Patterns and Internal Stability of Carbon, Nitrogen, and Phosphorus in Soils and Soil Microbial Biomass in Terrestrial Ecosystems in China: A Data Synthesis
Abstract
:1. Introduction
2. Materials and Methods
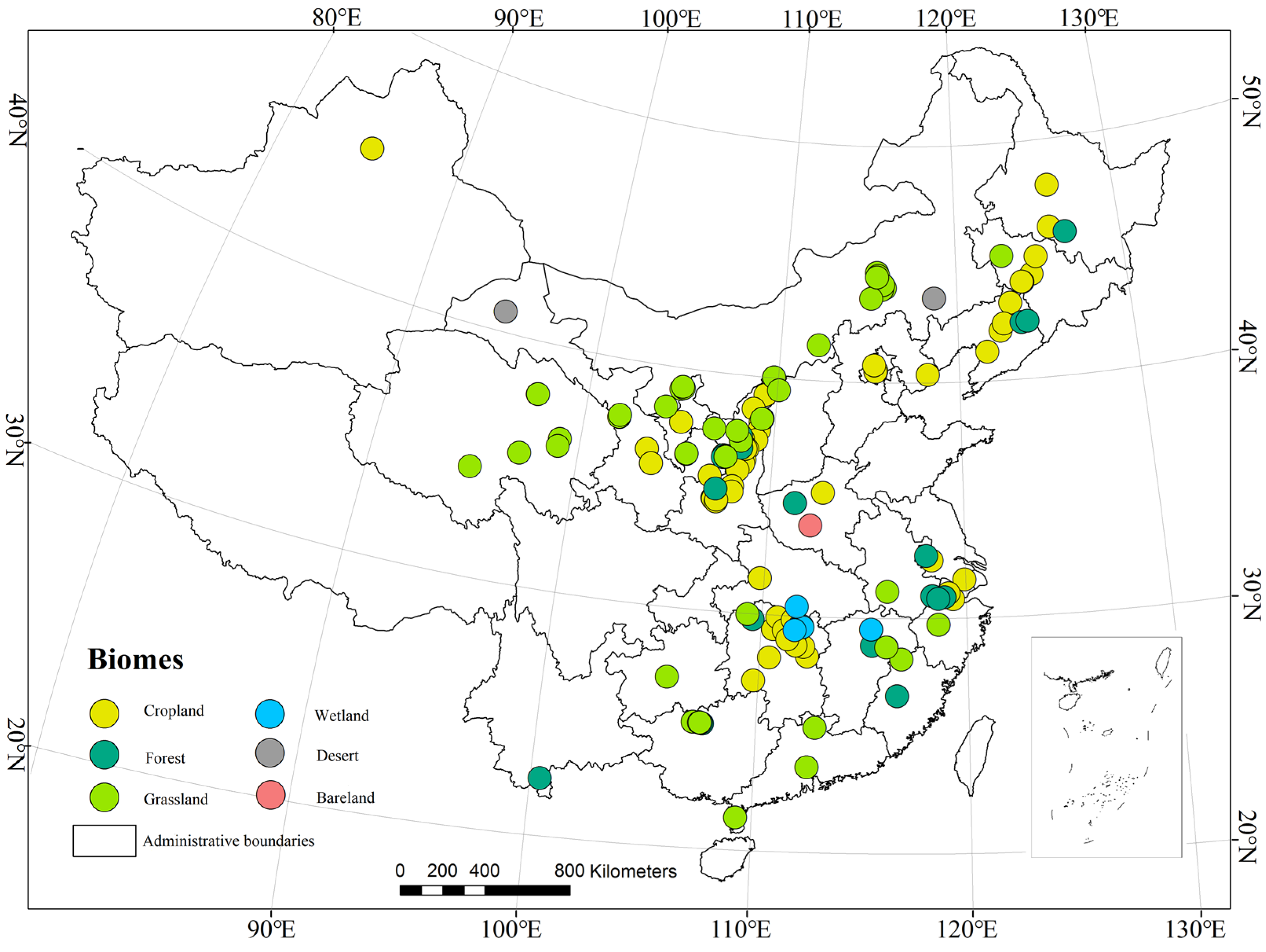
