How Does Radial Growth of Water-Stressed Populations of European Beech (Fagus sylvatica L.) Trees Vary under Multiple Drought Events?
Abstract
:1. Introduction
- to compare the radial growth between beech trees from dry and less-dry plots;
- to establish the influence of temperature, precipitation, and drought on radial growth of beech trees in dry and less-dry plots over the last 60 years;
- to compare the resistance, recovery, and resilience of beech trees between dry and less-dry plots after multiple drought years;
- to quantify the influence of neighborhood plant-plant competition, canopy openness, and water availability status (i.e., dry plots vs. less-dry plots) on the mean basal area increment in beech trees.
2. Materials and Methods
2.1. Study Locations and Sampling Design
2.2. Tree Ring Analysis
2.3. Soil Analyses
2.4. Climate Data and Quantification of Drought
2.5. Relating the Response of Annual Growth to Climate and Drought Intensity
2.6. Influence of Multiple Severe Drought Years on Resistance, Recovery, and Resilience of Growth
2.7. Comparing Resistance, Recovery, and Resilience with the Response Ratio
2.8. Light Measurement and Assessment of Plant-Plant Neighborhood Interactions
3. Results
3.1. Tree Ring Chronology, Size, and Growth Comparisons
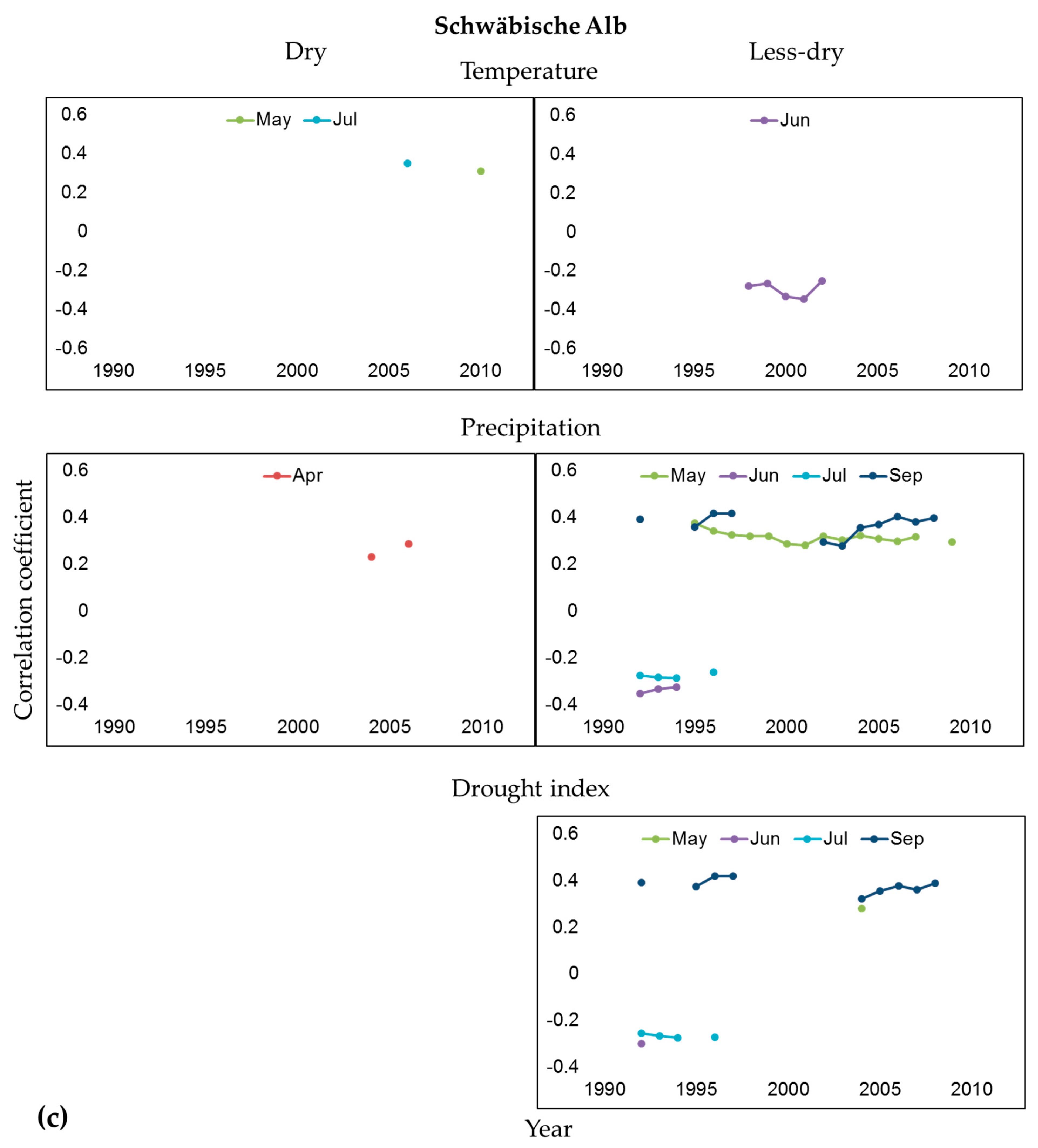
3.2. Influence of Climate and Drought on Radial Growth
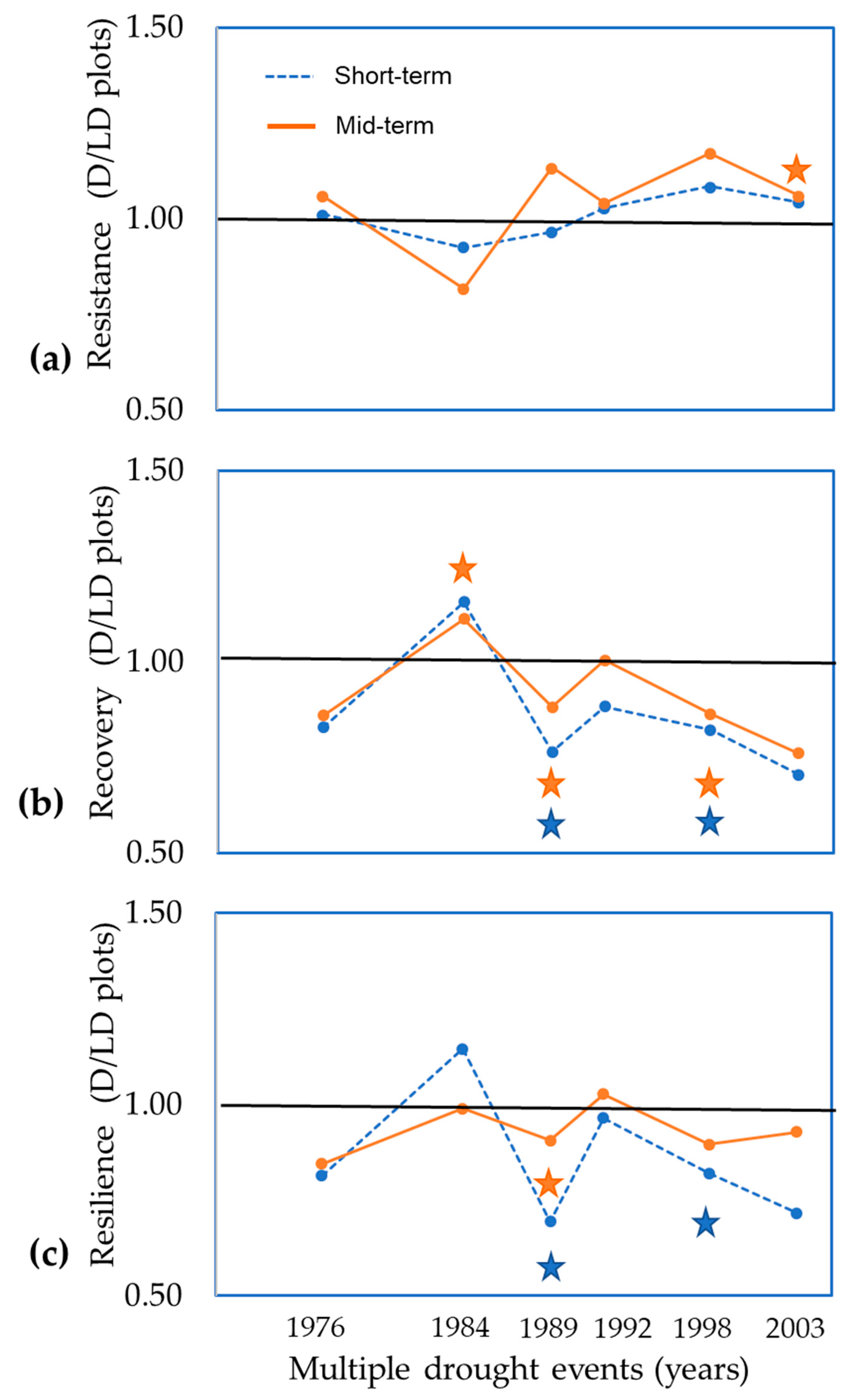
3.3. Comparisons of Resistance, Recovery, and Resilience of Trees
3.4. Stand Density and Composition of Study Locations, and Influence of Canopy Openness and Neighborhood Competition on Mean Basal Area Increment of Beech Trees
4. Discussion
4.1. Growth Difference in Beech Trees between Dry and Less-Dry Plots
4.2. Climate-Growth Relationships
4.3. Resistance, Recovery, and Resilience of Trees under Multiple Drought Events
4.4. Growth Reaction of Beech Trees under Drought Stress
