–196, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Brito, P.; Morales, D.; Wieser, G.; Jiménez, M.S. Spatial and seasonal variations in stem CO2 efflux of Pinus canariensis at their upper distribution limit. Trees 2010, 24, 523–531. [Google Scholar] [CrossRef]
- Zha, T.; Kellomäki, S.; Wang, K.-Y.; Ryyppö, A.; Niinistö, S. Seasonal and annual stem respiration of Scots pine trees under boreal conditions. Ann. Bot. 2004, 94, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Pallardy, S.G.; Kozlowski, T.T. Physiology of Woody Plants, 3rd ed.; Elsevier: Amsterdam, The Netheralnds; Boston, MA, USA, 2008; p. xiv. 454p. [Google Scholar]
- Azcón-Bieto, J.; Talón, M. Fundamentos de Fisiología Vegetal, 2nd ed.; McGraw-Hill Interamericana: Madrid, Spain, 2013; p. 651. [Google Scholar]
- Martínez-García, E.; Dadi, T.; Rubio, E.; García-Morote, F.A.; Andrés-Abellán, M.; López-Serrano, F.R. Aboveground autotrophic respiration in a Spanish black pine forest: Comparison of scaling methods to improve component partitioning. Sci. Total Environ. 2017, 580, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Salomon, R.; Valbuena-Carabana, M.; Gil, L.; McGuire, M.; Teskey, R.; Aubrey, D.; Gonzalez-Doncel, I.; Rodriguez-Calcerrada, J. Temporal and spatial patterns of internal and external stem CO2 fluxes in a sub-Mediterranean oak. Tree Physiol. 2016, 36, 1409–1421. [Google Scholar] [CrossRef] [Green Version]
- Ryan, M.G.; Linder, S.; Vose, J.M.; Hubbard, R.M. Dark Respiration of Pines. Ecol. Bull. 1994, 43, 50–63. [Google Scholar]
- Wittmann, C.; Pfanz, H.; Loreto, F.; Centritto, M.; Pietrini, F.; Alessio, G. Stem CO2 release under illumination: Corticular photosynthesis, photorespiration or inhibition of mitochondrial respiration? Plant Cell Environ. 2006, 29, 1149–1158. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Calcerrada, J.; López, R.; Salomón, R.; Gordaliza, G.G.; Valbuena-Carabaña, M.; Oleksyn, J.; Gil, L. Stem CO2 efflux in six co-occurring tree species: Underlying factors and ecological implications. Plant Cell Environ. 2015, 38, 1104–1115. [Google Scholar] [CrossRef]
- Gower, S.T. Patterns and Mechanisms of the Forest Carbon Cycle. Annu. Rev. Environ. Resour. 2003, 28, 169–204. [Google Scholar] [CrossRef]
- Rodríguez-Calcerrada, J.; Salomón, R.L.; Gordaliza, G.G.; Miranda, J.C.; Miranda, E.; de la Riva, E.G.; Gil, L. Respiratory costs of producing and maintaining stem biomass in eight co-occurring tree species. Tree Physiol. 2019, 39, 1838–1854. [Google Scholar] [CrossRef] [PubMed]
- Farjon, A. A Monograph of Cupressaceae and Sciadopitys; Royal Botanic Gardens, Kew: London, UK, 2005; p. 648. [Google Scholar]
- Adams, R.P. The Junipers of the World:The Genus Juniperus, 4th ed.; Trafford Publishing: Victoria, BC, Canada, 2014; p. 422. [Google Scholar]
- Mao, K.; Hao, G.; Liu, J.; Adams, R.P.; Milne, R.I. Diversification and biogeography of Juniperus (Cupressaceae): Variable diversification rates and multiple intercontinental dispersals. New Phytol. 2010, 188, 254–272. [Google Scholar] [CrossRef]
- Jiménez, J.F.; Werner, O.; Sánchez-Gómez, P.; Fernández, S.; Guerra, J. Genetic variations and migration pathway of Juniperus thurifera L. (Cupressaceae) in the western Mediterranean region. Isr. J. Plant Sci. 2003, 51, 11–22. [Google Scholar] [CrossRef]
