Abstract
Root-specific and leaf-specific biomarkers have been used for decades to identify the origin of organic materials in soils and sediments. However, quantitative approaches require appropriate knowledge about the fate of these indicator molecules during degradation. To clarify this issue, we performed a 1-year incubation experiment with fine root and leaf material of six temperate tree species: European ash (Fraxinus excelsior), European beech (Fagus sylvatica), Oak spec. (Quercus spec.), Linden spec. (Tilia spec.), Norway spruce (Picea abies) and Scots pine (Pinus sylvatica). Only one molecule, x,16-dihydroxy hexadecanoic acid (x,16-C16), could be validated as a general leaf-specific biomarker for the set of all species. For roots, no general root biomarker was found. Ester-bound tricosanol (C23-OH) could be validated for five out of six species; 20-hydroxy eicosanoic acid (ωC20) could be validated for four out of six species, leaving Norway spruce without a suitable root biomarker. The results of this study suggest that the validity of leaf- and root-derived ester-bound lipids as biomarkers is highly species dependent and does not always coincide with previous findings. Concentrations of root- and leaf-derived ester-bound lipids did not stay constant within 1 year of degradation and changed without a linear trend. The change of concentrations seems to be highly species dependent. This might be due to a different structure and arrangement of the individual monomers in cutin and suberin per species, and, therefore, a different accessibility of bond cleaving enzymes. The usefulness of root and leaf biomarkers is context dependent. Our results suggest that general assumptions about litter input to forest soils solely based on biomarker analysis have to be considered carefully.
1. Introduction
A growing body of studies emphasizes the significance of root-related inputs as precursor material for soil organic carbon (SOC) (e.g., [1,2,3,4]). The relative contribution of leaf and root litter to soil organic matter (SOM) formation is yet to be fully understood.
One approach to address the topic has been the use of distinctive molecules, called biomarkers, as indicators to trace back the origin of SOM in soils [5]. For this purpose, the aliphatic polyesters cutin and suberin seemed to be indicative for leaves and cork cells of bark and roots, respectively, making them a potent analytical tool for source identification in archeology, forensics, paleoclimate, paleoecology, climate change and soil science studies [6,7,8].
Cutin was reported to consist of ester-bound n-carboxylic acids (FAs), di-hydroxy FAs, and short-chain ω-hydroxy FAs [9,10]. Suberin was reported to consist of alkane dioic acids, and long-chain ω-hydroxy FAs (C20-C32) for roots [11,12,13].
Besides qualitative approaches, several quantitative attempts by utilization of the biomarker method to measure the contribution of root and leaf material to the soil organic carbon pool have also been conducted recently [14,15,16,17].
However, numerous studies have shown that the chemical composition of litter materials does not stay constant during degradation, suggesting the existence of compound-specific turnover rates [5,18,19,20]. Varying concentrations of indicative compounds over time could result in severe over- or underestimation of the amount of source materials. When degradation of a biomarker is decoupled from the degradation process of the source material, as indicated by varying degradation rates of both, this will limit its suitability as quantitative indicator for carbon storage from the specific source of origin stored in litter, soils or sediments.
Goñi and Hedges [21] reported a strong decrease of concentrations of cutin-derived aliphatic compounds in organic matter with age, after analyzing fresh leaves, litter and marine sediments. Angst et al. [22] showed a rapid initial degradation of cutin- and suberin-derived compounds of Norway spruce and European beech during a period of less than 3 months.
Opsahl and Benner [23] observed that under aquatic conditions concentrations of ester-bound hydroxy carboxylic acids, typical for cutin, doubled after 21 days of incubation relative to the source material some compounds reaching peak concentration after 6 months of incubation. After 1 year of incubation, concentrations did not follow a general trend, being higher in some cases, in other cases lower than the initial concentrations.
Recent findings suggest that the degradation pattern of specific molecules like lignin or n-alkanes differ between species and different experiments (e.g., [18,20,24]). Results of long-term degradation patterns of cutin- and suberin-derived ester-bound lipids in terrestrial environments are scarce.
The purpose of this study is to improve the understanding of the long-term decomposition of tree root- and leaf-derived ester-bound lipids and their contribution to SOM, as well as their suitability as quantitative indicators to estimate the contribution of fresh source materials to the carbon pool of forest soils.
For this purpose, we conducted an incubation experiment with roots and leaves of six different temperate European tree species under standardized laboratory conditions for a period of 1 year.
We focused on the following questions:
- (i)
- What is the initial composition of aliphatic biopolyesters in leaf and root litter of the selected tree species?
- (ii)
- Which compounds are suitable as indicator molecules for the distinction of leaf and root material from the respective species?
- (iii)
- What is the degradation pattern of the identified biomarkers during the decomposition of litter and root material?
2. Materials and Methods
2.1. Samples
The leaf and root litter material for the experiment was collected after leaf abscission in September 2010 in Hainich National Park (51°06′ N, 10°31′ E) which is located in central Germany. The mean annual temperature is 7.5 °C, with a mean annual precipitation of 670 mm [25]. The mean elevation of the sites is 350 m a.s.l. The forest grows on a Luvisol [26] developed from loess underlain by Triassic Limestone. The site has been well characterized by Langenbruch et al. [25].
Fine roots (<2 mm) and leaves were collected from six tree species: European ash (Fraxinus excelsior), European beech (Fagus sylvatica), Oak spec. (Quercus spec.), Linden spec. (Tilia spec.), Norway spruce (Picea abies) and Scots pine (Pinus sylvatica). Fine roots were taken from the topsoil (0–10 cm) around the adjacent tree. Freshly fallen senescent leaves of the deciduous species were sampled from the forest floor. Needles from the coniferous species were sampled from the branches. Fine roots were gently washed under water to remove attached soil material. All litter materials were dried at 40 °C and cut (<10 mm).