4.5. Impact of Neighborhood Competition and Canopy Openness on the Mean Growth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Breda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef] [Green Version]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogee, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Gessler, A.; Keitel, C.; Kreuzwieser, J.; Matyssek, R.; Seiler, W.; Rennenberg, H. Potential risks for European beech (Fagus sylvatica L.) in a changing climate. Trees-Struct. Funct. 2007, 21, 1–11. [Google Scholar] [CrossRef]
- Jump, A.S.; Hunt, J.M.; Penuelas, J. Rapid climate change-related growth decline at the southern range edge of Fagus sylvatica. Glob. Chang. Biol. 2006, 12, 2163–2174. [Google Scholar] [CrossRef] [Green Version]
- Ellenberg, H.; Leuschner, C. Vegetation Mitteleuropas mit den Alpen: In Ökologischer, Dynamischer und Historischer Sicht, 6., Überarb; Ulmer UTB: Stuttgart, Germany, 2010; p. 1357. [Google Scholar]
- Dittmar, C.; Zech, W.; Elling, W. Growth variations of Common beech (Fagus sylvatica L.) under different climatic and environmental conditions in Europe—A dendroecological study. For. Ecol. Manag. 2003, 173, 63–78. [Google Scholar] [CrossRef]
- Lebourgeois, F.; Breda, N.; Ulrich, E.; Granier, A. Climate-tree-growth relationships of European beech (Fagus sylvatica L.) in the French Permanent Plot Network (RENECOFOR). Trees-Struct. Funct. 2005, 19, 385–401. [Google Scholar] [CrossRef]
- Backes, K.; Leuschner, C. Leaf water relations of competitive Fagus sylvatica and Quercus petraea trees during 4 years differing in soil drought. Can. J. For. Res. 2000, 30, 335–346. [Google Scholar] [CrossRef]
- Scharnweber, T.; Manthey, M.; Criegee, C.; Bauwe, A.; Schröder, C.; Wilmking, M. Drought matters-Declining precipitation influences growth of Fagus sylvatica L. and Quercus robur L. in north-eastern Germany. For. Ecol. Manag. 2011, 262, 947–961. [Google Scholar] [CrossRef]