- Costa, M.; Gómez, F.; Morla, C.; Ollero, H. Caracterización fitoecológica de los sabinares albares de la Península Ibérica. Orsis Org. I Sist. 1993, 8, 79–93. [Google Scholar]
- García López, J.M.; Allué Camacho, C. Caracterización y potencialidades fitoclimáticas de la sabina albar (Juniperus thurifera L.) en la Península Ibérica. For. Syst. Investig. Agrar. Sist. Recur For. 2005, 14, 98–109. [Google Scholar] [CrossRef] [Green Version]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J. IPCC Climate Change 2013: The Physical Science Basis; Cambridge University Press, Ed.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- García Morote, F.A. Biomasa y productividad de la sabina albar (Juniperus thurifera L.) en El Campo de Montiel (Albacete). Ph.D. Thesis, Universidad de Castilla-La Mancha, Albacete, Spain, 2008. [Google Scholar]
- Garcia Morote, F.A.; Lopez Serrano, F.R.; Andres, M.; Rubio, E.; Gonzalez Jimenez, J.L.; de las Heras, J. Allometries, biomass stocks and biomass allocation in the thermophilic Spanish juniper woodlands of Southern Spain. For. Ecol. Manag. 2012, 270, 85–93. [Google Scholar] [CrossRef]
- Garcia-Morote, F.A.; Lopez-Serrano, F.R.; Andres, M.; Martinez-Garcia, E.; Lucas-Borja, M.; Dadi, T.; Candel, D.; Wic, C. Effects of woodland maturity, vegetation cover and season on enzymatic and microbial activity in thermophilic Spanish Juniper woodlands (Juniperus thurifera L.) of southern Spain. Eur. J. Soil Sci. 2012, 63, 579–591. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- FAO. FAO/UNESCO Soil Map of the World, Revised Legend; FAO: Rome, Italy, 1988. [Google Scholar]
- Xu, M.; DeBiase, T.; Qi, Y. A simple technique to measure stem respiration using a horizontally oriented soil chamber. Can. J. For. Res. Rev. Can. Rech. For. 2000, 30, 1555–1560. [Google Scholar] [CrossRef]
- Hoshmand, H.R. Design of Experiments for Agriculture and the Natural Sciences, 2nd ed.; Champan & Hall/CRC: New York, NY, USA, 2006; p. 437. [Google Scholar]
- Neter, J.; Kutner, M.; Wasserman, W.; Nachtsheim, C. Applied Linear Statistical Models, 4th ed.; McGraw-Hill-Irwin: Chicago, IL, USA, 1996; p. xv. 720p. [Google Scholar]
- Lavigne, M.B. Comparing stem respiration and growth of jack pine provenances from northern and southern locations. Tree Physiol. 1996, 16, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Belsley, D.A.; Kuh, E.; Welsch, R.E. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity; John Wiley & Sons, Ltd.: New York, NY, USA, 2013. [Google Scholar]
- Xu, M.; DeBiase, T.A.; Qi, Y.; Goldstein, A.; Liu, Z. Ecosystem respiration in a young ponderosa pine plantation in the Sierra Nevada Mountains, California. Tree Physiol. 2001, 21, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Davidson, E.A.; Janssens, I.A.; Luo, Y. On the variability of respiration in terrestrial ecosystems: Moving beyond Q10. Glob. Chang. Biol. 2006, 12, 154–164. [Google Scholar] [CrossRef]
- Amthor, J.S. The role of maintenance respiration in plant growth. Plant Cell Environ. 1984, 7, 561–569. [Google Scholar]
- Ryan, M.G.; Hubbard, R.M.; Pongracic, S.; Raison, R.J.; McMurtrie, R.E. Foliage, fine-root, woody-tissue and stand respiration in Pinus radiata in relation to nitrogen status. Tree Physiol. 1996, 16, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Litton, C.M.; Raich, J.W.; Ryan, M.G. Carbon allocation in forest ecosystems. Glob. Chang. Biol. 2007, 13, 2089–2109. [Google Scholar] [CrossRef] [Green Version]