2.2. Incubation Experiment
The plant litter was mixed with quartz sand, which was free of carbon and metal oxides (Zilverzand Exploitatie Beaujean B.V., Heerlen, The Netherlands). The mixing ratio of litter and sand was 1:9 (w/w). Samples of 100 g soil were incubated in 700 mL jars (n = 3) at 20 °C and 60% of WHC for 12 months in the dark. To obtain 60% of WHC, demineralized water was added. At the beginning of the experiment and after 1, 3, 6, 9 and 12 months of the experiment 200 mL of demineralized water were added to the samples to remove water soluble organic residues and metabolites. After 24 h at 2°C the water was decanted, the samples were dried back to 60% WHC and representative subsamples of 4 g from each jar were taken. The evolution of CO2 was recorded by placing the incubation containers with samples (n = 3 per sample type: six species, root and leaf/needle samples) into a Respicond apparatus (Nordgren Innovations, Bygdeå, Sweden). The CO2 analysis is based on hourly measurements of the electrical conductivity in 10 mL of 0.6 mol KOH solution placed inside the Respicond vessels [27]. The measurement periods lasted for at least 10 days. Subsequently, samples were freeze-dried (Scanvac Coolsafe 55, Labogene, Allerød, Denmark) and ground in a ball mill (Pulverisette-5, Fritsch GmbH, Idar-Oberstein, Germany). Carbon and nitrogen contents of the solid residues were determined by an elementar analyzer (VarioEl, Elementar Analysensysteme, Langenselbold, Germany). The sampling was destructive, hence a whole set of samples had to be prepared for each sampling date.
2.3. Extraction and Quantification of Root- and Leaf-Derived Biomarkers
2.3.1. Lipid Extraction
To remove free lipids, 0.2 g of each material were extracted using accelerated solvent extraction (ASE) in triplicates via a Dionex 200 ASE extractor with 11 mL extraction cells and dichloromethane (DCM/MeOH) (93:7 v/v) [26]. Extraction was carried out at 75 °C and 17,106 Pa with a heating phase of 5 min. and static extraction time of 20 min. [28]. The free lipids were not considered for this study as they were not expected to contain individual root or needle-specific compounds [5]. Hence, they were removed to avoid interference with the analysis of the ester-bound lipids.
2.3.2. Ester-Bound Lipid Extraction and Derivation
After extraction, the samples were subjected to base methanolysis to release ester bound lipids [29,30]. The extraction residues were refluxed (3 h, 20 mL of 1 M KOH/MeOH). After cooling, the suspension was centrifuged (20 min, 2500 rpm). Each soil residue was then extracted via sonication (15 min., 30 mL DCM/MeOH 1:1 v/v). The suspension was centrifuged again, and the supernatant was combined with the supernatant from the first centrifugation step. The combined extracts were acidified to pH 1 by addition of 6 M HCl. The solvent was removed by evaporation, the samples were redissolved sequentially in 50 mL ultra-pure water and DCM and added to separation funnels. Hydrolysis products were recovered via liquid–liquid extraction in a separation funnel. Anhydrous Na2SO4 was added to the combined DCM phase to remove water. The DCM extracts were concentrated by evaporation under N2, transferred to 2 mL glass vials and dried. Deuterated hexadecanoic acid was added as internal standard for relative quantification. Aliquots of the total extracts after solvent extraction and base hydrolysis were converted to trimethylsilyl (TMS) derivatives by reaction with N,O-bis-(trimethylsilyl) trifluoroacetamide (BSTFA) for 1 h at 70 °C. After derivatization, the solutions were dried once more under N2 to remove excess BSTFA, and re-dissolved in 200 μL cyclohexane [27]. Conversation of the hydroxy acids to the methyl ester trimethylsilyl ethers was inspected for completeness and was thus non-discriminatory.
2.3.3. Gas Chromatography–Mass Spectrometry (GC-MS)
Gas chromatography–mass spectrometry (GC/MS) analyses were performed on a ThermoQuest Trace GC 2000 gas chromatograph connected to a Finnigan Trace MS quadrupole mass spectrometer. The methanolysed samples were separated by cold on-column injection of 1 μL of the extract on an HP-5MS fused-silica capillary column (30 m × 0.25 mm id, 0.25 μm film thickness) at 45 °C. The GC operating conditions were as follows: temperature hold at 65 °C for 2 min., increase from 65 to 120 °C at a rate of 70 °C/min. After a hold time of 5 min., the temperature was increased at a rate of 5 °C/min. with a final isothermal hold at 325 °C for 15 min. Helium was used as carrier gas at a flow rate of 1.4 mL/min. The subsequent MS detection in full scan mode used a mass to charge ratio (m/z) of 50–650 with a cycle time of 0.65 s and followed electron impact ionization with an electron energy of 70 eV. From the chromatograms ester-bound lipids were identified by their mass spectra and retention times (e.g., [29,31]). For semi-quantitative interpretation the base peak areas for each component were compared to the peak areas from the base peak of the deuterated internal standard.
2.4. Statistical Analyses
Decomposition rate constants (k) of leaf and root litter of six temperate tree species were calculated using the exponential decay model of Olson [32]:
where x0 is the original litter weight, xt is the litter weight after a given period, and t is the time. Decomposition rates were compared by analysis of variance (ANOVA) followed by Tukey’s honestly significant difference (HSD) post hoc test (p < 0.05). All statistical analyses were carried out using SPSS 19.0 (IBM Corp., Armonk, NY, USA).