- Ammer, C.; Albrecht, L.; Borchert, H.; Brosinger, F.; Dittmar, C.; Elling, W.; Ewald, J.; Felbermeier, B.; von Gilsa, H.; Huss, J.; et al. Future suitability of beech (Fagus sylvatica L.) in Central Europe: Critical remarks concerning a paper of Rennenberg et al. (2004). Allg. Forst Jagdztg. 2005, 176, 60–67. [Google Scholar]
- Weber, P.; Bugmann, H.; Pluess, A.R.; Walthert, L.; Rigling, A. Drought response and changing mean sensitivity of European beech close to the dry distribution limit. Trees-Struct. Funct. 2013, 27, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Cavin, L.; Jump, A.S. Highest drought sensitivity and lowest resistance to growth suppression are found in the range core of the tree Fagus sylvatica L. not the equatorial range edge. Glob. Chang. Biol. 2017, 23, 362–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, T.; Saha, S.; Reif, A. Biomass equations for European beech growing on dry sites. iForest-Biogeosci. For. 2016, 9, 751–757. [Google Scholar] [CrossRef] [Green Version]
- Pretzsch, H.; Roetzer, T.; Matyssek, R.; Grams, T.E.E.; Haeberle, K.H.; Pritsch, K.; Kerner, R.; Munch, J.C. Mixed Norway spruce (Picea abies [L.] Karst) and European beech (Fagus sylvatica [L.]) stands under drought: From reaction pattern to mechanism. Trees-Struct. Funct. 2014, 28, 1305–1321. [Google Scholar] [CrossRef]
- Schulze, E.-D.; Beck, E.; Muller-Hohenstein, K. Plant Ecology; Springer: Berlin/Heidelberg, Germany, 2005; p. 702. [Google Scholar]
- Bolte, A.; Czajkowski, T.; Kompa, T. The north-eastern distribution range of European beech—A review. Forestry 2007, 80, 413–429. [Google Scholar] [CrossRef]
- Bertness, M.D.; Callaway, R. Positive interactions in communities. Trends Ecol. Evol. 1994, 9, 191–193. [Google Scholar] [CrossRef]
