Cross-Cultural Leadership Enables Collaborative Approaches to Management of Kauri Dieback in Aotearoa New Zealand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Aerial Surveillance
2.3. Ground Surveillance
2.4. Sampling for Phytophthora Agathidicida Presence
2.5. Mapping
2.6. Track Analysis
- The actual track network of the WRRP intersects a greater amount of kauri than a randomly generated track.
- The actual track network has a higher percentage of kauri dieback along it than the randomly generated track.
2.7. Replication
3. Results
3.1. Presence of Kauri Dieback
3.2. Track Analysis
4. Discussion
4.1. Survey of Distribution of Kauri Dieback
4.2. Cross-Cultural Management of Kauri Dieback and Rāhui—Merging Western Science and Mātaranga Indicators
- cultural quarantine—enable the forest to ‘self-heal’ without the presence of humans (or harmful human behaviours).
- exclusion zones—shifting most planned future recreation away from high-value ecosystem areas and towards the edge of the forest.
- ‘rolling openings’ of tracks—closing all tracks and only re-opening tracks that are strategically located and designed and upgraded to accommodate tikanga, ecological, biosecurity, engineering, and accessibility criteria.
- adaptive management—altering our methods and processes as new information emerges and to suit the situation or context.
- Monitoring—ongoing monitoring based on both scientific and cultural indicators.
- Research—ongoing research including whakapapa seed collections that represent an inter-generational response to the threat of kauri dieback.
4.3. Beyond Rāhui
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Royal, T.A.C. ‘Māori Creation Traditions’, Te Ara—The Encyclopedia of New Zealand. Available online: http://www.TeAra.govt.nz/en/maori-creation-traditions/print (accessed on 1 June 2021).
- Lee, D.E.; Bannister, J.M.; Lindqvist, J.K. Late Oligocene-Early Miocene leaf macrofossils confirm a long history of Agathis in New Zealand. N. Z. J. Bot. 2007, 45, 565–578. [Google Scholar] [CrossRef]
- Ecroyd, C.E. Biological flora of New Zealand 8. Agathis australis. Kauri. N. Z. J. Bot. 1982, 20, 17–36. [Google Scholar] [CrossRef]
- Ogden, J. The long-term conservation of forest diversity in New Zealand. Pac. Conserv. Biol. 1995, 2, 77–90. [Google Scholar] [CrossRef]
- Craig, J.; Anderson, S.; Clout, M.; Creese, B.; Mitchell, N.; Ogden, J.; Roberts, M.; Ussher, G. Conservation Issues in New Zealand. Annu. Rev. Ecol. Syst. 2000, 31, 61–78. [Google Scholar] [CrossRef]
- Jones, D.; Molloy, B.; Clements, M. Six new species of Pterostylis, R. Br. (Orchidaceae) from New Zealand. Orchadian 1997, 12, 266–281. [Google Scholar]
- Ahmed, M.; Ogden, J. Population dynamics of the emergent conifer Agathis australis (D. Don) Lindl. (kauri) in New Zealand. N. Z. J. Bot. 1987, 25, 217–229. [Google Scholar] [CrossRef]
- McKenzie, E.; Buchanan, P.; Johnston, P. Checklist of fungi on kauri (Agathis australis) in New Zealand. N. Z. J. Bot. 2002, 40, 269–296. [Google Scholar] [CrossRef]