xt/x0 = e−kt
3. Results
3.1. Litter Mass Loss
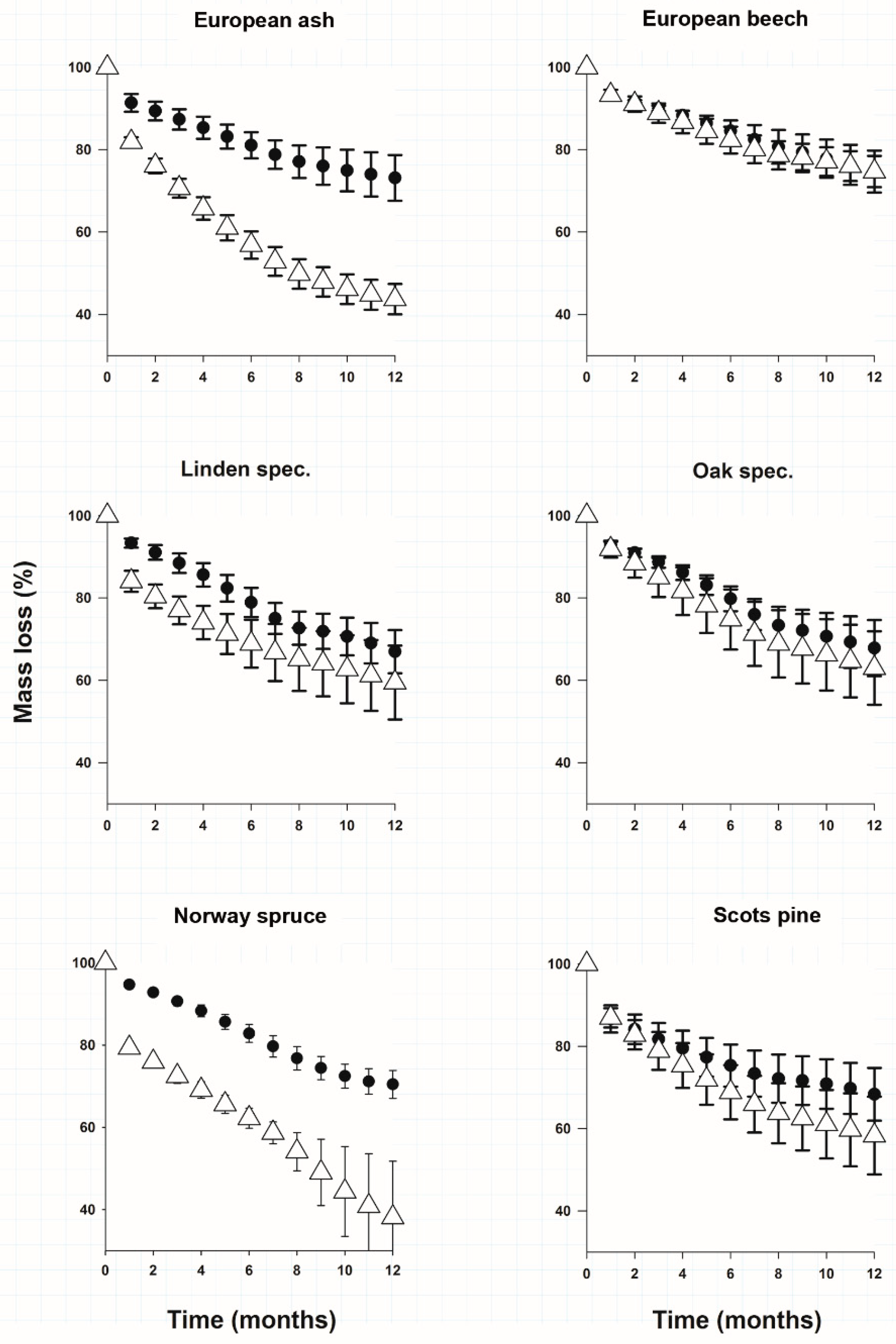
After 12 months of litter decomposition leaf and needle litter of the studied species had on average a higher carbon loss than root litter (Figure 1). The only exception was European beech litter material where carbon loss for root and leaf litter was similar (Figure 1). Norway spruce covered both extremes of mass loss of all litter types. Spruce needles had significantly the largest mass loss (Figure 1) and spruce roots on the other end of the scale lost only 27.3 ± 3.4% of their mass (Figure 1). In Table 1 we provide the calculated decomposition rate constants (k) for roots and leaves. We found no significant differences for decomposition rate constants (k) of roots between the species (Table 1). The decomposition rate constants of leaves could be grouped in 2 clusters (Table 1). Only the carbon loss and decomposition rate constants of spruce needles and ash leaves were significantly faster than those of their roots.
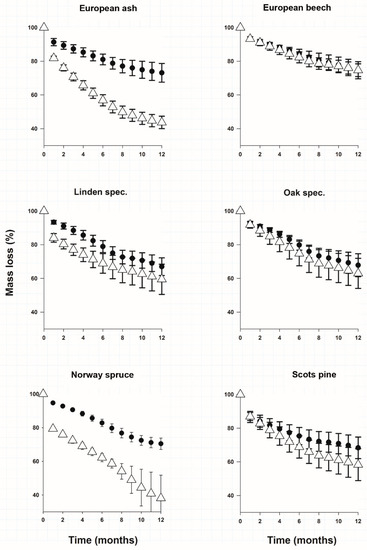
Figure 1.
Mean (±SE)% of initial mass remaining of each incubated material calculated from CO2 loss. White triangles represent needles and leaves, black dots represent roots.

Table 1.
Calculated decomposition rate constants (k) over 12 months from 6 different temperate tree species (means, SE, number of replicates = 3, coefficient of determination). Different letters represent significant differences (p < 0.05).
3.2. Initial Molecular Composition of Ester-Bound Lipids in Leaf- and Root-Derived Litter
After alkaline methanolysis 27 different ester-bound lipids were determined from fresh leaf and roots material, which were assigned as carboxylic acids, long-chain alcohols, α,ω-alkandioic acids, ω-hydroxy alkanoic acids, and mid-chain hydroxy alkanoic acids (Table S1). As a selection criterion, the only compounds considered to be indicative for their origin, were those which appeared in at least two samples from the three replicates. The released compounds covered chain-lengths of 15 to 24 carbon atoms and had, except for 7-hydroxy-1,15-pentadecanedioic acid (7-C15 DA) and tricosanol (C23-OH), an even number of carbon atoms (Table S1).
Compounds with 16 and 18 carbons dominated the composition of the extracted ester-bound lipids making up consistently more than 89% of all quantified molecules of the leaf and needle extracts (Table S1). Root extracts showed a significant difference between coniferous and broadleaf species. Compounds with 16 and 18 carbons contributed always more than 84% of the total extract for broadleaf species; for coniferous species, the amount was below 63% (Table S1).
Leaves contained on average 14 different ester-bound lipids. X,16-dihydroxy hexadecanoic acids (x,16-C16) were the most abundant compounds in leaves of five species: European ash, European beech, Oak, Norway spruce and Scots pine, with a share of more than 40% in the extract (Table S1). X,16-C16 was generally present in every leaf and needle sample and was always absent in root samples. Therefore, the selection criteria as a leaf and needle biomarker were successfully matched.