- Gärtner, S.; Reif, A.; Xystrakis, F.; Sayer, U.; Bendagha, N.; Matzarakis, A. The drought tolerance limit of Fagus sylvatica forest on limestone in southwestern Germany. J. Veg. Sci. 2008, 19, 757–768. [Google Scholar] [CrossRef]
- Douglass, A.E. Evidence of climatic effects in the annual rings of trees. Ecology 1920, 1, 24–32. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Academic Press: San Diego, CA, USA, 1976; p. 567. [Google Scholar]
- Schweingruber, F.H. Tree Rings and Environment: Dendroecology; Paul Haupt AG: Bern, Switzerland, 1996. [Google Scholar]
- Garcia-Suarez, A.M.; Butler, C.J.; Baillie, M.G.L. Climate signal in tree-ring chronologies in a temperate climate: A multi-species approach. Dendrochronologia 2009, 27, 183–198. [Google Scholar] [CrossRef] [Green Version]
- Breda, N.; Badeau, V. Forest tree responses to extreme drought and some biotic events: Towards a selection according to hazard tolerance? Comptes Rendus Geosci. 2008, 340, 651–662. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; p. 1132. [Google Scholar]
- Metz, J.; Annighöfer, P.; Schall, P.; Zimmermann, J.; Kahl, T.; Schulze, E.-D.; Ammer, C. Site-adapted admixed tree species reduce drought susceptibility of mature European beech. Glob. Chang. Biol. 2016, 22, 903–920. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, T.; Saha, S.; Matzarakis, A.; Reif, A. Influence of multiple biotic and abiotic factors on the crown die-back of European beech trees at their drought limit. Flora-Morphol. Distrib. Funct. Ecol. Plants 2017, 229, 58–70. [Google Scholar] [CrossRef]
- Gauer, J.; Aldinger, E. Mitteilungen des Vereins für Forstliche Standortskunde und Forstpflanzenzüchtung; Verein für Forstliche Standortskunde und Forstpflanzenzüchtung e.V.: Freiburg, Germany, 2005; Volume 43. [Google Scholar]
- DWD. Wetter und Klima aus Einer Hand; Deutscher Wetterdienst: Offenbach, Germany, 2015.
