Effects of Gap Size and Cardinal Directions on Natural Regeneration, Growth Dynamics of Trees outside the Gaps and Soil Properties in European Beech Forests of Southern Italy
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Sites
2.2. Gap Opening
2.3. Soil Analysis
2.3.1. Measurement of Soil Temperature, Soil Moisture and Photosynthetically Active Radiation
2.3.2. Measurement of Soil Chemical and Physical Properties
2.4. Natural Regeneration, Tree Sampling, Ring Width and BAI Analyses
2.5. Statistical Analysis
3. Results
3.1. Soil Properties
3.1.1. Soil Temperature, Soil Moisture and PAR
3.1.2. Soil Chemical and Physical Properties
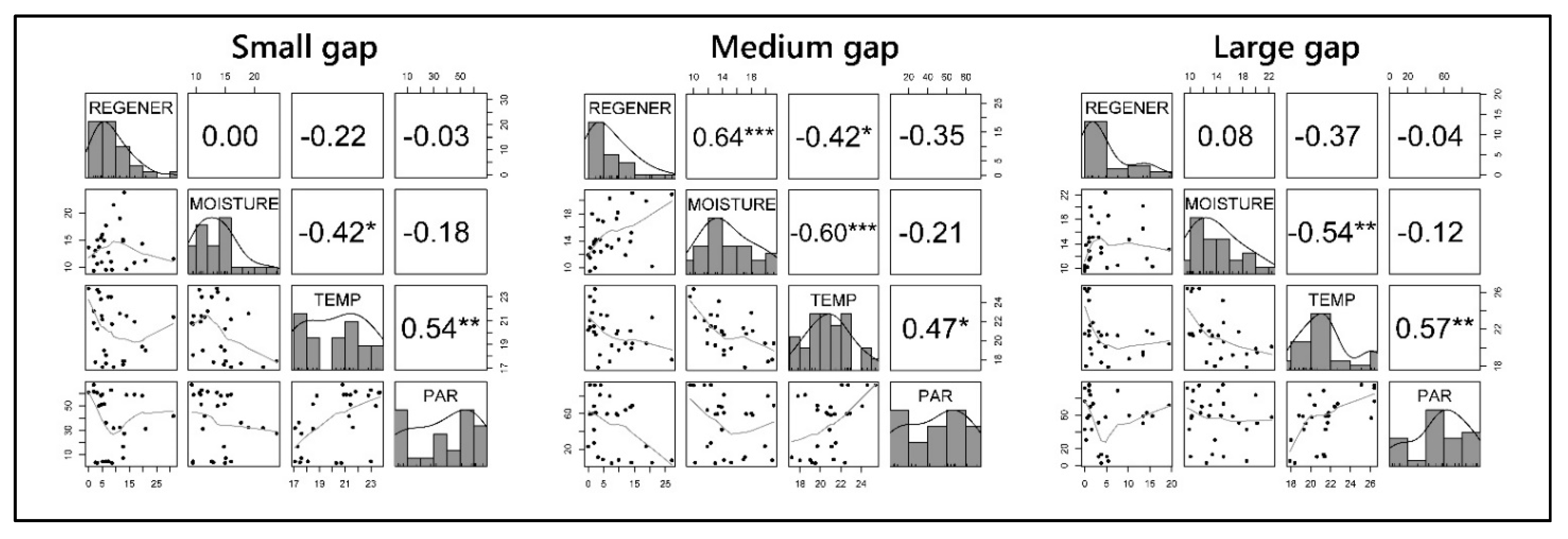
3.2. Natural Regeneration
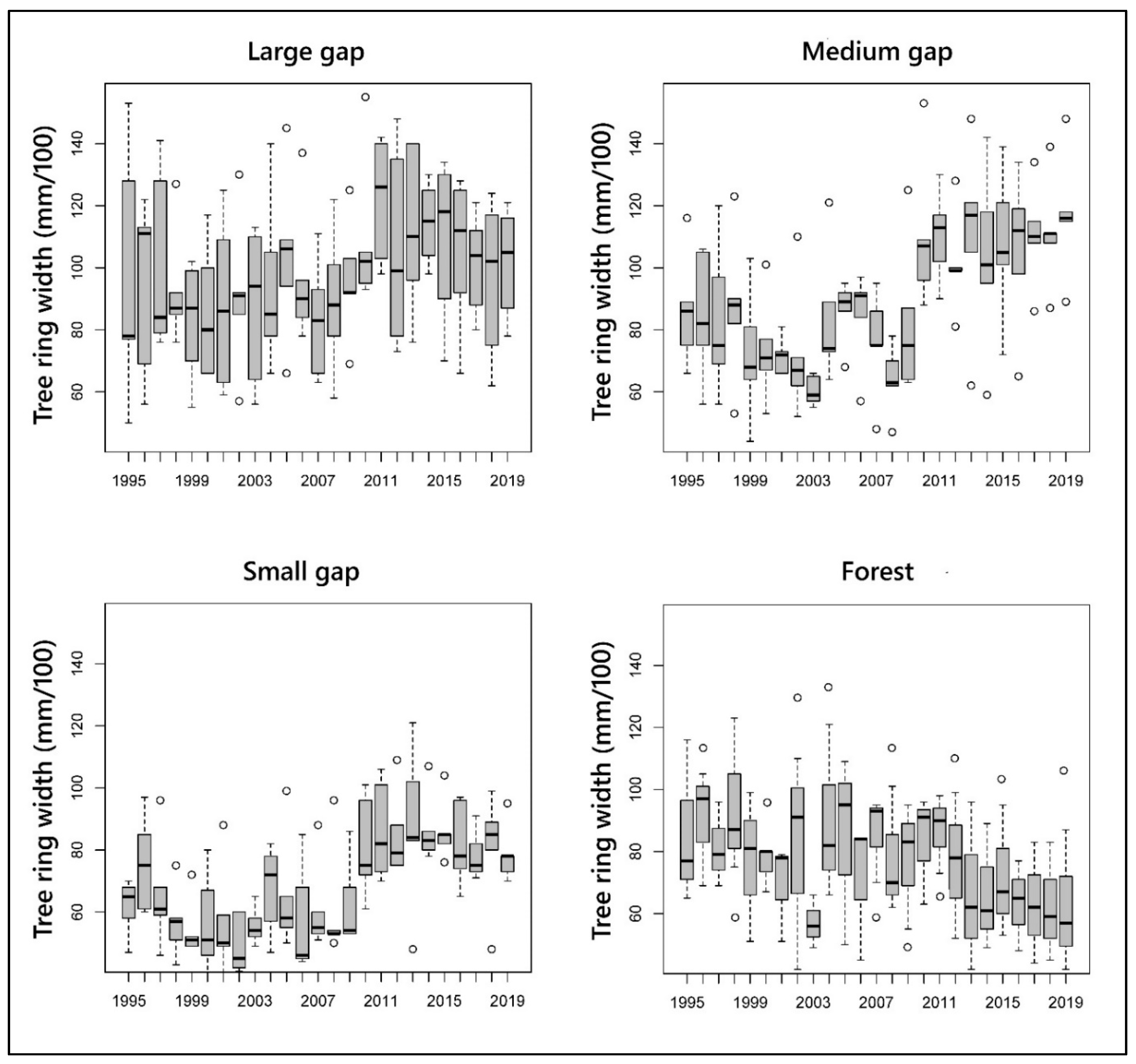
3.3. BAI Values and Tree Ring Width
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Puettmann, K.J.; Coates, K.D.; Messier, C. A Critique of Silviculture; Managing for Complexity; Island Press: Washington, DC, USA, 2009. [Google Scholar]