2.1. Distribution of Types of Terrestrial Ecosystems in China
2.2. Data Acquisition
2.3. Data Analysis
3. Results
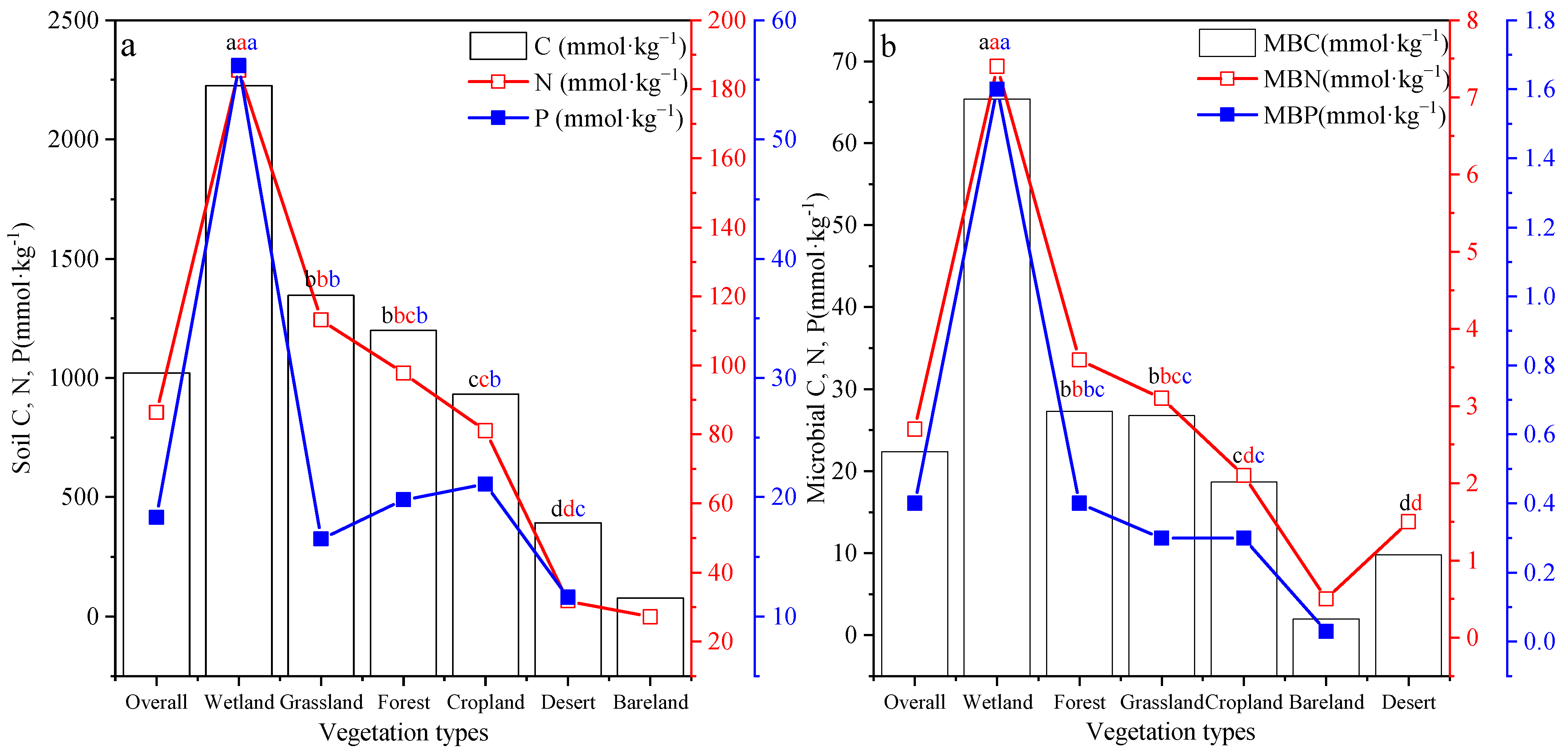
3.1. Summary of Soil and Soil Microbial Biomass Carbon (C), Nitrogen (N), and