- Padamsee, M.; Johansen, R.B.; Stuckey, S.A.; Williams, S.E.; Hooker, J.E.; Burns, B.R.; Bellgard, S.E. The arbuscular mycorrhizal fungi colonising roots and root nodules of New Zealand kauri Agathis australis. Fungal Biol. 2016, 120, 807–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvester, W.; Orchard, T. The biology of kauri (Agathis australis) in New Zealand. Production, biomass, carbon storage, and litter fall in four forest remnants. N. Z. J. Bot. 1999, 37, 553–571. [Google Scholar] [CrossRef] [Green Version]
- Wyse, S.V.; Burns, B.R. Effects of Agathis australis (New Zealand kauri) leaf litter on germination and seedling growth differs among plant species. N. Z. J. Bot. 2013, 37, 178–183. [Google Scholar]
- Bradshaw, R.E.; Bellgard, S.E.; Black, A.; Burns, B.R.; Gerth, M.L.; McDougal, R.L.; Scott, P.M.; Waipara, N.W.; Weir, B.S.; Williams, N.M.; et al. Phytophthora agathidicida: Research progress, cultural perspectives and knowledge gaps in the control and management of kauri dieback in New Zealand. Plant Pathol. 2020, 69, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Steward, G.A.; Beveridge, A.E. A review of New Zealand kauri (Agathis australis (D. Don) Lindl.): Its ecology, history, growth and potential for management for timber. N. Z. J. For. Sci. 2010, 40, 33–59. [Google Scholar]
- Beever, R.E.; Waipara, N.W.; Ramsfield, T.D.; Dick, M.A.; Horner, I.J. Kauri (Agathis australis) under threat from Phytophthora. Phytophthoras For. Nat. Ecosyst. 2009, 74, 74–85. [Google Scholar]
- Weir, B.S.; Paderes, E.P.; Anand, N.; Uchida, J.Y.; Pennycook, S.R.; Bellgard, S.E.; Beever, R.E. A taxonomic revision of Phytophthora Clade 5 including two new species, Phytophthora agathidicida and P. cocois. Phytotaxa 2015, 205, 21–38. [Google Scholar] [CrossRef]
- Waipara, N.; Hill, S.; Hill, L.; Hough, E.; Horner, I. Surveillance methods to determine tree health, distribution of kauri dieback disease and associated pathogens. N. Z. Plant Prot. 2013, 66, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Horner, I.J.; Hough, E.G. Pathogenicity of four Phytophthora species on kauri: In-vitro and glasshouse trials. N. Z. Plant Prot. 2014, 67, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Beever, R.E.; Tsai, S.; Waipara, N.W.; Dick, M.A.; Ramsfield, T.D. Pathogenicity of Phytophthora Taxon Agathis. In Proceedings of the 5th IUFRO Working Party S07.02.09, Phytophthora Diseases in Forests and Natural Ecosystems, Rotorua, New Zealand, 8–12 March 2010; p. 2. [Google Scholar]
- Gadgil, P.D. Phytophthora heveae, a pathogen of kauri. N. Z. J. For. Sci. 1974, 4, 59–63. [Google Scholar]
- Beachman, J. The Introduction and Spread of Kauri Dieback Disease in New Zealand: Did Historic Forestry Operations Play a Role? Ministry for Primary Industries Report; Ministry for Primary Industries: Wellington, New Zealand, 2017; ISBN 978-1-77665-669-1.
- Waipara, N.W.; Davis, A.; Hill, S.L.; Brooks, J.; Pengelly, M.; Barr, J.A.; Bellgard, S.E.; Beever, R.E. Management of Kauri Dieback and Phytophthora Taxon Agathis. In Proceedings of the 5th IUFRO Working Party S07.02.09, Phytophthora Diseases in Forests and Natural Ecosystems, Rotorua, New Zealand, 8–12 March 2010; p. 41. [Google Scholar]
- Kauri Protection Programme. Kauri and Kauri Dieback Distribution. Available online: https://www.kauriprotection.co.nz/kauri-maps/ (accessed on 1 June 2021).