- Federal Office of Meteorology and Climatology. MeteoSwiss; Federal Office of Meteorology and Climatology: Zürich, Switzerland, 2015.
- Turc, L. Estimation of irrigation water requirements, potential evapotranspiration: A simple climatic formula evolved up to date. Ann. Agron. 1961, 12, 13–49. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Biondi, F.; Qeadan, F. Inequality in paleorecords. Ecology 2008, 89, 1056–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, E.R.; Krusic, P.J. Program ARSTAN: A Tree-Ring Standardization Program Based on Detrending and Autoregressive Time Series Modeling, with Interactive Graphics; Tree-Ring Laboratory Lamont Doherty Earth Observatory of Columbia University Palisades: New York, NY, USA, 2007. [Google Scholar]
- Chakraborty, T. Effect of Soil Drought on Vitality and Growth on Juvenile and Understorey Beech (Fagus sylvatica L.) Trees: Case Study from a Rocky Gneiss Outcrop near Freiburg, Black Forest, Germany. Master’s Thesis, Albert-Ludwigs-University of Freiburg, Freiburg, Germany, 2010; p. 86. Available online: https://freidok.uni-freiburg.de/data/8066 (accessed on 1 January 2021).
- Chakraborty, T.; Saha, S.; Reif, A. Decrease in available soil water storage capacity reduces vitality of young understorey European beeches (Fagus sylvatica L.)—A case study from the Black Forest, Germany. Plants 2013, 2, 676–698. [Google Scholar] [CrossRef]
- FAO. Guidelines for Soil Description; FAO of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Arbeitskreis Standortskartierung in der Arbeitsgemeinschaft Forsteinrichtung. Forstliche Standortsaufnahme: Begriffe, Definitionen, Einteilungen, Kennzeichnungen, Erläuterungen, 7th ed.; IHW-Verlag: Eching, Germany, 2016. [Google Scholar]
- Matzarakis, A.; Rutz, F.; Mayer, H. Modelling radiation fluxes in simple and complex environments—Application of the RayMan model. Int. J. Biometeorol. 2007, 51, 323–334. [Google Scholar] [CrossRef]
- Spinoni, J.; Naumann, G.; Vogt, J.; Barbosa, P. European drought climatologies and trends based on a multi-indicator approach. Glob. Planet. Chang. 2015, 127, 50–57. [Google Scholar] [CrossRef]
- Biondi, F.; Waikul, K. DENDROCLIM2002: A C++ program for statistical calibration of climate signals in tree-ring chronologies. Comput. Geosci. 2004, 30, 303–311. [Google Scholar] [CrossRef]
- Piovesan, G.; Biondi, F.; Di Filippo, A.; Alessandrini, A.; Maugeri, M. Drought-driven growth reduction in old beech (Fagus sylvatica L.) forests of the central Apennines, Italy. Glob. Chang. Biol. 2008, 14, 1265–1281. [Google Scholar] [CrossRef]