Phosphorus (P) Concentrations in Different Ecosystem Types
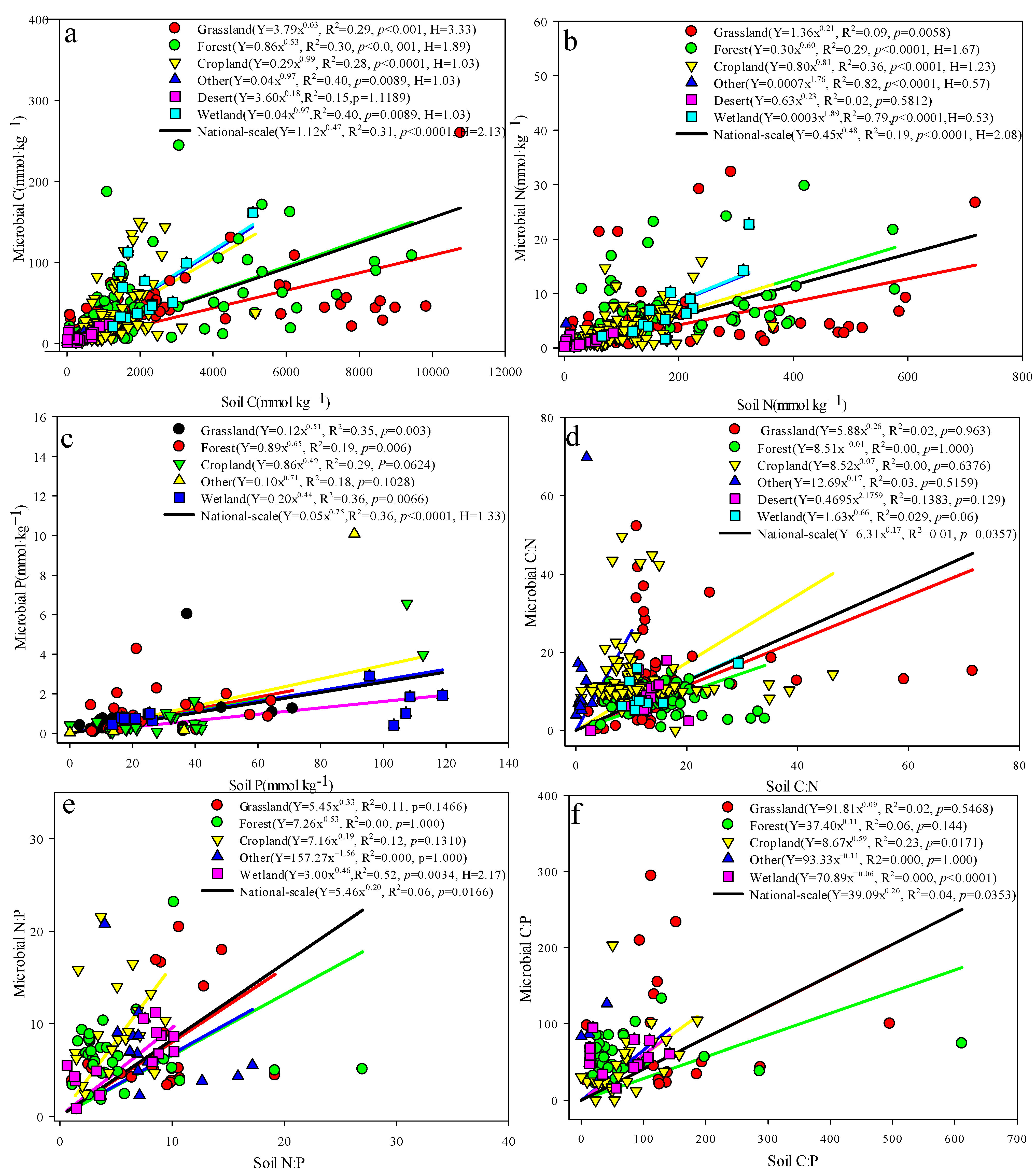
3.2. Correlation Analysis of Soil and Soil Microbial Biomass C, N, and P Concentrations and Their Ecological Stoichiometric Characteristics