- Hill, L.M.W.; Waipara, N.W. Phytophthora agathidicida sampling of symptomatic kauri in close proximity to Tāne Mahuta, Waipoua Forest Unpublished Report to Te Kawerau ā Maki. Unpublished work. 2018. [Google Scholar]
- Jung, T.; Pérez-Sierra, A.; Durán, A.; Jung, M.H.; Balci, Y.; Scanu, B. Canker and decline diseases caused by soil- and airborne Phytophthora species in forests and woodlands. Persoonia 2018, 40, 182–220. [Google Scholar] [CrossRef] [Green Version]
- Brasier, C.M. The biosecurity threat to the UK and global environment from international trade in plants. Plant Pathol. 2008, 57, 792–808. [Google Scholar] [CrossRef]
- Hill, L.M.W.; Waipara, N.W.; Horner, I.J. Ongoing monitoring of kauri dieback risk with Auckland Councils Regional Park network. Unpublished work. 2017. [Google Scholar]
- Jung, T.; Scanu, B.; Brasier, C.M.; Webber, J.; Milenkovíc, I.; Corcobado, T.; Tomšovský, M.; Pánek, M.; Bakonyi, J.; Maia, C.; et al. A survey in natural forest ecosystems of Vietnam reveals high diversity of both new and described Phytophthora Taxa including P. ramorum. Forests 2020, 11, 93. [Google Scholar] [CrossRef] [Green Version]
- Nowak, K.J.; Trzewik, A.; Tułacz, D.; Orlikowska, T.; Orlikowski, L.B. Characterization of Polish Phytophthora lacustris Isolates Obtained from Water Environments. Pol. J. Environ. Stud. 2015, 24, 619–630. [Google Scholar] [CrossRef]
- Riddell, C.E.; Dun, H.F.; Elliot, M.; Armstrong, A.C.; Clark, M.; Forster, J.; Hedley, P.E.; Green, S. Detection and spread of Phytophthora austrocedri within infected Juniperus communis woodland and diversity of co-associated Phytophthoras as revealed by metabarcoding. For. Pathol. 2020, 50, e12602. [Google Scholar] [CrossRef]
- Hill, L.M.W.; Hjelm, K.F.; Bellgard, S.B.; Winkworth, R. Kauri Dieback Disease Surveillance of Watercare’s Proposed Replacement Water Treatment Plant Site at Waima Catchment. Resource Consent Hearing Report. 2020. Available online: https://wslpwstoreprd.blob.core.windows.net/kentico-media-libraries-prod/watercarepublicweb/media/watercare-media-library/huia/biosense_kauri_dieback_disease_surveillance_report_nov_2020.pdf (accessed on 20 November 2021).
- Lambert, S.; Waipara, N.W.; Black, A.; Mark-Shadbolt, M.; Wood, W. Indigenous biosecurity: Maori Responses to Kauri Dieback and Myrtle Rust in Aotearoa New Zealand. In The Human Dimensions of Forest and Tree Health: Global Perspectives; Urquhart, J., Marzano, M., Potter, C., Eds.; Springer International Publishing: Amsterdam, The Netherlands, 2018; pp. 109–137. [Google Scholar]
- Garnett, S.; Burgess, N.; Fa, J.; Fernández-Llamazares, Á.; Molnár, Z.; Robinson, C.; Watson, J.; Zander, K.; Austin, B.; Brondízio, E.; et al. A spatial overview of the global importance of Indigenous lands for conservation. Nat. Sustain. 2018, 1, 369–374. [Google Scholar] [CrossRef]
- Schuster, R.; Germain, R.R.; Bennett, J.R.; Reo, N.J.; Arcese, P. Vertebrate biodiversity on indigenous-managed lands in Australia, Brazil, and Canada equals that in protected areas. Environ. Sci. Policy 2019, 101, 1–6. [Google Scholar] [CrossRef]
- Abdullahi, J.; Usman, I.; Samaila, G.; Zuni, A. Importance of Indigenous Knowledge in Biodiversity Conservation: A Case Study of Communities Surrounding Kpashimi Forest Reserve, Niger State, Nigeria. J. Environ. Sci. Toxicol. Food Technol. 2013, 6, 10–17. [Google Scholar] [CrossRef]