The most abundant compounds in linden leaves were 18-hydroxy octadecenoic acid (ωC18:1) with a proportion of more than 43% of the released compounds and x,16-C16 acids with a proportion of 24% (Table S1). 9,10-Epoxy-18-hydroxy octadecanoic acid (ω-9,10-epoxy-C18) was present in leaves of Linden, Norway spruce and Scots pine and absent in the corresponding root samples (Table S1). Therefore, in a set of these three species it would be valid as a leaf biomarker. ω-9,10-epoxy-C18 was present in roots of European ash, European beech and Oak (Table S1).
On average roots released 17 different ester-bound lipids. ωC18:1 was the most abundant compound in the roots of three species Linden spec., Norway spruce and Scots Pine with a proportion of more than 40% in the extract (Table S1). 9,10-epoxy-18C18 was the most abundant compound in roots of European beech and Oak with more than 37% in the extract. The most abundant compound in roots of European ash was 1,18-octadecenedioic acid (C18:1 DA) (Table S1). Therefore, no compound fulfilled the selection criteria to be a general root biomarker.
Tricosanol (C23-OH) appeared in root extracts of 5 species, being only absent in roots of Norway spruce and every leaf or needle sample. C23-OH would therefore be suitable as a root biomarker for roots of European ash, European beech, Oak, Scots pine and Linden ωC20 was only present in needle extracts of Norway spruce and Scots pine, making it a suitable biomarker for 4 out of 6 species. Root samples of the two coniferous species could be identified by 1,20-Eicosanedioic acid (C20-DA), which only appeared in samples of Norway spruce and Scots pine roots.
3.3. Degradation of Ester-Bound Lipids
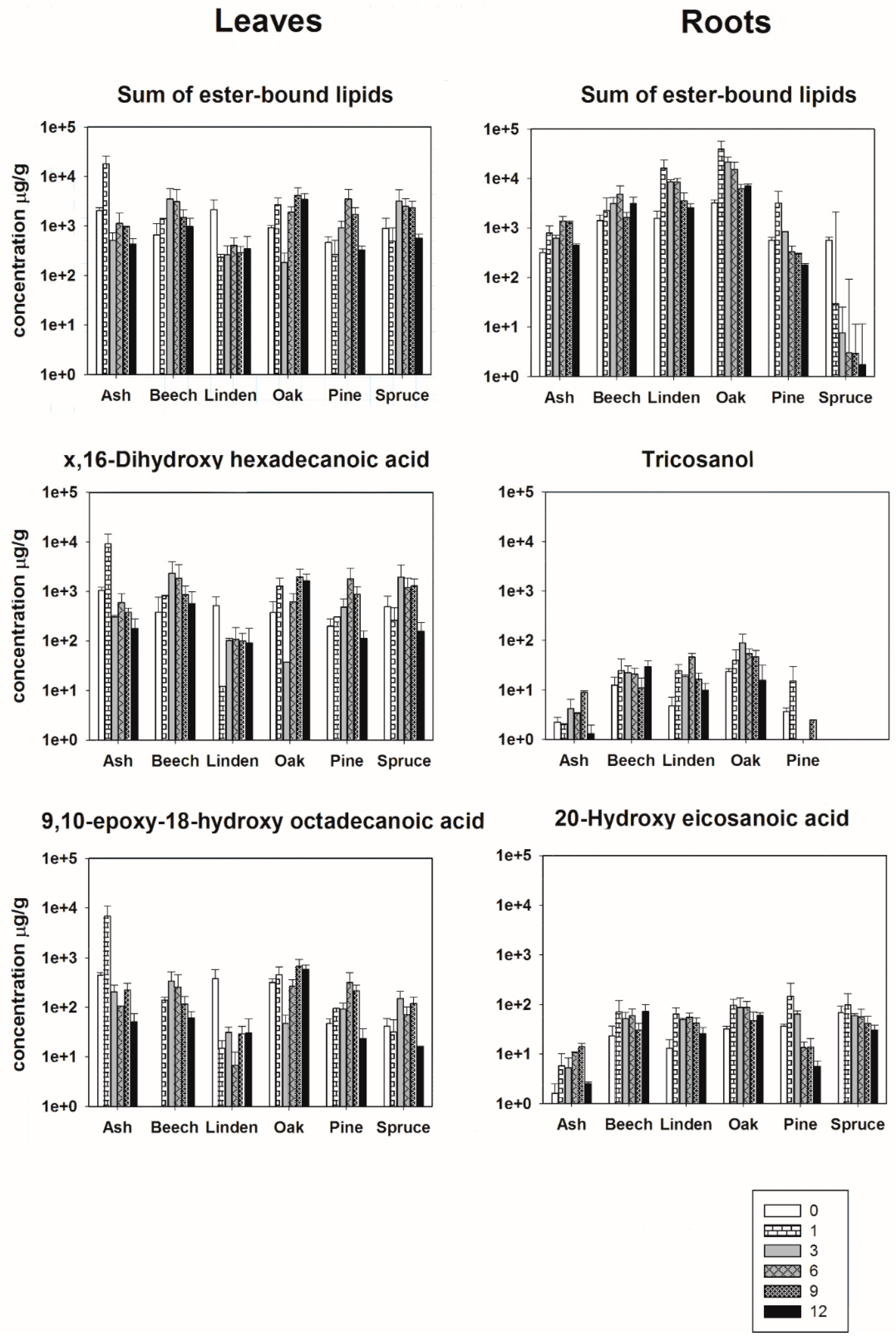
The degradation of leaf-derived ester-bound lipids did not show a general trend for all species (Figure 2). Disregarding leaves of Linden, the total concentration of extracted ester-bound lipids was higher after several months relatively to the starting point during the decomposition process. For the leaves of European ash, the maximum concentrations were reached after 1 month, for European beech and Norway spruce after 3 months, for Scots pine after 6 months, for Oak after 9 months (Figure 2). After the maximum concentration, values decreased again. Interestingly, leaves of European beech and Oak still released a higher total concentration of lipids after 1 year of decomposition than the fresh material (Figure 2). The degradation trend of the leaf biomarker x,16-C16 and ω-9,10-epoxy-18-C18 showed the same degradation pattern as the total concentration of ester-bound lipids, explained by the fact that x,16-C16 and ω-9,10-epoxy-18-C18 were highly abundant molecules in the total extract. Depending on the species the maximum concentration of x,16-C16 was 4–9 times higher, respectively, than the initial concentration. For ω-9,10-epoxy-18-C18 the maximum concentration was 2–15 times higher depending on the species (Figure 2).