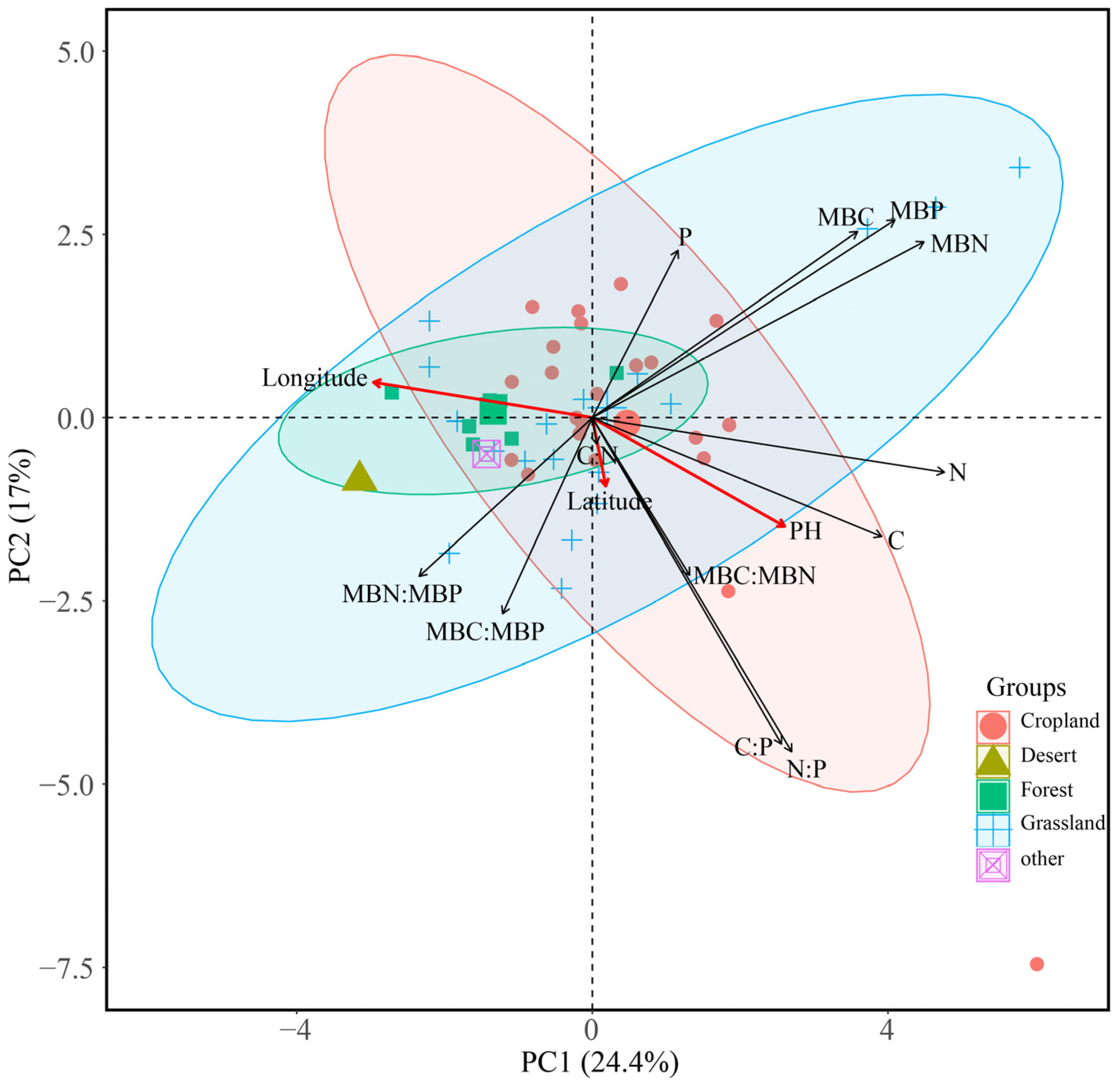
3.3. Relationships between Environmental Factors and Ecological Stoichiometry of Soil and Soil Microbial Biomass
3.4. Characteristics of the Internal Stability of Soil Microbial C, N and P
4. Discussion
4.1. Is There a “Redfield-like” Ratio for Soil and Soil Microbial Biomass C:N:P in China’s Terrestrial Ecosystems?
4.2. Can Soil Microbial Biomass N:P Be Used as a Tool to Evaluate Nutrient Limitations?
4.3. What Are the Factors Affecting the C:N:P Ratio of Soil and Soil Microbial Biomass in China?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michaels, A.F. The ratios of life. Science 2003, 300, 906–907. [Google Scholar] [CrossRef]
- Li, Z.; Tian, D.; Wang, B.; Wang, J.; Wang, S.; Chen, H.Y.H.; Xu, X.; Wang, C.; He, N.; Niu, S. Microbes drive global soil nitrogen mineralization and availability. Glob. Change Biol. 2019, 25, 1078–1088. [Google Scholar] [CrossRef]
- Tao, Y. Fire Interference on Forest Soil Microbial Communities and the Mechanism: A Review. Sci. Silvae Sin. 2013, 49, 146–157. [Google Scholar]
- An, S.S.; Mentler, A.; Acosta-Martínez, V.; Blum, W. Soil microbial parameters and stability of soil aggregate fractions under different grassland communities on the Loess Plateau, China. Biologia 2009, 64, 424–427. [Google Scholar] [CrossRef]
- Trivedi, P.; Anderson, I.C.; Singh, B.K. Microbial modulators of soil carbon storage: Integrating genomic and metabolic knowledge for global prediction. Trends Microbiol. 2013, 21, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Thornton, P.E.; Post, W.M. A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Global Ecol. Biogeogr. 2013, 22, 737–749. [Google Scholar] [CrossRef]
- Redfield, A. The biological control of chemical factors in the environment. Am. Sci. 1958, 46, 205–221. [Google Scholar]
- Schindler, D.W. Balancing planets and molecules. Nature 2003, 423, 225–226. [Google Scholar] [CrossRef]
- Zeng, D.H.; Chen, G.S. Ecological stoichiometry: A science to explore the complexity of living systems. Chin. J. Plant Ecol. 2005, 29, 1007–1019. [Google Scholar]
- Wang, X.; Song, Y.; Huo, G.; Zhang, F. Research advances of soils and plants ecological stoichiometry. J. Dalian Minzu Univ. 2016, 18, 437–442. [Google Scholar]
- Zhang, Z.; Xue, Z.; Liu, X. Is There a Redfield-Type C:N:P Ratio in Chinese Wetland Soils? Acta Pedol. Sin. 2016, 53, 1160–1169. [Google Scholar]