- Pfanz, H.; Aschan, G. The Existence of Bark and Stem Photosynthesis in Woody Plants and Its Significance for the Overall Carbon Gain. An Eco-Physiological and Ecological Approach. In Progress in Botany: Genetics Physiology Systematics Ecology; Esser, K., Lüttge, U., Kadereit, J.W., Beyschlag, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 477–510. [Google Scholar]
- Maseyk, K.; GrÜNzweig, J.M.; Rotenberg, E.; Yakir, D.A.N. Respiration acclimation contributes to high carbon-use efficiency in a seasonally dry pine forest. Glob. Chang. Biol. 2008, 14, 1553–1567. [Google Scholar] [CrossRef]
- Wang, W.; Yang, F.; Zu, Y.; Wang, H.; Takagi, K.; Sasa, K.; Koike, T. Stem respiration of a larch (Larix gmelini) plantation in Northeast China. Acta Bot. Sin. 2003, 45, 1387–1397. [Google Scholar]
- Aponte, C.; Marañón, T.; García, L.V. Microbial C, N and P in soils of Mediterranean oak forests: Influence of season, canopy cover and soil depth. Biogeochemistry 2010, 101, 77–92. [Google Scholar] [CrossRef] [Green Version]
- Flexas, J.; Galmes, J.; Ribas-Carbo, M.; Medrano, H. The Effects of Water Stress on Plant Respiration; Lambers, H., Ribas-Carbo, M., Eds.; Springer: Dordrecht, The Netheralnds, 2005; Volume 18. [Google Scholar]
- Guzmán-Delgado, P.; Fernández, V.; Venturas, M.; Rodríguez-Calcerrada, J.; Gil, L. Surface properties and physiology of Ulmus laevis and U. minor samaras: Implications for seed development and dispersal. Tree Physiol. 2017, 37, 815–826. [Google Scholar] [CrossRef]
- Bosc, A.; De Grandcourt, A.; Loustau, D. Variability of stem and branch maintenance respiration in a Pinus pinaster tree. Tree Physiol. 2003, 23, 227–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Querejeta Mercader, J.I.; Egerton-Warburton, L.M.; Allen, M.F. Hydraulic lift may buffer rhizosphere hyphae against the negative effects of severe soil drying in a California Oak savanna. Soil Biol. Biochem. 2007, 39, 409–417. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Zu, Y.; Li, X.; Koike, T. Characteristics of the temperature coefficient, Q10, for the respiration of non-photosynthetic organs and soils of forest ecosystems. Front. For. China 2006, 1, 125–135. [Google Scholar] [CrossRef]
- Atkin, O.K.; Bruhn, D.; Hurry, V.M.; Tjoelker, M.G. The hot and the cold: Unravelling the variable response of plant respiration to temperature. Funct Plant Biol. 2005, 32, 87–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambers, H.; Oliveira, R.S. Plant Physiological Ecology, 3rd ed.; Springer, C., Ed.; Springer International Publishing: Cham, Switzerland, 2019; p. 736. [Google Scholar]
- Ryan, M.G.; Gower, S.T.; Hubbard, R.M.; Waring, R.H.; Gholz, H.L.; Cropper, W.P.; Running, S.W. Woody tissue maintenance respiration of four conifers in contrasting climates. Oecologia 1995, 101, 133–140. [Google Scholar] [CrossRef] [PubMed]





| Characteristics of Woodland | Young Woodland | Mature Woodland |
|---|---|---|
| Mean age (years) | 66 ± 4 | 170 ± 5 |
| Woodland density (trees ha−1) | 308 ± 40 | 95 ± 12 |
| Juniper cover (%) | 65 ± 23 | 32 ± 14 |