- Conti, F.; Abbate, G.; Alessandrini, A.; Blasi, C. An Annotated Checklist of the Italian Vascular Flora; Palombi Editore: Roma, Italy, 2005. [Google Scholar]
- Marziliano, P.A.; Antonucci, S.; Tognetti, R.; Chirici, G.; Corona, P.; Lombardi, F. Factors affecting the quantity and type of tree-related microhabitats in Mediterranean mountain forests of high nature value. iForest 2021, 14, 250–259. [Google Scholar] [CrossRef]
- Nocentini, S.; Buttoud, G.; Ciancio, O.; Corona, P. Managing forests in a changing world: The need for a systemic approach. A review. For. Syst. 2017, 26, eR01. [Google Scholar] [CrossRef]
- Gardiner, B.; Blennow, K.; Carnus, J.M.; Fleischer, P.; Ingemarson, F.; Landmann, G.; Lindner, M.; Marzano, M.; Nicoll, B.; Orazio, C.; et al. Destructive Storms in European Forests: Past and Forthcoming Impacts. European Forest Institute: Atlantic European Regional Office. Final Report to DG Environment (07.0307/2009/SI2.540092/ETU/B.1). 2010, p. 138. Available online: https://www.researchgate.net/publication/234080766_Destructive_storms_in_European_forests_past_and_forthcoming_impacts (accessed on 4 November 2021).