- Hacket-Pain, A.J.; Cavin, L.; Friend, A.D.; Jump, A.S. Consistent limitation of growth by high temperature and low precipitation from range core to southern edge of European beech indicates widespread vulnerability to changing climate. Eur. J. Res. 2016, 135, 897–909. [Google Scholar] [CrossRef] [Green Version]
- Dorado-Linan, I.; Zorita, E.; Martinez-Sancho, E.; Gea-Izquierdo, G.; Di Filippo, A.; Gutierrez, E.; Levanic, T.; Piovesan, G.; Vacchiano, G.; Zang, C.; et al. Large-scale atmospheric circulation enhances the Mediterranean East-West tree growth contrast at rear-edge deciduous forests. Agric. For. Meteorol. 2017, 239, 86–95. [Google Scholar] [CrossRef]
- Kozlowski, T.T.; Pallardy, S.G. Growth Control in Woody Plants; Academic Press: San Diego, CA, USA, 1997; p. 641. [Google Scholar]
- van der Maaten, E. Climate sensitivity of radial growth in European beech (Fagus sylvatica L.) at different aspects in southwestern Germany. Trees-Struct. Funct. 2012, 26, 777–788. [Google Scholar] [CrossRef] [Green Version]
- Lloret, F.; Keeling, E.G.; Sala, A. Components of tree resilience: Effects of successive low-growth episodes in old ponderosa pine forests. Oikos 2011, 120, 1909–1920. [Google Scholar] [CrossRef]
- Sohn, J.A.; Kohler, M.; Gessler, A.; Bauhus, J. Interactions of thinning and stem height on the drought response of radial stem growth and isotopic composition of Norway spruce (Picea abies). Tree Physiol. 2012, 32, 1199–1213. [Google Scholar] [CrossRef] [Green Version]
- Sohn, J.; Saha, S.; Bauhus, J. Potential of forest thinning to mitigate drought stress: A meta-analysis. For. Ecol. Manag. 2016, 380, 261–273. [Google Scholar] [CrossRef]
- Osenberg, C.W.; Sarnelle, O.; Cooper, S.D. Effect size in ecological experiments: The application of biological models in meta-analysis. Am. Nat. 1997, 150, 798–812. [Google Scholar] [CrossRef]
- Gurevitch, J.; Hedges, L.V. Statistical issues in ecological meta-analyses. Ecology 1999, 80, 1142–1149. [Google Scholar] [CrossRef]
- Saha, S.; Kuehne, C.; Kohnle, U.; Brang, P.; Ehring, A.; Geisel, J.; Leder, B.; Muth, M.; Petersen, R.; Peter, J. Growth and quality of young oaks (Quercus robur and Quercus petraea) grown in cluster plantings in central Europe: A weighted meta-analysis. For. Ecol. Manag. 2012, 283, 106–118. [Google Scholar] [CrossRef]