- Blodau, C. Carbon cycling in peatlands-A review of processes and controls. Environ. Rev. 2002, 10, 111–134. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Tian, H.; Chen, G.; Zhang, C.; Melillo, J.M.; Hall, C.A. Pattern and variation of C:N:P ratios in China’s soils: A synthesis of observational data. Biogeochemistry 2010, 98, 139–151. [Google Scholar] [CrossRef]
- Yang, Z.F.; Yu, T.; Hou, Q.Y.; Xia, X.; Feng, H.; Huang, C.; Wang, L.; Lv, Y.; Zhang, M. Geochemical evaluation of land quality in China and its applications. J. Geochem. Explor. 2014, 139, 122–135. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, P.; Chen, Y.M. Soil C:N:P stoichiometry in plantations of N-fixing black locust and indigenous pine, and secondary oak forests in Northwest China. J. Soils Sediments 2018, 18, 1478–1489. [Google Scholar] [CrossRef]
- Liu, J.; Gou, X.; Zhang, F.; Bian, R.; Yin, D. Spatial patterns in the C: N: P stoichiometry in Qinghai spruce and the soil across the Qilian Mountains, China. Catena 2021, 196, 104814. [Google Scholar] [CrossRef]
- Wang, H.; Ren, T.; Müller, K.; Van Zwieten, L.; Wang, H.; Feng, H.; Xu, C.; Yun, F.; Ji, X.; Yin, Q.; et al. Soil type regulates carbon and nitrogen stoichiometry and mineralization following biochar or nitrogen addition. Sci. Total Environ. 2021, 753, 141645. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, H.; Yao, X.; Zeng, W.; Wang, W. Latitudinal and depth patterns of soil microbial biomass carbon, nitrogen, and phosphorus in grasslands of an agro-pastoral ecotone. Land Degrad. Dev. 2021, 32, 3833–3846. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, J.; Xu, X.; Qin, X. Stoichiometry of soil microbial biomass carbon and microbial biomass nitrogen in China’s temperate and alpine grasslands. Eur. J. Soil Biol. 2017, 83, 1–8. [Google Scholar] [CrossRef]
- Zhou, W.; Gang, C.; Zhou, L.; Chen, Y.; Li, J.; Ju, W.; Odeh, I. Dynamic of grassland vegetation degradation and its quantitative assessment in the Northwest China. Acta Oecol. 2014, 55, 86–96. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Wu, J.S.; Brookes, P.C. Measuring soil microbial biomass using an automated procedure. Soil Biol. Biochem. 2011, 43, 873–876. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002; pp. 225–226. 439p. [Google Scholar]
- Hood, J.M.; Sterner, R. Diet mixing: Do animals integrate growth or resources across temporal heterogeneity? Am. Nat. 2010, 176, 651–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persson, J.; Fink, P.; Goto, A. To be or not to be what you eat: Regulation of stoichiometric homeostasis among autotrophs and heterotrophs. Oikos 2010, 119, 741–751. [Google Scholar] [CrossRef]