- Halim, A.A.; Othman, N.; Ismail, S.R.; Jawan, J.A.; Ibrahim, N.N. Indigenous Knowledge and Biodiversity Conservation in Sabah, Malaysia. Int. J. Soc. Sci. Humanit. 2013, 159–163. [Google Scholar] [CrossRef]
- IPBES. Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Brondizio, E.S., Settele, J., Díaz, S., Ngo, H.T., Eds.; IPBES Secretariat: Bonn, Germany, 2019. [Google Scholar] [CrossRef]
- Wehi, P.; Beggs, J.; McAllister, T. Ka mua, ka muri: The inclusion of mātauranga Māori in New Zealand ecology. N. Z. J. Ecol. 2019, 43, 1–8. [Google Scholar] [CrossRef]
- McAllister, T.; Beggs, J.; Ogilvie, S.; Kirikiri, R.; Black, A.; Wehi, P. Kua takoto te mānuka: Mātauranga Māori in New Zealand ecology. N. Z. J. Ecol. 2020, 43, 1–7. [Google Scholar] [CrossRef]
- Best, E. Notes on the custom of rāhui: Its application and manipulation, as also its supposed powers, its rites, invocations and superstitions. J. Polyn. Soc. 1904, 13, 83–88. [Google Scholar]
- Marsden, M.; Royal, T.A.C. The Woven Universe: Selected Writings of Rev. Māori Marsden (Non-Fiction); Estate of Rev. Māori Marsden: Ótaki, New Zealand, 2003; p. 50. [Google Scholar]
- Maxwell, K.H.; Penetito, W. How the Use of Rāhui for Protecting Taonga Has Evolved over Time. MAI Review, Intern Research Report. 2007. Available online: http://www.review.mai.ac.nz/mrindex/MR/article/download/58/58-69-1-PB.pdf (accessed on 1 June 2021).
- Hill, L.M.W. An investigation into the distribution and spread of Kauri dieback disease within the Waitākere Ranges. Auckland Council Internal Report. Unpublished work. 2011. [Google Scholar]
- Lindsay, H.; Wild, C.; Byers, S.; Riddell, J. Auckland Protection Strategy; Nature Heritage Fund: Wellington, New Zealand, 2009.
- Denyer, K.; Cutting, M.; Campbell, G.; Green, C.; Hilton, M. Waitākere Ecological District. Survey Report for the Protected Natural Areas Programme; Auckland Regional Council: Auckland, New Zealand, 1993.
- Jamieson, A.; Bassett, I.E.; Hill, L.M.W.; Hill, S.; Davis, A.; Waipara, N.W.; Hough, E.G.; Horner, I.J. Aerial surveillance to detect kauri dieback in New Zealand. N. Z. Plant Protec. 2014, 67, 60–65. [Google Scholar] [CrossRef]
- Jamieson, A. Summary Report: Aerial Resurvey for Kauri Dieback in the Waitākere Regional Parkland. Auckland Council Internal Report. Unpublished work. 2016. [Google Scholar]
- Hill, L.M.W.; Stanley, R.; Hammon, C.; Waipara, N.W. Kauri Dieback Report 2017: An Investigation into the Distribution of Kauri Dieback, and Implications for its Future Management, within the Waitākere Ranges Regional Park. Auckland Council: Auckland, New Zealand. Available online: https://www.kauridieback.co.nz/media/1516/7-auckland-council-waitakere-ranges-report-v2-final-aug17.pdf (accessed on 28 June 2021).
- Horner, I.J.; Hough, E.G. Phosphorous acid for controlling Phytophthora taxon Agathis in kauri: Glasshouse trials. N. Z. Plant Protec. 2013, 66, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Horner, I.J.; Hough, E.G.; Horner, M.B. Forest efficacy trials on phosphite for control of kauri dieback. N. Z. Plant Protec. 2015, 68, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Te Kawerau ā Maki. Waitākere Rahui. Available online: http://tekawerau.iwi.nz/node/13 (accessed on 1 June 2021).