- Thom, D.; Seidl, R.; Steirer, G.; Krehan, H.; Formayer, H. Slow and fast drivers of the natural disturbance regime in Central European forest ecosystems. For. Ecol. Manag. 2013, 307, 293–302. [Google Scholar] [CrossRef]
- Thom, D.; Seidl, R. Natural disturbance impacts on ecosystem services and biodiversity in temperate and boreal forests. Biol. Rev. 2016, 91, 760–781. [Google Scholar] [CrossRef]
- Kulakowski, D.; Svoboda, M.; Bebi, P. The central role of disturbances in mountain forests of Europe. For. Ecol. Manag. 2017, 388, 1–2. [Google Scholar] [CrossRef]
- Orman, O.; Wrzesinski, P.; Dobrowolska, D.; Szewczyk, J. Regeneration growth and crown architecture of European beech and silver fir depend on gap characteristics and light gradient in the mixed montane old-growth stands. For. Ecol. Manag. 2021, 482, 118866. [Google Scholar] [CrossRef]
- Bottero, A.; Garbarino, M.; Dukic, V.; Govedar, Z.; Lingua, E.; Nagel, T.A.; Motta, R. Gapphase dynamics in the old-growth forest of Lom, Bosnia and Herzegovina. Silva Fenn. 2011, 45, 875–887. [Google Scholar] [CrossRef] [Green Version]
- Nagel, T.A.; Svoboda, M. Gap disturbance regime in an old-growth Fagus–Abies forest in the Dinaric Mountains, Bosnia-Herzegovina. Can. J. For. Res. 2008, 38, 2728–2737. [Google Scholar] [CrossRef] [Green Version]
- Rentch, J.S.; Schuler, T.M.; Nowacki, G.J.; Beane, N.R.; Ford, W.M. Canopy gap dynamics of second-growth red spruce-northern hardwood stands in West Virginia. For. Ecol. Manag. 2010, 260, 1921–1929. [Google Scholar] [CrossRef]
- Muscolo, A.; Bagnato, S.; Sidari, M.; Mercurio, R. A review of the roles of forest canopy gaps. J. For. Res. 2014, 25, 725–736. [Google Scholar] [CrossRef]
- Jaloviar, P.; Sedmáková, D.; Pittner, J.; Danková, L.J.; Kucbel, S.; Sedmákand, R.; Saniga, M. Gap structure and regeneration in the mixed old-growth forests of National Nature Reserve Sitno, Slovakia. Forests 2020, 11, 81. [Google Scholar] [CrossRef] [Green Version]
- Schliemann, S.; Bockheim, J. Methods for studying treefall gaps: A review. For. Ecol. Manag. 2011, 261, 1143–1151. [Google Scholar] [CrossRef]
- Marziliano, P.A.; Coletta, V.; Scuderi, A.; Scalise, C.; Menguzzato, G.; Lombardi, F. Forest structure of a maple old-growth stand: A case study on the Apennines mountains (Southern Italy). J. Mt. Sci. 2017, 14, 1329–1340. [Google Scholar] [CrossRef]
- Muscolo, A.; Settineri, G.; Bagnato, S.; Mercurio, R.; Sidari, M. Use of canopy gap openings to restore coniferous stands in Mediterranean environment. iForest 2017, 10, 322–327. [Google Scholar] [CrossRef] [Green Version]
- Košulič, O.; Michalko, R.; Hula, V.; Heneberg, P. Impact of canopy openness on spider communities: Implications for conservation management of formerly coppiced Oak forests. PLoS ONE 2016, 11, e0148585. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.D.; Cheng, C.C.; Chang, C.C.; Lin, C.; Chang, K.C.; Chuang, Y.C. Gap shape classification using landscape indices and multivariate statistics. Sci. Rep. 2016, 6, 38217. [Google Scholar] [CrossRef] [PubMed]
- Ritter, E.; Dalsgaard, L.; Einhorn, K.S. Light, temperature and soil moisture regimes following gap formation in a semi-natural beech-dominated forest in Denmark. For. Ecol. Manag. 2005, 206, 1533. [Google Scholar] [CrossRef]
- Rozenbergar, D.; Mikac, S.; Anic, I.; Diaci, J. Gap regeneration patterns in relationship to light heterogeneity in two old-growth beech fir forest reserves in South East Europe. Forestry 2007, 80, 431–443. [Google Scholar]
- Gray, A.N.; Spies, T.A.; Pabst, R.J. Canopy gaps affect long-term patterns of tree growth and mortality in mature and old-growth forests in the Pacific Northwest. For. Ecol. Manag. 2012, 281, 111–120. [Google Scholar] [CrossRef]
- Stan, A.B.; Daniels, L.D. Growth releases across a natural canopy gap-forest gradient in old-growth forests. For. Ecol. Manag. 2014, 313, 98–103. [Google Scholar] [CrossRef]
- Orman, O.; Dobrowolska, D.; Szwagrzyk, J. Gap regeneration patterns in Carpathian old-growth mixed beech forests—Interactive effects of spruce bark beetle canopy disturbance and deer herbivory. For. Ecol. Manag. 2018, 430, 451–459. [Google Scholar] [CrossRef]