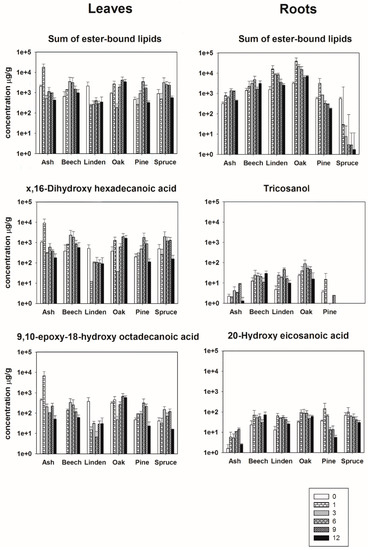
Figure 2.
Bars represent the mean concentration (µg/g OC) of selected biomarkers (n-carboxylic, α,ω-diacids, ω-hydroxy and mid-chain substituted hydroxy acids) of the incubated materials at the different sampling dates (month after incubation). Error bars indicate SEM.
Additionally, the degradation of root-derived ester-bound lipids did not show a general trend for all species over time (Figure 2). With exception of roots of Norway spruce, the total concentration of extracted ester-bound lipids consistently increased relatively to the starting point during the decomposition process. For the roots of Linden, Oak and Scots pine, the maximum concentrations were reached after 1 month, and for European ash and European beech maximum concentrations were reached after 6 months (Figure 2).
The degradation pattern of C23-OH and ωC20 differed per species and were different from the trend of the total concentration of ester-bound lipids showing maximum concentrations at different phases of the degradation (Figure 2). The maximum concentration of C23-OH was between 1,4 and 4,9 times higher than the initial concentration. The maximum concentration of ωC20 during 1 year of decomposition was between two, three and four times higher than the initial concentration (Figure 2).
4. Discussion
4.1. Composition of Ester-Bound Lipids in Leaf- and Root-Derived Litter
The results, showing leaf- and needle-derived ester-bound lipids to be dominated by a chain length of 16 and 18 carbons, are consistent with previous findings (e.g., Goñi and Hedges, 1990b [33]). Whilst compounds with 16 and 18 carbons were dominating the plant extracts, we found compounds with chain length up to 24 carbons in the leaf material. X,16-C16, which dominated all leaf and needle material, was completely absent in roots, which would confirm its leaf biomarker status [34,35].
However, x,16-C16 was detected previously by Hamer et al. [36] and Nierop et al. [37] in plant roots. Holloway [38] detected x,16-C16 in suberin of Oak and European beech. Matzke and Riederer [39] reported the presence of Dihydroxy fatty acids in Suberin of European beech and European oak (Quercus robur).
Therefore, we would not consider x,16-C16 as a general leaf biomarker; however, in this set of litter materials it was indicative for leaf material and would be suitable as a leaf biomarker in this study.
In our study, we could not detect one molecule which was exclusively present in root samples, the crucial selection criterion to be suitable as root biomarker (Figure 2). Ester-bound tricosanol (C23-OH) could be validated for five out of six species. To our knowledge ester-bound C23-OH has not been used earlier as biomarker for plant roots in geosciences. However, C23-OH has been reported earlier to be a constituent of suberin [40].
α,β-alkanedioic acids were regarded by some authors to be exclusively present in suberized tissue [11,41]. However, hexadecane-1,16-dioic acid (C16-DA) appeared in leaf and needle material of 4 species. Additionally, other studies showed that in fact α,β-alkanedioic acids can be important constituents of leaf cuticles [42,43] making up to 40% of cuticular biomass. It was proven earlier, that suberized cells do appear in leaves, fruits, twigs and bark of trees as well [44,45].
Additionally, long-chain ω-hydroxy fatty acids (Cn > 18) were frequently used in previous studies as root biomarker [14,15,16,46]. However, in our study we found ωC20 in needle material of Norway spruce and Scots pine. As these compounds appear as well in needles and in root material of two species, assumptions about the origin of organic matter in soils based on ωC20 is not possible in this case. The presence of ester-bound ωC20 in leaf material, shoots [15,43] and isolated cutin [47] is not new and has been published earlier. In addition, we observed ωC22 in Oak leafes, and needles of Norway spruce. Furthermore, ωC22 was absent in Ash roots, which makes it ineligible as a general root biomarker in this study.
ω-9,10-epoxy-C18 has been reported to be a constituent in cutin and suberin [48]. However, as it is valid as leaf biomarker for 3 species of our incubation study, it was included in our selection of leaf indicator molecules.
The results of our study indicate that the attempt in geosciences to use cutin- and suberin-derived monomers qualitatively as leaf and root biomarkers might not be accurate, due to the general unspecific molecular signature of leaf- and root-derived compounds [36]. Therefore, the identification of specific compounds for the respective study area should be preferred over the transfer of data about leaf and root composition from previous studies.
4.2. Degradation of Ester-Bound Lipids
The measured concentrations of selected ester-bound lipids in roots were always higher after 1 month of litter degradation than in the fresh litter material (Figure 2). For leaf material, the trend was similar with only four exceptions. Opsahl and Benner [23] who observed that concentrations of di-hydroxy fatty acids and ω-hydroxy fatty acids in leaves tripled within the first two month of litter degradation, have already reported these findings.
Increasing concentrations of ester-bound compounds during litter degradation have previously been explained by increasing partial depolymerization of the polymers and therefore enhanced extractability during sample preparation [49]. The accessibility of the alkaline methanol decisive for hydrolysis and subsequential extraction of the cutin- and suberin-derived monomers, is determined by the remaining degree of polymerization, surface area, porosity, sheathing by barrier structures, lignin and hemicellulose content of the degraded litter material [50].