- Stuff News. Calls to Shut Large Auckland Regional Park to Stop Kauri Extinction. Available online: https://www.stuff.co.nz/environment/95615785/calls-to-shut-large-auckland-regional-park-to-stop-kauri-extinction (accessed on 1 June 2021).
- Timberbiz. Waitākere Ranges Besieged by Kauri Dieback. Available online: https://www.timberbiz.com.au/Waitakere-ranges-besieged-by-kauri-dieback/ (accessed on 1 June 2021).
- Science Media Centre. Kauri Dieback in the Waitākere Ranges—Expert Reaction. Available online: https://www.sciencemediacentre.co.nz/2017/08/10/kauri-dieback-Waitakere-ranges-expert-reaction/ (accessed on 1 June 2021).
- Radio New Zealand. Rāhui on Waitākere Ranges to Protect Kauri. Available online: https://www.rnz.co.nz/news/national/345250/rahui-on-Waitakere-ranges-to-protect-kauri (accessed on 1 June 2021).
- Te Ao Māori News. Local Iwi Are Fighting to Protect Kauri for Future Generations. Available online: https://www.teaomaori.news/local-iwi-are-fighting-protect-kauri-future-generations (accessed on 1 June 2021).
- New Zealand Herald. Waitākeres Rahui Keeping People Away-iwi. Available online: https://www.nzherald.co.nz/nz/Waitakeres-rahui-keeping-people-away-iwi/BDM5LXV5CEQYSEZEEFTERHFMBE/ (accessed on 1 June 2021).
- Ministry for Primary Industries. Kauri Dieback Disease Controlled Areas. Available online: https://www.mpi.govt.nz/biosecurity/long-term-biosecurity-management-programmes/kauri-dieback-disease-control/kauri-dieback-controlled-areas/ (accessed on 1 June 2021).
- Our Auckland. What is Happening with the Waitākere Ranges and Hunua Ranges Regional Park Closures? Available online: https://ourauckland.aucklandcouncil.govt.nz/news/2018/5/kauri-dieback-closures-what-you-need-to-know/ (accessed on 1 June 2021).
- New Zealand Herald. Waitākere Ranges to Close in May to Control Kauri Dieback Disease. Available online: https://www.nzherald.co.nz/nz/Waitākere-ranges-to-close-in-may-to-control-kauri-dieback-disease/M3DQ62JUYV775HQJ5MSYA2TZ2A/ (accessed on 1 June 2021).
- Bassett, I.E.; Horner, I.J.; Hough, E.G.; Wolber, F.B.; Egeter, B.; Stanley, M.C.; Krull, C.R. Ingestion of infected roots by feral pigs provides a minor vector pathway for kauri dieback disease Phytophthora agathidicida. Forestry 2017, 90, 640–648. [Google Scholar] [CrossRef] [Green Version]
- De Lange, P.J.; Rolfe, J.R.; Barkla, J.W.; Courtney, S.P.; Champion, P.D.; Perrie, L.R.; Beadel, S.M.; Ford, K.A.; Breitwieser, I.; Schönberger, I.; et al. Conservation Status of New Zealand Indigenous Vascular Plants; Department of Conservation: Wellington, New Zealand, 2018.
- Auckland Council. Rēti Matawhāiti mō te Taiao Māori. Natural Environment Targeted Rate. Available online: https://www.aucklandcouncil.govt.nz/environment/what-we-do-to-help-environment/Pages/natural-environment-targeted-rate.aspx (accessed on 1 June 2021).
- National Science Challenges. New Zealand’s Biological Heritage. Ngā Rākau Taketake. Available online: https://bioheritage.nz/research/saving-our-iconic-trees/ (accessed on 1 June 2021).