| Net primary productivity (t ha−1 year−1) | 1.91 ± 0.14 | 0.44 ± 0.01 |
| Total biomass (t ha−1) | 30.8 ± 2.6 | 7.6 ± 0.6 |
| Litter fall (t ha−1 year−1) | 0.98 ± 0.13 | 0.24 ± 0.05 |
| LAI (m2 m−2) | 1.03 | 0.32 |
| Mean diameter at breast height (cm) | 13.3 ± 0.7 | 16.7 ± 0.5 |
| Stem diameter growth (mm year−1) | 2.96 ± 1.32 | 1.50 ± 1.12 |
| Sapwood (%) at breast height (1.30 m) | 73.3 ± 8.2 | 55.9 ± 4.5 |
| Soil taxonomy (FAO; [28]) | Calcaric cambisol | Lithic leptosol |
| Mean soil depth | 0.42 ± 0.04 | 0.10 ± 0.02 |
| Soil texture (sand; %) | 50 ± 5 | 58 ± 5 |
| Soil texture (clay; %) | 28 ± 1 | 12 ± 1 |
| Soil pH | 8.3 ± 0.1 | 8.6 ± 0.1 |
| Soil organic C (%) | 2.7 ± 0.8 | 3.7 ± 1.1 |
| Apparent density of soil (g cm−3) | 1.37 ± 0.01 | 1.54 ± 0.02 |
| Seasons | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Effects | Total Annual | Winter | Spring | Summer | Autumn | |||||
| F | p | LSD | p | LSD | p | LSD | p | LSD | p | |
| W | 16.9 | <0.00 | 0.74 | 0.72 | 0.34 | <0.00 | 0.61 | <0.00 | 0.60 | 0.29 |
| J (W) | 1.18 | 0.29 | - | - | - | - | - | - | - | - |
| S | 28.6 | <0.00 | - | - | - | - | - | - | - | - |
| W × S | 3.32 | 0.02 | - | - | - | - | - | - | - | - |
| Main Effects | Levels | Es (μmol CO2 m−2 s−1) |
|---|---|---|
| W | Young woodland | 1.95 ± 0.11 a |
| (Woodland type) | Mature woodland | 1.26 ± 0.11 b |
| S | Winter | 0.84 ± 0.15 a |
| (Season) | Spring | 2.33 ± 0.09 b |
| Summer | 1.92 ± 0.15 c | |
| Autumn | 1.31 ± 0.15 d |
| Parameters | Coefficients | Standard Error | t | p-Value |
|---|---|---|---|---|
| Intercept | −0.350 | 0.0623 | −5.62 | 0.000 |
| Ts/10 | 0.684 | 0.0375 | 18.18 | 0.000 |
| W = Mature | −0.129 | 0.0875 | −1.48 | 0.139 |
| (Ts/10) × (W = Mature) | −0.113 | 0.0543 | −2.07 | 0.038 |
| Source | Sum of Squares | df | Mean Square | F | p-Value |
|---|---|---|---|---|---|
| Ts/10 | 71.66 | 1 | 71.66 | 560.1 | 0.000 |
| Intercepts | 4.95 | 1 | 4.95 | 38.7 | 0.000 |
| Slopes | 0.55 | 1 | 0.55 | 4.3 | 0.038 |
| Model | 77.17 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García Morote, F.A.; Andrés Abellán, M.; Rubio, E.; Pérez Anta, I.; García Saucedo, F.; López Serrano, F.R. Stem CO2 Efflux as an Indicator of Forests’ Productivity in Relict Juniper Woodlands (Juniperus thurifera L.) of Southern Spain. Forests 2021, 12, 1340. https://doi.org/10.3390/f12101340
García Morote FA, Andrés Abellán M, Rubio E, Pérez Anta I, García Saucedo F, López Serrano FR. Stem CO2 Efflux as an Indicator of Forests’ Productivity in Relict Juniper Woodlands (Juniperus thurifera L.) of Southern Spain. Forests. 2021; 12(10):1340. https://doi.org/10.3390/f12101340
Chicago/Turabian StyleGarcía Morote, Francisco Antonio, Manuela Andrés Abellán, Eva Rubio, Iván Pérez Anta, Francisco García Saucedo, and Francisco Ramón López Serrano. 2021. "Stem CO2 Efflux as an Indicator of Forests’ Productivity in Relict Juniper Woodlands (Juniperus thurifera L.) of Southern Spain" Forests 12, no. 10: 1340. https://doi.org/10.3390/f12101340
APA StyleGarcía Morote, F. A., Andrés Abellán, M., Rubio, E., Pérez Anta, I., García Saucedo, F., & López Serrano, F. R. (2021). Stem CO2 Efflux as an Indicator of Forests’ Productivity in Relict Juniper Woodlands (Juniperus thurifera L.) of Southern Spain. Forests, 12(10), 1340. https://doi.org/10.3390/f12101340