- Dobrowolska, D.; Boncina, A.; Klumpp, R. Ecology and silviculture of silver fir (Abies alba Mill.): A review. J. For. Res. 2017, 22, 326–335. [Google Scholar] [CrossRef]
- Schwarz, J.A.; Bauhus, J. Benefits of mixtures on growth performance of silver fir (Abies alba) and European beech (Fagus sylvatica) increase with tree size without reducing drought tolerance. Front. For. Glob. Chang. 2019, 2, 79. [Google Scholar] [CrossRef]
- Zeibig, A.; Diaci, J.; Wagner, S. Gap disturbance patterns of a Fagus sylvatica virgin forest remnant in the mountain vegetation belt of Slovenia. For. Snow Landsc. Res. 2005, 79, 69–80. [Google Scholar]
- Drößler, L.; von Lüpke, B. Canopy gaps in two virgin beech forest reserves in Slovakia. J. For. Sci. 2005, 51, 446–457. [Google Scholar] [CrossRef] [Green Version]
- Kral, K.; Danek, P.; Janík, D.; Krůcek, M.; Vrska, T. How cyclical and predictable are Central European temperate forest dynamics in terms of development phases? J. Veg. Sci. 2018, 29, 84–97. [Google Scholar] [CrossRef] [Green Version]
- Nagel, T.A.; Svoboda, M.; Rugani, T.; Diaci, J. Gap regeneration and replacement patterns in an old-growth Fagus–Abies forest of Bosnia–Herzegovina. Plant Ecol. 2010, 208, 307–318. [Google Scholar] [CrossRef]
- Diaci, J.; Adamic, T.; Rozman, A. Gap recruitment and partitioning in an old-growth beech forest of the Dinaric Mountains: Influences of light regime, herb competition and browsing. For. Ecol. Manag. 2012, 285, 20–28. [Google Scholar] [CrossRef]
- Čater, M.; Diaci, J.; Roženbergar, D. Gap size and position influence variable response of Fagus sylvatica L. and Abies alba Mill. For. Ecol. Manag. 2014, 325, 128–135. [Google Scholar] [CrossRef]
- Hammond, M.E.; Pokorný, R. Effects of gap size on natural regeneration and microenvironmental soil conditions in European beech (Fagus sylvatica L.) and Norway spruce (Picea abies (L.) Karst) dominated mixed forest. Plant Soil Environ. 2020, 66, 607–615. [Google Scholar] [CrossRef]
- FAO World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. In World Soil Resources Reports; No. 106; FAO: Roma, Italy, 2014. [Google Scholar]
- Rivas-Martinez, S. Clasificacìon Bioclimàtica de la tierra. Folia Bot. Madritensis 1996, 16, 1–32. [Google Scholar]
- Ciancio, O.; Iovino, F.; Mendicino, V.; Menguzzato, G.; Nicolaci, A.; Nocentini, S. Structure and management of Aleppo pine forests. Options Méditerranéennes Ser. A 2008, 75, 61–72. [Google Scholar]
- Nocentini, S. Structure and management of beech (Fagus sylvatica L.) forests in Italy. iForest 2009, 2, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Russo, D.; Marziliano, P.A.; Macrì, G.; Proto, A.R.; Zimbalatti, G.; Lombardi, F. Does thinning intensity affect wood quality? An analysis of Calabrian pine in Southern Italy using a non-destructive acoustic method. Forests 2019, 10, 303. [Google Scholar] [CrossRef] [Green Version]
- Marziliano, P.A.; Tognetti, R.; Lombardi, F. Is tree age or tree size reducing height increment in Abies alba Mill. at its southernmost distribution limit? Ann. For. Sci. 2019, 76, 17. [Google Scholar] [CrossRef] [Green Version]
- Russo, D.; Marziliano, P.A.; Macrì, G.; Zimbalatti, G.; Tognetti, R.; Lombardi, F. Tree growth and wood quality in pure vs. mixed-species stands of European beech and Calabrian pine in Mediterranean mountain forests. Forests 2020, 11, 6. [Google Scholar] [CrossRef] [Green Version]
- Ciancio, O.; Iovino, F.; Menguzzato, G.; Nicolaci, A.; Nocentini, S. Structure and growth of a small group selection forest of Calabrian pine in Southern Italy: A hypothesis for continuous cover forestry based on traditional silviculture. For. Ecol. Manag. 2006, 224, 229–234. [Google Scholar] [CrossRef]
- Arseneault, J.E.; Saunders, M.R.; Seymour, R.S.; Wagner, R.G. First decadal response to treatment in a disturbance-based silviculture experiment in Maine. For. Ecol. Manag. 2011, 262, 404–412. [Google Scholar] [CrossRef]