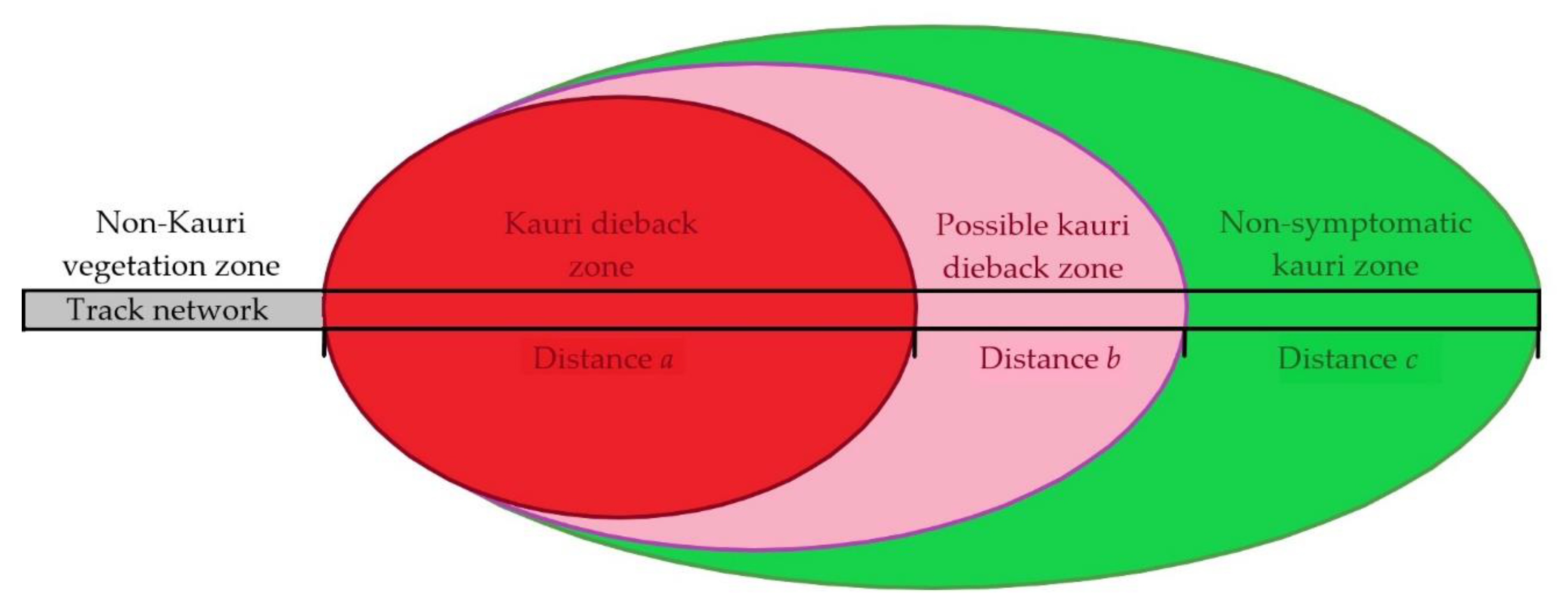





| Zone | Characteristics |
|---|---|
| Kauri dieback zone | Based on aerial and ground surveillance of symptomology and/or confirmed presence of Phytophthora agathidicida (PA) via soil sample bioassay |
| Possible kauri dieback zone | Minor symptomology expression but PA presence not confirmed via soil sample bioassay |
| Non-symptomatic kauri zone | Generated by existing kauri vegetation layer, enhanced by information from aerial and ground surveillance. No symptoms of kauri dieback or detection of PA |
| Track Network | Distance through Kauri Zone (KM) | |||
|---|---|---|---|---|
| Kauri with Kauri Dieback | Kauri with Possible Kauri Dieback | Non-Symptomatic Kauri | Total (Through All Kauri Zones) | |
| Simulation 1 | 6.666786 | 1.164078 | 32.645228 | 40.476092 |
| Simulation 2 | 5.736607 | 1.621335 | 20.553284 | 27.911226 |
| Simulation 3 | 6.284685 | 1.821385 | 24.36214 | 32.46821 |
| Simulation 4 | 7.794471 | 1.670616 | 34.597778 | 44.062865 |
| Simulation 5 | 3.790022 | 1.129356 | 28.914713 | 33.834091 |
| Simulation 6 | 8.4538 | 1.3453 | 34.845865 | 44.644965 |
| Simulation 7 | 7.2473 | 1.8842 | 29.71371 | 38.84521 |
| Simulation 8 | 5.0758 | 1.9918 | 31.2043 | 38.2719 |
| Simulation 9 | 9.6211 | 2.0786 | 29.1681 | 40.8678 |
| Simulation 10 | 5.56871 | 1.9338 | 27.72909 | 35.2576 |
| Mean +/− 99% confidence intervals | 6.62 +/− 1.76 | 1.67 +/− 0.36 | 29.4 +/− 4.56 | 37.7 +/− 5.44 |
| Actual track network | 20.94442888 | 7.567073535 | 54.74658001 | 83.25808243 |
| Track Network | Distance through Kauri Zone (%) | ||
|---|---|---|---|
| Kauri with Kauri Dieback | Kauri with Possible Kauri Dieback | Non-Symptomatic Kauri | |
| Simulation 1 | 16.4709231 | 2.8759644 | 80.6531125 |
| Simulation 2 | 20.5530456 | 5.8088993 | 73.6380552 |
| Simulation 3 | 19.3564259 | 5.6097487 | 75.0338254 |