- Xing, W.; Wu, H.; Shi, Q.; Hao, B.; Liu, H.; Wang, Z.; Liu, G. Multielement stoichiometry of submerged macrophytes across Yunnan plateau lakes (China). Sci. Rep. 2015, 5, 10186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C:N:P stoichiometry in forest worldwide: Implications of terrestrial Redfield-type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Güsewell, S. N/P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Hu, L.J.; Shao, M.A.; Yang, W.Z. Relationship between vegetation spatial collocations and soil moisture spatial heterogeneities in the Loess Plateau area. Acta Pratacul. Sin. 2004, 13, 14–20. [Google Scholar]
- Li, B.; Yang, C.; Lin, P. Ecology; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Zhu, Q.L.; Xing, Y.Y.; Zhang, H.; An, S. Soil ecological stoichiometry under different vegetation area on loess hilly-gully region. Acta Ecol. Sin. 2013, 33, 4674–4682. [Google Scholar]
- Ren, C.; Zhao, F.; Kang, D.; Yang, G.; Han, X.; Tong, X.; Feng, Y.; Ren, G. Linkages of C:N:P stoichiometry and bacterial community in soil following afforestation of former farmland. For. Ecol. Manag. 2016, 376, 59–66. [Google Scholar] [CrossRef]
- Lovett, G.M.; Weathers, K.C.; Arthur, M.A. Control of nitrogen loss from forested watersheds by soil carbon: Nitrogen ratio and tree species composition. Ecosystems 2002, 5, 712–718. [Google Scholar] [CrossRef]
- Sistla, S.A.; Appling, A.P.; Lewandowska, A.M.; Taylor, B.N.; Wolf, A.A. Stoichio-metric flexibility in response to fertilization along gradients of environmental and organismal nutrient richness. Oikos 2015, 124, 949–959. [Google Scholar] [CrossRef]
- Malchair, S.; Carnol, M. Microbial biomass and C and N transformations in forest floors under European beech, sessile oak, Norway spruce and Douglas-fir at four temperate forest sites. Soil Biol. Biochem. 2009, 41, 831–839. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.K.; Jin, G.Z. Seasonal dynamics of soil microbial biomass in six forest types in Xiaoxing’an Mountains, China. Acta Ecol. Sin. 2014, 34, 451–459. [Google Scholar]
- Bui, E.N.; Henderson, B.L. C:N:P stoichiometry in Australian soils with respect to vegetation and environmental factors. Plant Soil 2013, 373, 553–568. [Google Scholar] [CrossRef]
- Walter, H.; Box, E. Global classification of natural terrestrial ecosystems. Vegetatio 1976, 32, 75–81. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, L.; Chen, Y.; Yang, J.; An, S. Ecological stoihiometry characteristics of Robinia pseudoacacia forest soil in different latitudes of Loess Plateau. Acta Pedol. Sin. 2013, 50, 818–825. [Google Scholar]