- Comeau, P.G.; Gendron, F.; Letchford, T. A comparison of several methods for estimating light under a paper birch mixed wood stand. Can. J. For. Res. 1998, 28, 1843–1850. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analysis of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Kjeldahl, J. Methode zur bestimmung des stickstoffs in organischen Körpern. Z Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef] [Green Version]
- Kaminsky, R.; Muller, W.H. The extraction of soil phytotoxin using a neutral EDTA solution. Soil Sci. 1977, 124, 205–210. [Google Scholar] [CrossRef]
- Kaminsky, R.; Muller, W.H. A recommendation against the use of alkaline soil extraction in the study of allelopathy. Plant Soil 1978, 49, 641–645. [Google Scholar] [CrossRef]
- Box, J.D. Investigation of the Folin-Ciocalteau reagent for the determination of polyphenolic substances in natural waters. Water Res. 1983, 17, 511–525. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Muscolo, A.; Sidari, M.; Mercurio, R. Variations in soil chemical properties and microbial biomass in artificial gaps in Silver fir stands. Eur. J. For. Res. 2007, 126, 59–65. [Google Scholar] [CrossRef]
- Marziliano, P.A.; Menguzzato, G.; Scuderi, A.; Corona, P. Simplified methods to inventory the current annual increment of forest standing volume. iForest 2012, 5, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Diaci, J. Regeneration dynamics in a Norway spruce plantation on a silver fir-beech forest site in the Slovenian Alps. For. Ecol. Manag. 2002, 161, 27–38. [Google Scholar] [CrossRef]
- Gagnon, J.L.; Jokela, E.J.; Moser, W.K.; Huber, D.A. Dynamics of artificial regeneration in gaps within a longleaf pine flatwoods ecosystem. For. Ecol. Manag. 2003, 172, 133–144. [Google Scholar] [CrossRef]
- Mihók, B.; Gálhidy, L.; Kelemen, K.; Standovár, T. Study of gap-phase regeneration in a managed beech forest: Relations between tree regeneration and light, substrate features and cover of ground vegetation. Acta Silv. Lign. Hung. 2005, 1, 25–38. [Google Scholar]
- Raymond, P.; Munson, A.D.; Ruel, J.C.; Coates, K.D. Spatial patterns of soil, microclimate, light, regeneration, and growth within silvicultural gaps of mixed tolerant hardwood–white pine stands. Can. J. For. Res. 2006, 36, 639–651. [Google Scholar] [CrossRef]
- Diaci, J.; Gyoerek, N.; Gliha, J.; Nagel, T.A. Response of Quercus robur L. seedlings to north-south asymmetry of light within gaps in floodplain forests of Slovenia. Ann. For. Sci. 2008, 65, 105. [Google Scholar] [CrossRef]
- Denslow, J.S. Tropical rainforest gaps and tree species diversity. Annu. Rev. Ecol. Syst. 1987, 18, 431–451. [Google Scholar] [CrossRef]
- Zhang, X.R.; Tan, X.F.; Wang, R.Q.; Xu, N.N.; Guo, W.H. Effects of soil moisture and light intensity on ecophysiological characteristics of Amorpha fruticosa seedlings. J. For. Res. 2013, 24, 293–300. [Google Scholar] [CrossRef]
- Malcom, D.C.; Mason, W.; Clarke, G.C. The transformation of conifer forests in Britain regeneration, gap size and silvicultural systems. For. Ecol. Manag. 2001, 151, 7–23. [Google Scholar] [CrossRef]
- Sariyildiz, T. Effects of gap-size classes on long-term litter decomposition rates of beech, oak and chestnut species at high elevations in Northeast Turkey. Ecosystems 2008, 11, 841–853. [Google Scholar] [CrossRef]
- Gray, A.N.; Spies, T.A.; Easter, M.J. Microclimate and soil moisture responses to gap formation in coastal Douglas-fir forests. Can. J. For. Res. 2002, 32, 332–343. [Google Scholar] [CrossRef] [Green Version]
- Muscolo, A.; Sidari, M.; Mercurio, R. Influence of gap size on organic matter decomposition, microbial biomass and nutrient cycle in Calabrian pine (Pinus laricio Poiret) stands. For. Ecol. Manag. 2007, 242, 412–418. [Google Scholar] [CrossRef]
- Bauhus, J. C and N mineralization in an acid forest soil along a gap-stand gradient. Soil Biol. Biochem. 1996, 28, 923–932. [Google Scholar] [CrossRef]