| Simulation 4 | 17.689433 | 3.7914375 | 78.5191294 |
| Simulation 5 | 11.2017846 | 3.3379233 | 85.4602921 |
| Simulation 6 | 18.9356179 | 3.0133297 | 78.0510523 |
| Simulation 7 | 18.6568691 | 4.8505337 | 76.4925972 |
| Simulation 8 | 13.2624719 | 5.2043405 | 81.5331875 |
| Simulation 9 | 23.5420062 | 5.0861558 | 71.371838 |
| Simulation 10 | 15.7943536 | 5.5585179 | 78.6471286 |
| Mean +/− 99% confidence intervals | 17.5 +/− 3.66 | 4.51 +/− 1.17 | 77.9 +/− 4.19 |
| Actual track network | 25.1560308 | 9.0886954 | 65.7552737 |
| Actual average for WRRP as a whole | 18.95 | 4.62 | 76.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill, L.; Ashby, E.; Waipara, N.; Taua-Gordon, R.; Gordon, A.; Hjelm, F.; Bellgard, S.E.; Bodley, E.; Jesson, L.K. Cross-Cultural Leadership Enables Collaborative Approaches to Management of Kauri Dieback in Aotearoa New Zealand. Forests 2021, 12, 1671. https://doi.org/10.3390/f12121671
Hill L, Ashby E, Waipara N, Taua-Gordon R, Gordon A, Hjelm F, Bellgard SE, Bodley E, Jesson LK. Cross-Cultural Leadership Enables Collaborative Approaches to Management of Kauri Dieback in Aotearoa New Zealand. Forests. 2021; 12(12):1671. https://doi.org/10.3390/f12121671
Chicago/Turabian StyleHill, Lee, Edward Ashby, Nick Waipara, Robin Taua-Gordon, Aleesha Gordon, Fredrik Hjelm, Stanley E. Bellgard, Emma Bodley, and Linley K. Jesson. 2021. "Cross-Cultural Leadership Enables Collaborative Approaches to Management of Kauri Dieback in Aotearoa New Zealand" Forests 12, no. 12: 1671. https://doi.org/10.3390/f12121671
APA StyleHill, L., Ashby, E., Waipara, N., Taua-Gordon, R., Gordon, A., Hjelm, F., Bellgard, S. E., Bodley, E., & Jesson, L. K. (2021). Cross-Cultural Leadership Enables Collaborative Approaches to Management of Kauri Dieback in Aotearoa New Zealand. Forests, 12(12), 1671. https://doi.org/10.3390/f12121671