In such a case, cutin- and suberin-cleaving enzymes would require a previous auxiliary action of accessory enzymes such as a second possible process to explain increasing concentrations of ester-bound lipids with mid-chain-substituted hydroxy alkanoic acids and long-chain dicarboxylic acids is their secondary formation by enzymatic oxidation [51]. Enzymes of the group cytochrome P450 monooxygenases, which can be produced by insects, yeasts, filamentous fungi, and bacteria, have been reported to alter the structure of straight chain lipids [52]. Whether this oxidation pathway is in fact relevant for ester-bound lipids has to our knowledge not been investigated earlier, but it seems plausible.
The change of concentrations of leaf- and needle-derived biomarkers differs more strongly between species than between different molecules (Figure 2). These findings are in agreement with observations by Opsahl and Benner [23], who observed that concentrations of di-hydroxy fatty acids and ω-hydroxy fatty acids in leaves tripled within the first two months of litter degradation.
As the degradation patterns of the leaf-derived compounds differ for each species, most likely this might be due to a different structure and arrangement of the individual monomers in the leaf cutin per species and therefor a different accessibility of bond cleaving enzymes [53,54]. Species dependent degradability of biopolymers, while to our knowledge not reported before for suberin and cutin, has earlier been reported for cellulose, hemicellulose, lignin and starch [24,55,56,57,58]. Measured biomarker concentrations in plant litter indicate the quantity of the biomarker per mass unit litter. As organic matter consists of a complex three-dimensional structure of polymers, the observed dynamic of biomarker concentrations could be the simple consequence of a species-specific structure of the cutin and suberin matrix.
It was previously assumed, that cutin- and suberin-derived monomers are precursor materials for more slowly degraded constituents of the SOM pool [59]. However, the knowledge of degradation products of cutin and suberin is still fragmentary. Further studies are needed to unravel underlying processes governing the entire decomposition chain and their specific degradation pathways. This would make a significant contribution to the question of reliability of root and leaf biomarkers.
5. Conclusions
In this study one molecule, x,16-dihydroxy hexadecanoic acid (x,16-C16) proved to be suited as a general leaf-specific biomarker for the set with all species. For roots no general root biomarker was found. The suitability of certain molecules as an indicative biomarker strongly depends on the selection of species. We conclude, as the number of species increases, the probability to find an indicative molecule decreases. The analysis of root and leaf biomarker method to identify precursor materials of SOM seems to be most suitable for single-species tree stands.
Several studies showed previously that degradation of plant material causes changing concentrations of its individual components. In this study under controlled laboratory conditions, we could show that the degradation pattern of leaf- and root-derived molecules do not follow a general trend but show species dependent pattern. The quantitative application of root and leaf biomarkers in soils, sediments or litter layers therefore is associated with a high level of uncertainty. Only a detailed knowledge of the degradation characteristics and the specificity of a certain biomarker ensures the appropriate interpretation of its ecological significance.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/f12121732/s1, Table S1: Concentration of ester-bound lipids in fresh plant litter (means ± 1SE, n = 3).
Author Contributions
Conceptualization: J.G.A., B.J., H.F.J. and K.K.; Methodology: B.J. and K.K.; resources, investigation, analysis: J.G.A.; funding acquisition: K.K.; validation: J.G.A., B.J. and K.K.; writing: J.G.A., B.J., H.F.J. and K.K.; supervision: B.J. and K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Acknowledgments
Agnieszka Grzybowska is gratefully thanked for assistance and Joke Westerveld for technical support in the lab. We are especially grateful to the National Park administration to sample the plant material for this study in Hainich National Park (Germany) and the Zilverzand Exploitatie Beaujean B.V. (The Netherlands) for providing highly pure quartz sand. Wang Xiang and Thimo Klotzbücher are especially thanked for their diligent assistance during fieldwork.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rasse, D.P.; Rumpel, C.; Dignac, M.-F. Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 2005, 269, 341–356. [Google Scholar] [CrossRef]
- Pisani, O.; Lin, L.H.; Lun, O.O.Y.; Lajtha, K.; Nadelhoffer, K.; Simpson, A.J.; Simpson, M. Long-term doubling of litter inputs accelerates soil organic matter degradation and reduces soil carbon stocks. Biogeochemistry 2016, 127, 1–14. [Google Scholar] [CrossRef]
- Lajtha, K.; Bowden, R.D.; Crow, S.; Fekete, I.; Kotroczó, Z.; Plante, A.; Simpson, M.J.; Nadelhoffer, K.J. The detrital input and removal treatment (DIRT) network: Insights into soil carbon stabilization. Sci. Total Environ. 2018, 640, 1112–1120. [Google Scholar] [CrossRef]
- Sokol, N.W.; Bradford, M.A. Microbial formation of stable soil carbon is more efficient from belowground than aboveground input. Nat. Geosci. 2019, 12, 46–53. [Google Scholar] [CrossRef]
- Amelung, W.; Brodowski, S.; Sandhage-Hofmann, A.; Bol, R. Chapter 6 combining biomarker with stable isotope analyses for assessing the transformation and turnover of soil organic matter. Adv. Agron. 2008, 100, 155–250. [Google Scholar] [CrossRef]