- Wright, E.F.; Coates, K.D.; Bartemucci, P. Regeneration from seed of six tree species in the interior cedar-hemlock forests of British Columbia as affected by substrate and canopy gap position. Can. J. For. Res. 1998, 28, 1352–1364. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Plant-soil interactions in Mediterranean forest and shrublands: Impacts of climatic change. Plant Soil 2013, 365, 1–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zirlewangen, D.; von Wilpert, K. Modelling water and ion fluxes in a highly structured, mixed species stand. For Ecol. Manag. 2001, 143, 27–37. [Google Scholar] [CrossRef]
- Zhu, J.; Matsuzaki, T.; Lee, F.; Gonda, Y. Effect of gap size created by thinning on seedling emergency, survival and establishment in a coastal pine forest. For. Ecol. Manag. 2003, 182, 339–354. [Google Scholar] [CrossRef]
- Ochiai, Y.; Okuda, S.; Sato, A. The influence of canopy gap size on soil water condition in a deciduous broad-leaved secondary forest in Japan. J. Jpn For. Soc. 1994, 76, 308–314. [Google Scholar]
- Mihók, B.; Gálhidy, L.; Kenderes, K.; Standovár, T. Gap Regeneration Patterns in a Semi-natural Beech Forest Stand in Hungary. Acta Silv. Lign. Hung. 2007, 3, 31–45. [Google Scholar]
- Albanesi, E.; Gugliotta, O.I.; Mercurio, I.; Mercurio, R. Effects of gap size and within-gap position on seedlings establishment in silver fir stands. iForest 2008, 1, 55–59. [Google Scholar] [CrossRef]
- Latif, Z.A.; Blackburn, G.A. The effects of gap size on some microclimate variables during late summer and autumn in a temperate broadleaved deciduous forest. Int. J. Biometeorol. 2010, 54, 119–129. [Google Scholar] [CrossRef]
- Kučera, A.; Holik, L.; Cerro, E.M.; Petříček, J. Effect of gap size and forest type on mineral nitrogen forms under different soil properties. J. For. Res. 2020, 31, 375–386. [Google Scholar] [CrossRef]
- Dai, X. Influence of light conditions in canopy gaps on forest regeneration: A new gap light index and its application in a boreal forest in east central Sweden. For. Ecol. Manag. 1996, 84, 187–197. [Google Scholar] [CrossRef]
- Buckman, H.O.; Brady, N.C. The Nature and Properties of Soils; The Macmillian Co.: London, UK, 1969. [Google Scholar]
- Prescott, C.E.; Hope, G.D.; Blevins, L.L. Effect of gap size on litter decomposition and soil nitrate concentrations in a high elevation spruce-fir forest. Can. J. For. Res. 2003, 33, 2210–2220. [Google Scholar] [CrossRef]
- Ritter, E.; Vesterdal, L. Gap formation in Danish beech (Fagus sylvatica) forests of low management intensity: Soil moisture and nitrate in soil solution. Eur. J. For. Res. 2006, 125, 139–150. [Google Scholar] [CrossRef]
- Scharenbroch, B.C.; Bockheim, J.G. Gaps and soil C dynamics in old growth northern hardwoodhemlock forests. Ecosystems 2008, 11, 426–441. [Google Scholar] [CrossRef]
- Denslow, J.S.; Ellison, A.M.; Sanford, R.E. Treefall gap size effects on above-and below-ground processes in a tropical wet forest. J. Ecol. 1998, 86, 597–609. [Google Scholar] [CrossRef]
- Zhang, Q.; Zak, J.C. Effects of gap size on litter decomposition and microbial activity in a subtropical forest. Ecology 1995, 76, 2196–2204. [Google Scholar] [CrossRef]
- Taylor, B.R.; Parsons, W.J.F.; Parkinson, D. Decomposition of Populus tremuloides leaf litter accelerated by addition of Alnus crispa litter. Can. J. For. Res. 1989, 19, 674–679. [Google Scholar] [CrossRef]
- Muscolo, A.; Mallamaci, C.; Sidari, M.; Mercurio, R. Effects of gap size and soil chemical properties on the natural regeneration in black pine (Pinus nigra Arn.) stands. Tree For. Sci. Biotechnol. 2011, 5, 65–71. [Google Scholar]
- Nagel, T.A.; Mikac, S.; Dolinar, M.; Klopcic, M.; Keren, S.; Svoboda, M.; Diac, J.; Boncina, A.; Paulic, V. The natural disturbance regime in forests of the Dinaric Mountains: A synthesis of evidence. For. Ecol. Manag. 2017, 388, 29–42. [Google Scholar] [CrossRef]
- Hammond, M.E.; Pokorný, R. Preliminary assessment of effect of disturbance on natural regeneration in gaps of different sizes. J. For. Sci. 2020, 66, 185–196. [Google Scholar] [CrossRef]
- Mountford, E.P.; Savill, P.S.; Bebber, D.P. Patterns of regeneration and ground vegetation associated with canopy gaps in a managed beechwood in southern England. Forestry 2006, 79, 389–408. [Google Scholar] [CrossRef]