- Rosenberg, M.S.; Adams, D.C.; Gurevitch, J. MetaWin: Statistical Software for Meta-Analysis; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Dawkins, H.C.; Field, D.R.B. A Long-Term Surveillance System for British Woodland Vegetation; Commonwealth Forestry Institute: Oxford, UK, 1978. [Google Scholar]
- Clark, D.A.; Clark, D.B. Life history diversity of canopy and emergent trees in a neotropical rain forest. Ecol. Monogr. 1992, 62, 315–344. [Google Scholar] [CrossRef]
- Regent Instruments. WinSCANOPY: For Canopy Analysis; Regent Instruments Inc.: Quebec, QC, Canada, 2008. [Google Scholar]
- Coomes, D.A.; Allen, R.B. Effects of size, competition and altitude on tree growth. J. Ecol. 2007, 95, 1084–1097. [Google Scholar] [CrossRef]
- Hegyi, F. A simulation model for managing jack-pine stands. In Growth Models for Tree and Stand Simulation; Fries, J., Ed.; Royal College of Forestry: Stockh, Sweden, 1974; pp. 74–76. [Google Scholar]
- Pretzsch, H. Forest Dynamics, Growth, and Yield; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Aranda, I.; Gil, L.; Pardos, J.A. Water relations and gas exchange in Fagus sylvatica L. and Quercus petraea (Mattuschka) Liebl. in a mixed stand at their southern limit of distribution in Europe. Trees-Struct. Funct. 2000, 14, 344–352. [Google Scholar] [CrossRef]
- Meier, I.C.; Leuschner, C. Belowground drought response of European beech: Fine root biomass and carbon partitioning in 14 mature stands across a precipitation gradient. Glob. Chang. Biol. 2008, 14, 2081–2095. [Google Scholar] [CrossRef]
- Cavin, L.; Mountford, E.P.; Peterken, G.F.; Jump, A.S. Extreme drought alters competitive dominance within and between tree species in a mixed forest stand. Funct. Ecol. 2013, 27, 1424–1435. [Google Scholar] [CrossRef]
- Barigah, T.S.; Charrier, O.; Douris, M.; Bonhomme, M.; Herbette, S.; Ameglio, T.; Fichot, R.; Brignolas, F.; Cochard, H. Water stress-induced xylem hydraulic failure is a causal factor of tree mortality in beech and poplar. Ann. Bot. 2013, 112, 1431–1437. [Google Scholar] [CrossRef]
- Czajkowski, T.; Kuhling, M.; Bolte, A. Impact of the 2003 summer drought on growth of beech sapling natural regeneration (Fagus sylvatica L.) in north-eastern Central Europe. Allg. Forst Jagdztg. 2005, 176, 133–143. [Google Scholar]
- Hacket-Pain, A.J.; Friend, A.D.; Lageard, J.G.A.; Thomas, P.A. The influence of masting phenomenon on growth-climate relationships in trees: Explaining the influence of previous summers’ climate on ring width. Tree Physiol. 2015, 35, 319–330. [Google Scholar] [CrossRef]