- Simoneit, B.R. A review of current applications of mass spectrometry for biomarker/molecular tracer elucidations. Mass Spectrom. Rev. 2005, 24, 719–765. [Google Scholar] [CrossRef]
- Evershed, R.P. Organic residue analysis in archaeology: The archaeological biomarker revolution. Archaeometry 2008, 50, 895–924. [Google Scholar] [CrossRef]
- Owens, P.N.; Blake, W.H.; Gaspar, L.; Gateuille, D.; Koiter, A.J.; Lobb, D.A.; Petticrew, E.L.; Reiffarth, D.G.; Smith, H.G.; Woodward, J.C. Fingerprinting and tracing the sources of soils and sediments: Earth and ocean science, geoarchaeological, forensic, and human health applications. Earth-Sci. Rev. 2016, 162, 1–23. [Google Scholar] [CrossRef] [Green Version]
- E Kolattukudy, P. Structure, biosynthesis, and biodegradation of cutin and suberin. Annu. Rev. Plant Physiol. 1981, 32, 539–567. [Google Scholar] [CrossRef]
- Philippe, G.; Sørensen, I.; Jiao, C.; Sun, X.; Fei, Z.; Domozych, D.S.; Rose, J.K. Cutin and suberin: Assembly and origins of specialized lipidic cell wall scaffolds. Curr. Opin. Plant Biol. 2020, 55, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Nierop, K.G.J.; Verstraten, J.M. Rapid molecular assessment of the bioturbation extent in sandy soil horizons under pine using ester-bound lipids by on-line thermally assisted hydrolysis and methylation-gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.; Simpson, M.J. Sources and composition of hydrolysable aliphatic lipids and phenols in soils from western Canada. Org. Geochem. 2006, 37, 385–407. [Google Scholar] [CrossRef]
- Graça, J. Suberin: The biopolyester at the frontier of plants. Front. Chem. 2015, 3, 62. [Google Scholar] [CrossRef]
- Angst, G.; John, S.; Mueller, C.W.; Kögel-Knabner, I.; Rethemeyer, J. Tracing the sources and spatial distribution of organic carbon in subsoils using a multi-biomarker approach. Sci. Rep. 2016, 6, 29478. [Google Scholar] [CrossRef] [Green Version]
- Armas-Herrera, C.M.; Dignac, M.-F.; Rumpel, C.; Arbelo, C.D.; Chabbi, A. Management effects on composition and dynamics of cutin and suberin in topsoil under agricultural use. Eur. J. Soil Sci. 2016, 67, 360–373. [Google Scholar] [CrossRef]
- Mendez-Millan, M.; Dignac, M.-F.; Rumpel, C.; Derenne, S. Can cutin and suberin biomarkers be used to trace shoot and root-derived organic matter? A molecular and isotopic approach. Biogeochemistry 2010, 106, 23–38. [Google Scholar] [CrossRef]
- Vidal, A.; Quenea, K.; Alexis, M.; Derenne, S. Molecular fate of root and shoot litter on incorporation and decomposition in earthworm casts. Org. Geochem. 2016, 101, 1–10. [Google Scholar] [CrossRef]
- Klotzbücher, T.; Kaiser, K.; Guggenberger, G.; Gatzek, C.; Kalbitz, K. A new conceptual model for the fate of lignin in decomposing plant litter. Ecology 2011, 92, 1052–1062. [Google Scholar] [CrossRef]
- Zech, M.; Pedentchouk, N.; Buggle, B.; Leiber, K.; Kalbitz, K.; Marković, S.B.; Glaser, B. Effect of leaf litter degradation and seasonality on D/H isotope ratios of n-alkane biomarkers. Geochim. Cosmochim. Acta 2011, 75, 4917–4928. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.L.; Jansen, B.; van Loon, E.E.; Wiesenberg, G.L.B. Transformation of n-alkanes from plant to soil: A review. SOIL Discuss. 2021, 7, 785–809. [Google Scholar] [CrossRef]
- Goñi, M.A.; Hedges, J.I. The diagenetic behavior of cutin acids in buried conifer needles and sediments from a coastal marine environment. Geochim. Cosmochim. Acta 1990, 54, 3083–3093. [Google Scholar] [CrossRef]
- Angst, G.; Heinrich, L.; Kögel-Knabner, I.; Mueller, C.W. The fate of cutin and suberin of decaying leaves, needles and roots–Inferences from the initial decomposition of bound fatty acids. Org. Geochem. 2016, 95, 81–92. [Google Scholar] [CrossRef]
- Opsahl, S.; Benner, R. Early diagenesis of vascular plant tissues: Lignin and cutin decomposition and biogeochemical implications. Geochim. Cosmochim. Acta 1995, 59, 4889–4904. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Plant Litter, Decomposition, Humus Formation, Carbon Sequestration, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–341. [Google Scholar]
- Langenbruch, C.; Helfrich, M.; Flessa, H. Effects of beech (Fagus sylvatica), ash (Fraxinus excelsior) and lime (Tilia spec.) on soil chemical properties in a mixed deciduous forest. Plant Soil 2012, 352, 389–403. [Google Scholar] [CrossRef] [Green Version]
- WRB. World Reference Base for Soil Resources: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2014. [Google Scholar]
- Nordgren, A. Apparatus for the continuous, long-term monitoring of soil respiration rate in large numbers of samples. Soil Biol. Biochem. 1988, 20, 955–957. [Google Scholar] [CrossRef]
- Jansen, B.; Nierop, K.G.; Hageman, J.A.; Cleef, A.M.; Verstraten, J.M. The straight-chain lipid biomarker composition of plant species responsible for the dominant biomass production along two altitudinal transects in the Ecuadorian Andes. Org. Geochem. 2006, 37, 1514–1536. [Google Scholar] [CrossRef]
- Hunneman, D.; Eglinton, G. The constituent acids of gymnosperm cutins. Phytochemistry 1972, 11, 1989–2001. [Google Scholar] [CrossRef]
- Spielvogel, S.; Prietzel, J.; Leide, J.; Riedel, M.; Zemke, J.; Kögel-Knabner, I. Distribution of cutin and suberin biomarkers under forest trees with different root systems. Plant Soil 2014, 381, 95–110. [Google Scholar] [CrossRef]
- Järvinen, R.; Silvestre, A.J.; Holopainen, U.; Kaimainen, M.; Nyyssölä, A.; Gil, A.M.; Neto, C.P.; Lehtinen, P.; Buchert, J.; Kallio, H. Suberin of potato (Solanum tuberosum Var. Nikola): Comparison of the effect of cutinase CcCut1 with chemical depolymerization. J. Agric. Food Chem. 2009, 57, 9016–9027. [Google Scholar] [CrossRef] [Green Version]