- Gálhidy, L.; Mihók, B.; Hagyó, A.; Rajkai, K.; Standovár, T. Effects of gap size and associated changes in light and soil moisture on the understorey vegetation of a Hungarian beech forest. Plant Ecol. 2018, 183, 133–145. [Google Scholar] [CrossRef]
- Feldmann, E.; Glatthorn, J.; Ammer, C.; Leuschner, C. Regeneration dynamics following the formation of understory gaps in a Slovakian beech virgin forest. Forests 2018, 11, 585. [Google Scholar] [CrossRef]
- Wagner, S.; Fischer, H.; Huth, F. Canopy effects on vegetation caused by harvesting and regeneration treatments. Eur. J. For. Res. 2011, 130, 17–40. [Google Scholar] [CrossRef]
- Bílek, L.; Remeš, J.; Podrázský, V.; Rozenbergar, D.; Diaci, J.; Zahradník, D. Gap regeneration in near-natural European beech forest stands in Central Bohemia—The role of heterogeneity and micro-habitat factors. Dendrobiology 2014, 71, 59–71. [Google Scholar] [CrossRef] [Green Version]
- Čater, M.; Diaci, J. Divergent response of European beech, silver fir and Norway spruce advance regeneration to increased light levels following natural disturbance. For. Ecol. Manag. 2017, 399, 206–212. [Google Scholar] [CrossRef]
- Vilhar, U.; Roženbergar, D.; Simončič, P.; Diaci, J. Variation in irradiance, soil features and regeneration patterns in experimental forest canopy gaps. Ann. For. Sci. 2015, 72, 253–266. [Google Scholar] [CrossRef] [Green Version]
- Stiers, M.; Willim, K.; Seidel, D.; Ammer, C.; Kabal, M.; Stillhard, J.; Annighöfer, P. Analyzing spatial distribution patterns of European beech (Fagus sylvatica L.) regeneration in dependence of canopy openings. Forests 2019, 10, 637. [Google Scholar] [CrossRef] [Green Version]
- Sedmáková, D.; Sedmák, R.; Bosela, M.; Ježík, M.; Blaženec, M.; Hlásny, T.; Marušák, R. Growth climate responses indicate shifts in the competitive ability of European beech and Norway spruce under recent climate warming in East-Central Europe. Dendrochronologia 2019, 54, 37–48. [Google Scholar] [CrossRef]
- Farahat, E.; Linderholm, H.W. Growth-climate relationship of European beech at its northern distribution limit. Eur. J. For. Res. 2018, 137, 619–629. [Google Scholar] [CrossRef]
- Hobi, M.L.; Commarmot, B.; Bugmann, H. Pattern and process in the largest primeval beech forest of Europe (Ukrainian Carpathians). J. Veg. Sci. 2015, 26, 323–336. [Google Scholar] [CrossRef]
- Feldmann, E.; Drößler, L.; Hauck, M.; Kucbel, S.; Pichler, V.; Leuschner, C. Canopy gap dynamics and tree understory release in a virgin beech forest, Slovakian Carpathians. For. Ecol. Manag. 2018, 415, 38–46. [Google Scholar] [CrossRef]
- Yamamoto, S.I. Forest gap dynamics and tree regeneration. J. For. Res. 2000, 5, 223–229. [Google Scholar] [CrossRef]




| Gap | Position | pH | Sand % | Silt % | Clay % | Textural Class | CaCO3 |
|---|---|---|---|---|---|---|---|
| Small | CEN | 5.5 ± 0.13 | 81 ± 3 | 11 ± 2 | 8 ± 1 | Loamy-sand | 0.0 |
| North | 5.6 ± 0.09 | 81 ± 3 | 11 ± 2 | 8 ± 1 | Loamy-sand | 0.0 | |
| East | 5.8 ± 0.10 | 81 ± 3 | 11 ± 2 | 8 ± 1 | Loamy-sand | 0.0 | |
| South | 5.5 ± 0.11 | 81 ± 3 | 11 ± 2 | 8 ± 1 | Loamy-sand | 0.0 | |
| West | 5.6 ± 0.07 | 81 ± 3 | 11 ± 2 | 8 ± 1 | Loamy-sand | 0.0 | |
| Forest | 5.8 ± 0.09 | 81 ± 3 | 11 ± 2 | 8 ± 1 | Loamy-sand | 0.0 | |
| Medium | CEN | 5.7 ± 0.14 | 80 ± 4 | 10 ± 1 | 10 ± 2 | Loamy-sand | 0.0 |
| North | 5.9 ± 0.08 | 80 ± 4 | 10 ± 1 | 10 ± 2 | Loamy-sand | 0.0 | |
| East | 5.7 ± 0.13 | 80 ± 4 | 10 ± 1 | 10 ± 2 | Loamy-sand | 0.0 | |
| South | 5.5 ± 0.09 | 80 ± 4 | 10 ± 1 | 10 ± 2 | Loamy-sand | 0.0 | |
| West | 5.7 ± 0.10 | 80 ± 4 | 10 ± 1 | 10 ± 2 | Loamy-sand | 0.0 | |
| Forest | 5.6 ± 0.13 | 80 ± 4 | 10 ± 1 | 10 ± 2 | Loamy-sand | 0.0 | |
| Large | CEN | 5.7 ± 0.16 | 78 ± 3 | 16 ± 2 | 8 ± 1 | Loamy-sand | 0.0 |
| North | 5.9 ± 0.05 | 78 ± 3 | 16 ± 2 | 8 ± 1 | Loamy-sand | 0.0 | |
| East | 5.5 ± 0.12 | 78 ± 3 | 16 ± 1 | 8 ± 1 | Loamy-sand | 0.0 | |
| South | 5.6 ± 0.12 | 78 ± 3 | 16 ± 2 | 8 ± 1 | Loamy-sand | 0.0 | |
| West | 5.8 ± 0.10 | 78 ± 2 | 16 ± 2 | 8 ± 1 | Loamy-sand | 0.0 | |
| Forest | 5.5 ± 0.13 | 78 ± 1 | 16 ± 2 | 8 ± 1 | Loamy-sand | 0.0 |
| Small | Medium | Large | Forest | ||
|---|---|---|---|---|---|