- Bai, J.; Deng, W.; Zhu, Y.; Luan, Z.; Zhang, Y. Spatial distribution characteristics and ecological of effects of carbon and nitrogen of soil in Huolin River catchment wetland. J. Appl. Ecol. 2003, 14, 1494–1498. [Google Scholar]
- Li, T.; Deng, Q.; Yuan, Z.Y.; Jiao, F. Latitudinal changes in plant stoichiometric and soil C, N, P stoichiometry in Loess Plateau. Environ. Sci. 2015, 36, 2988–2996. [Google Scholar]
- Tu, X.; Cao, J.; Han, Y.; Shen, Z.; Zhang, B. Storage and spatial distribution of organic and inorganic carbon in the topsoil of Loess Plateau. J. Arid Land Resour. Environ. 2012, 26, 114–118. [Google Scholar]
- Díaz-Ravia, M.; Acea, M.J.; Carballas, T. Seasonal changes in microbial biomass and nutrient flush in forest soils. Biol. Fert. Soils 1995, 19, 220–226. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Pendall, E.; Morgan, J.A.; Blumenthal, D.M.; Carrillo, Y.; LeCain, D.R.; Follett, R.F.; Williams, D.G. Climate change alters stoichiometry of phosphorus and nitrogen in a semiarid grassland. New Phytol. 2012, 196, 807–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djukic, I.; Zehetner, F.; Watzinger, A.; Horacek, M.; Gerzabek, M.H. In situcarbon turnover dynamics and the role of soil microorganisms therein: A climate warming study in an Alpine ecosystem. FEMS Microbiol. Ecol. 2013, 83, 112–124. [Google Scholar] [CrossRef] [Green Version]
- Drake, J.E.; Darby, B.A.; Giasson, M.A.; Kramer, M.A.; Phillips, R.P.; Finzi, A.C. Stoichiometry constrains microbial response to root exudation: Insights from a model and a field experiment in a temperate forest. Biogeosciences 2013, 10, 821–838. [Google Scholar] [CrossRef] [Green Version]
- Ren, C.; Zhao, F.; Shi, Z.; Chen, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Differential responses of soil microbial biomass and carbon-degrading enzyme activities to altered precipitation. Soil Biol. Biochem. 2017, 115, 1–10. [Google Scholar] [CrossRef]
- Chen, Y.L.; Chen, L.Y.; Peng, Y.F.; Ding, J.Z.; Li, F.; Yang, G.B.; Kou, D.; Liu, L.; Fang, K.; Zhang, B.B.; et al. Linking microbial C: N: P stoichiometry to microbial community and abiotic factors along a 3500 km grassland transect on the Tibetan Plateau. Glob. Ecol. Biogeogr. 2016, 25, 1416–1427. [Google Scholar] [CrossRef]