- Olson, J.S. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef] [Green Version]
- Goñi, M.A.; Hedges, J.I. Cutin-derived CuO reaction products from purified cuticles and tree leaves. Geochim. Cosmochim. Acta 1990, 54, 3065–3072. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Ziegler, F.; Riederer, M.; Zech, W. Distribution and decomposition pattern of cutin and suberin in forest soils. J. Plant Nutr. Soil Sci. 1989, 152, 409–413. [Google Scholar] [CrossRef]
- Wang, J.-J.; Bowden, R.D.; Lajtha, K.; Washko, S.; Wurzbacher, S.J.; Simpson, M.J. Long-term nitrogen addition suppresses microbial degradation, enhances soil carbon storage, and alters the molecular composition of soil organic matter. Biogeochemistry 2019, 142, 299–313. [Google Scholar] [CrossRef] [Green Version]
- Hamer, U.; Rumpel, C.; Dignac, M.-F. Cutin and suberin biomarkers as tracers for the turnover of shoot and root derived organic matter along a chronosequence of Ecuadorian pasture soils. Eur. J. Soil Sci. 2012, 63, 808–819. [Google Scholar] [CrossRef]
- Nierop, K.G.; Naafs, D.F.; Verstraten, J.M. Occurrence and distribution of ester-bound lipids in Dutch coastal dune soils along a pH gradient. Org. Geochem. 2003, 34, 719–729. [Google Scholar] [CrossRef]
- Holloway, P.J. Some variations in the composition of suberin from the cork layers of higher plants. Phytochemistry 1983, 22, 495–502. [Google Scholar] [CrossRef]
- Matzke, K.; Riederer, M. A comparative study into the chemical constitution of cutins and suberins from Picea abies (L.) Karst., Quercus robur L., and Fagus sylvatica L. Planta 1991, 185, 233–245. [Google Scholar] [CrossRef]
- Graça, J.; Pereira, H. Suberin structure in potato periderm: Glycerol, long-chain monomers, and glyceryl and feruloyl dimers. J. Agric. Food Chem. 2000, 48, 5476–5483. [Google Scholar] [CrossRef]
- Crow, S.E.; Lajtha, K.; Filley, T.R.; Swanston, C.W.; Bowden, R.D.; Caldwell, B.A. Sources of plant-derived carbon and stability of organic matter in soil: Implications for global change. Glob. Chang. Biol. 2009, 15, 2003–2019. [Google Scholar] [CrossRef]
- Bonaventure, G.; Beisson, F.; Ohlrogge, J.; Pollard, M. Analysis of the aliphatic monomer composition of polyesters associated with Arabidopsis epidermis: Occurrence of octadeca-cis-6, cis-9-diene-1,18-dioate as the major component. Plant J. 2004, 40, 920–930. [Google Scholar] [CrossRef]
- Franke, R.; Briesen, I.; Wojciechowski, T.; Faust, A.; Yephremov, A.; Nawrath, C.; Schreiber, L. Apoplastic polyesters in Arabidopsis surface tissues–A typical suberin and a particular cutin. Phytochemistry 2005, 66, 2643–2658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean, B.B.; Kolattukudy, P.E. Synthesis of suberin during wound-healing in jade leaves, tomato fruit, and bean pods’. Plant Physiol. 1976, 58, 411–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espelie, K.E.; Davis, R.W.; Kolattukudy, P.E. Composition, ultrastructure and function of the cutin- and suberin-containing layers in the leaf, fruit peel, juice-sac and inner seed coat of grapefruit (Citrus paradisi Macfed.). Planta 1980, 149, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.; Shunthirasingham, C.; Simpson, M.J. A comparison of plant and microbial biomarkers in grassland soils from the Prairie Ecozone of Canada. Org. Geochem. 2005, 36, 425–448. [Google Scholar] [CrossRef]
- Kallio, H.; Nieminen, R.; Tuomasjukka, S.; Hakala, M. Cutin composition of five finnish berries. J. Agric. Food Chem. 2006, 54, 457–462. [Google Scholar] [CrossRef]
- Holloway, P.J.; Deas, A.H.B. Epoxyoctadecanoic acids in plant cutins and suberins. Phytochemistry 1973, 12, 1721–1735. [Google Scholar] [CrossRef]
- Viikari, L.; Kantelinen, A.; Buchert, J.; Puls, J. Enzymatic accessibility of xylans in lignocellulosic materials. Appl. Microbiol. Biot. 1994, 41, 124–129. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Kim, S.-U.; Kim, K.-R.; Kim, J.-W.; Kim, S.; Kwon, Y.-U.; Oh, D.-K.; Park, J.-B. Microbial synthesis of plant oxylipins from γ-linolenic acid through designed biotransformation pathways. J. Agric. Food Chem. 2015, 63, 2773–2781. [Google Scholar] [CrossRef]
- Van Bogaert, I.N.A.; Groeneboer, S.; Saerens, K.; Soetaert, W. The role of cytochrome P450 monooxygenases in microbial fatty acid metabolism. FEBS J. 2010, 278, 206–221. [Google Scholar] [CrossRef]
- Himmel, M.E.; Ding, S.-Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [Green Version]
- Mansfield, S.; Mooney, C.; Saddler, J.N. Substrate and enzyme characteristics that limit cellulose hydrolysis. Biotechnol. Prog. 1999, 15, 804–816. [Google Scholar] [CrossRef]
- Buléon, A.; Colonna, P.; Planchot, V.; Ball, S. Starch granules: Structure and biosynthesis. Int. J. Biol. Macromol. 1998, 23, 85–112. [Google Scholar] [CrossRef] [Green Version]
- Hedges, J.I.; Cowie, G.L.; Ertel, J.R.; Barbour, R.J.; Hatcher, P.G. Degradation of carbohydrates and lignins in buried woods. Geochim. Cosmochim. Acta 1985, 49, 701–711. [Google Scholar] [CrossRef]
- Lynd, L.R.; Weimer, P.J.; van Zyl, W.H.; Pretorius, I.S. Microbial cellulose utilization: Fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 2002, 66, 506–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puls, J. Chemistry and biochemistry of hemicelluloses: Relationship between hemicellulose structure and enzymes required for hydrolysis. Macromol. Symp. 1997, 120, 183–196. [Google Scholar] [CrossRef]
- Feng, X.; Xu, Y.; Jaffé, R.; Schlesinger, W.H.; Simpson, M. Turnover rates of hydrolysable aliphatic lipids in Duke Forest soils determined by compound specific 13C isotopic analysis. Org. Geochem. 2010, 41, 573–579. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).