| OM | CEN | 6.2 ± 0.8 | 7.1 ± 0.8 | 6.3 ± 1.1 | 8.9 ± 2.1 |
| North | 5.5 ± 1.1 | 7.2 ± 0.9 | 6.5 ± 1.0 | ||
| East | 5.6 ± 1.3 | 7.6 ± 1.0 | 6.0 ± 0.7 | ||
| South | 7.6 ± 2.2 | 7.6 ± 1.2 | 6.5 ± 0.9 | ||
| West | 6.9 ± 1.1 | 7.1 ± 1.3 | 5.8 ± 2.5 | ||
| WSP | CEN | 170 ± 23 | 177 ± 35 | 174 ± 31 | 148 ± 15 |
| North | 180 ± 28 | 178 ± 17 | 185 ± 41 | ||
| East | 179 ± 13 | 171 ± 34 | 178 ± 29 | ||
| South | 177 ± 24 | 174 ± 36 | 179 ± 34 | ||
| West | 179 ± 13 | 176 ± 38 | 185 ± 30 | ||
| C/N | CEN | 27 ± 6.5 | 26 ± 3.9 | 19 ± 3.3 | 21 ± 4.7 |
| North | 26 ± 9.7 | 28 ± 7.4 | 19 ± 2.6 | ||
| East | 29 ± 5.0 | 26 ± 2.5 | 20 ± 4.1 | ||
| South | 23 ± 9.7 | 23 ± 2.7 | 15 ± 1.8 | ||
| West | 22 ± 7.1 | 27 ± 5.2 | 20 ± 4.4 | ||
| FDA | CEN | 40 ± 3.4 | 50 ± 8.3 | 41 ± 11.5 | 61 ± 5.6 |
| North | 36 ± 3.9 | 51 ± 8.1 | 39 ± 8.7 | ||
| East | 44 ± 9.5 | 49 ± 7.5 | 39 ± 8.1 | ||
| South | 54 ± 8.0 | 59 ± 5.9 | 48 ± 4.5 | ||
| West | 41 ± 4.7 | 40 ± 6.4 | 38 ± 10.1 | ||
| DHA | CEN r | 7.7 ± 2.1 | 9.1 ± 5.1 | 8.3 ± 0.8 | 15.0 ± 5.3 |
| North | 7.0 ± 1.5 | 9.6 ± 1.9 | 7.9 ± 1.4 | ||
| East | 8.6 ± 3.0 | 8.5 ± 2.0 | 8.9 ± 1.5 | ||
| South | 10 ± 5.3 | 12 ± 2.0 | 11 ± 3.0 | ||
| West | 8.0 ± 2.4 | 8.8 ± 3.4 | 8.4 ± 3.1 | ||
| C | CENr | 3.6 ± 1.1 | 4.1 ± 1.2 | 3.7 ± 0.8 | 5.2 ± 1.6 |
| North | 3.2 ± 0.9 | 4.2 ± 0.7 | 3.8 ± 1.2 | ||
| East | 3.2 ± 1.3 | 4.4 ± 1.4 | 3.5 ± 0.8 | ||
| South | 3.6 ± 2.3 | 4.4 ± 0.7 | 3.8 ± 1.1 | ||
| West | 3.3 ± 0.5 | 4.1 ± 1.0 | 3.4 ± 1.0 | ||
| N | CEN | 1.3 ± 0.2 | 1.6 ± 0.2 | 2.0 ± 1.0 | 2.4 ± 0.3 |
| North | 1.2 ± 0.2 | 1.5 ± 03 | 2.0 ± 0.9 | ||
| East | 1.1 ± 0.3 | 1.7 ± 0.4 | 1.7 ± 0.4 | ||
| South | 1.6 ± 0.1 | 1.9 ± 0.2 | 2.5 ± 0.4 | ||
| West | 1.5 ± 0.3 | 1.5 ± 0.3 | 1.7 ± 0.5 | ||
| K+ | CEN | 147 ± 75.5 | 212 ± 144.3 | 192 ± 103.5 | 312 ± 68.3 |
| North | 160 ± 80.1 | 238 ± 161.1 | 187 ± 99.6 | ||
| East | 160 ± 62.3 | 244 ± 75.4 | 177 ± 74.3 | ||
| South | 260 ± 94.7 | 268 ± 80.1 | 190 ± 100.4 | ||
| West | 150 ± 78.3 | 250 ± 101.6 | 167 ± 92.8 | ||
| MBC | CEN | 3100 ± 295 | 3900 ± 311 | 2010 ± 279 | 3570 ± 294 |
| North | 3370 ± 175 | 4400 ± 293 | 1700 ± 313 | ||
| East | 3500 ± 221 | 4770 ± 315 | 1990 ± 274 | ||
| South | 3990 ± 337 | 4660 ± 339 | 2400 ± 364 | ||
| West | 3010 ± 365 | 4220 ± 359 | 1900 ± 240 |
| Estimate | Std. Error | z Value | Pr(>|z|) | Sig. | |
|---|---|---|---|---|---|
| Intercept | 1.8973 | 0.1790 | 10.599 | <2.0e-16 | *** |
| GAP SIZE | 0.0014 | 0.0003 | 5.407 | 6.4e-08 | *** |
| Center | 0.7012 | 0.0946 | 7.409 | 1.3e-13 | *** |
| East | 0.3037 | 0.1487 | 2.043 | 4.1e-02 | * |
| North | −0.2742 | 0.1660 | −1.652 | 9.8e-02 | - |
| South | 0.4828 | 0.1448 | 3.334 | 9.0e-04 | ** |
| West | 0.0264 | 0.1560 | 0.169 | 8.6e-01 | - |
| Large Gap | Medium Gap | Small Gap | FOREST | |
|---|---|---|---|---|
| Ring Width 1999–2009 (mm yr−1) | 0.94 ± 0.22 | 0.74 ± 0.19 | 0.58 ± 0.14 | 0.81 ± 0.19 |
| Ring Width 2010–2019 (mm yr−1) | 1.14 ± 0.25 | 1.16 ± 0.24 | 0.85 ± 0.18 | 0.72 ± 0.17 |
| z-value | 3.853 | 7.863 | 7.542 | 1.899 |
| p-value | <0.0001 | <0.0001 | <0.0001 | 0.058 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagnato, S.; Marziliano, P.A.; Sidari, M.; Mallamaci, C.; Marra, F.; Muscolo, A. Effects of Gap Size and Cardinal Directions on Natural Regeneration, Growth Dynamics of Trees outside the Gaps and Soil Properties in European Beech Forests of Southern Italy. Forests 2021, 12, 1563. https://doi.org/10.3390/f12111563
Bagnato S, Marziliano PA, Sidari M, Mallamaci C, Marra F, Muscolo A. Effects of Gap Size and Cardinal Directions on Natural Regeneration, Growth Dynamics of Trees outside the Gaps and Soil Properties in European Beech Forests of Southern Italy. Forests. 2021; 12(11):1563. https://doi.org/10.3390/f12111563
Chicago/Turabian StyleBagnato, Silvio, Pasquale A. Marziliano, Maria Sidari, Carmelo Mallamaci, Federica Marra, and Adele Muscolo. 2021. "Effects of Gap Size and Cardinal Directions on Natural Regeneration, Growth Dynamics of Trees outside the Gaps and Soil Properties in European Beech Forests of Southern Italy" Forests 12, no. 11: 1563. https://doi.org/10.3390/f12111563