- Lambers, H.; Shane, M.W.; Cramer, M.D.; Pearse, S.J.; Veneklaas, E.J. Root structure and functioning for efficient acquisition of phosphorus: Matching morphological and physiological traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [CrossRef] [Green Version]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.Q. Ecological C:N:P Stoichiometry of Soil Microorganisms and Its Driving Factors in Grassland of Inner Mongolia. Master’s Thesis, Nanjing Normal University, Nanjing, China, 2019. [Google Scholar]




| Different Ecosystems | C:N | C:P | N:P | MBC:MBN | MBC:MBP | MBN:MBP |
|---|---|---|---|---|---|---|
| Grassland | 11.92 ± 9.19 ab | 143.36 ± 253.32 a | 13.08 ± 17.96 a | 14.53 ± 10.29 b | 181.85 ± 241.91 a | 10.61 ± 14.35 b |
| Forest | 8.43 ± 3.35 ab | 92.54 ± 130.09 a | 13.83 ± 22.76 a | 14.12 ± 5.77 b | 97.56 ± 196.69 b | 6.07 ± 4.49 bc |
| Cropland | 12.01 ± 10.35 ab | 113.85 ± 296.64 a | 8.89 ± 8.46 a | 12.60 ± 17.09 b | 73.03 ± 52.09 bc | 6.29 ± 3.98 b |
| Desert | 12.56 ± 3.31 a | 34.97 ± 26.62 a | 2.88 ± 2.09 a | 8.55 ± 3.79 c | 1.48 ± 0.99 c | 0.20 ± 0.20 d |
| Wetland | 16.94 ± 1.89 b | Na | Na | 26.96 ± 16.97 a | 2.23 ± 17.50 c | 2.32 ± 0.88 a |
| Other | 8.11 ± 3.99 ab | 63.19 ± 25.50 a | 7.33 ± 4.57 a | 12.29 ± 4.40 bc | 58.57 ± 41.32 bc | 4.72 ± 3.18 cd |
| Overall | 10.69 | 100.19 | 10.33 | 13.7 | 85.15 | 9.23 |
| Region | Soil Nutrients | Soil Microbial Biomass | Citations | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C:N | C:P | N:P | C:N:P | C:N | C:P | N:P | C:N:P | ||
| Global | 14.3 ± 0.5 * | 186.0 ± 12.9 * | 13.1 ± 0.8 * | 186:13:01 | 8.6 ± 0.3 | 59.5 ± 3.6 | 6.9 ± 0.4 | 60:07:01 | Cleveland & Liptzin [13] |
| 16.4 * | 286.5 * | 17.5 * | 287:17:01 | 7.6 | 42.4 | 5.6 | 42:06:01 | Xu et al. [6] | |
| China | 11.9 ± 0.1 | 61.0 ± 0.9 | 5.2 ± 0.1 | 60:05:01 | - | - | - | - | Tian et al. [14] |
| 10.7 | 100.2 | 10.3 | 66:05:01 | 13.7 * | 85.2 * | 9.2 * | 22:02:01 | Our study | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Fang, F.; Tang, H. Patterns and Internal Stability of Carbon, Nitrogen, and Phosphorus in Soils and Soil Microbial Biomass in Terrestrial Ecosystems in China: A Data Synthesis. Forests 2021, 12, 1544. https://doi.org/10.3390/f12111544
Pan Y, Fang F, Tang H. Patterns and Internal Stability of Carbon, Nitrogen, and Phosphorus in Soils and Soil Microbial Biomass in Terrestrial Ecosystems in China: A Data Synthesis. Forests. 2021; 12(11):1544. https://doi.org/10.3390/f12111544
Chicago/Turabian StylePan, Yunlong, Fei Fang, and Haiping Tang. 2021. "Patterns and Internal Stability of Carbon, Nitrogen, and Phosphorus in Soils and Soil Microbial Biomass in Terrestrial Ecosystems in China: A Data Synthesis" Forests 12, no. 11: 1544. https://doi.org/10.3390/f12111544