- Krahl-Urban, B.; Papke, H.; Peters, K.; Schimansky, C. Forest Decline: Cause-Effect Research in the United States of North America and the Federal Republic of Germany; Assessment Group for Biology, Ecology and Energy of the Juelich Research Center for the US Environmental Agency and German Ministery of Research and Technology: Jülich, Germany, 1988.
- Vacchiano, G.; Hacket-Pain, A.; Turco, M.; Motta, R.; Maringer, J.; Conedera, M.; Drobyshev, I.; Ascoli, D. Spatial patterns and broad-scale weather cues of beech mast seeding in Europe. New Phytol. 2017, 215, 595–608. [Google Scholar] [CrossRef] [Green Version]
- Mund, M.; Kutsch, W.L.; Wirth, C.; Kahl, T.; Knohl, A.; Skomarkova, M.V.; Schulze, E.D. The influence of climate and fructification on the inter-annual variability of stem growth and net primary productivity in an old-growth, mixed beech forest. Tree Physiol. 2010, 30, 689–704. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Plazaola, J.I.; Esteban, R.; Hormaetxe, K.; Fernandez-Marin, B.; Becerril, J.M. Photoprotective responses of Mediterranean and Atlantic trees to the extreme heat-wave of summer 2003 in Southwestern Europe. Trees-Struct. Funct. 2008, 22, 385–392. [Google Scholar] [CrossRef]
- Aranda, I.; Gil, L.; Pardos, J. Seasonal water relations of three broadleaved species (Fagus sylvatica L, Quercus petraea (Mattuschka) Liebl and Quercus pyrenaica Willd) in a mixed stand in the centre of the Iberian Peninsula. For. Ecol. Manag. 1996, 84, 219–229. [Google Scholar] [CrossRef]
- Hampe, A.; Petit, R.J. Conserving biodiversity under climate change: The rear edge matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolte, A.; Czajkowski, T.; Cocozza, C.; Tognetti, R.; de Miguel, M.; Psidova, E.; Ditmarova, L.; Dinca, L.; Delzon, S.; Cochard, H.; et al. Desiccation and mortality dynamics in seedlings of different European beech (Fagus sylvatica L.) populations under extreme drought conditions. Front. Plant Sci. 2016, 7, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutschick, V.P.; BassiriRad, H. Extreme events as shaping physiology, ecology, and evolution of plants: Toward a unified definition and evaluation of their consequences. New Phytol. 2003, 160, 21–42. [Google Scholar] [CrossRef]
- Vospernik, S. Basal area increment models accounting for climate and mixture for Austrian tree species. For. Ecol. Manag. 2021, 480, 21. [Google Scholar] [CrossRef]

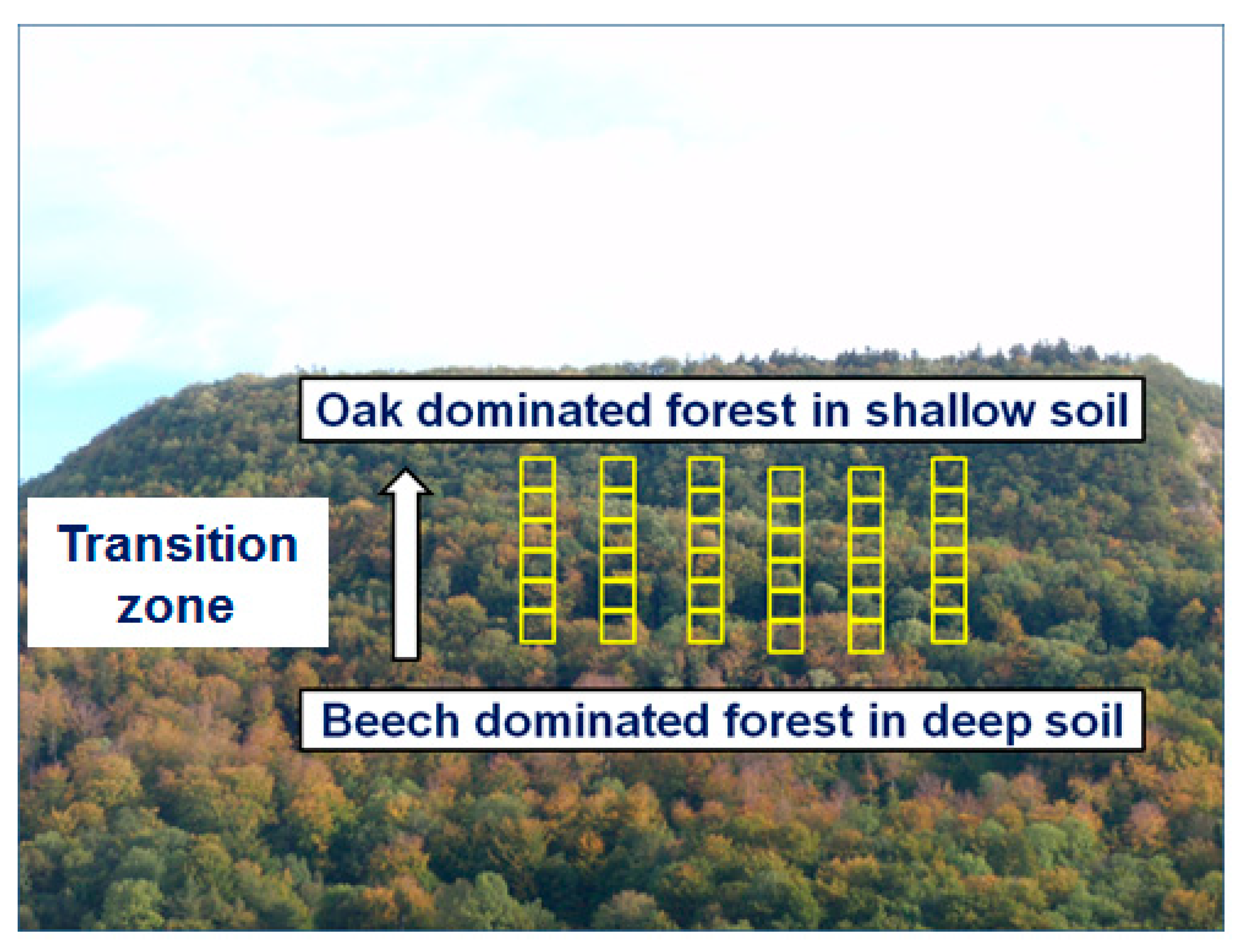
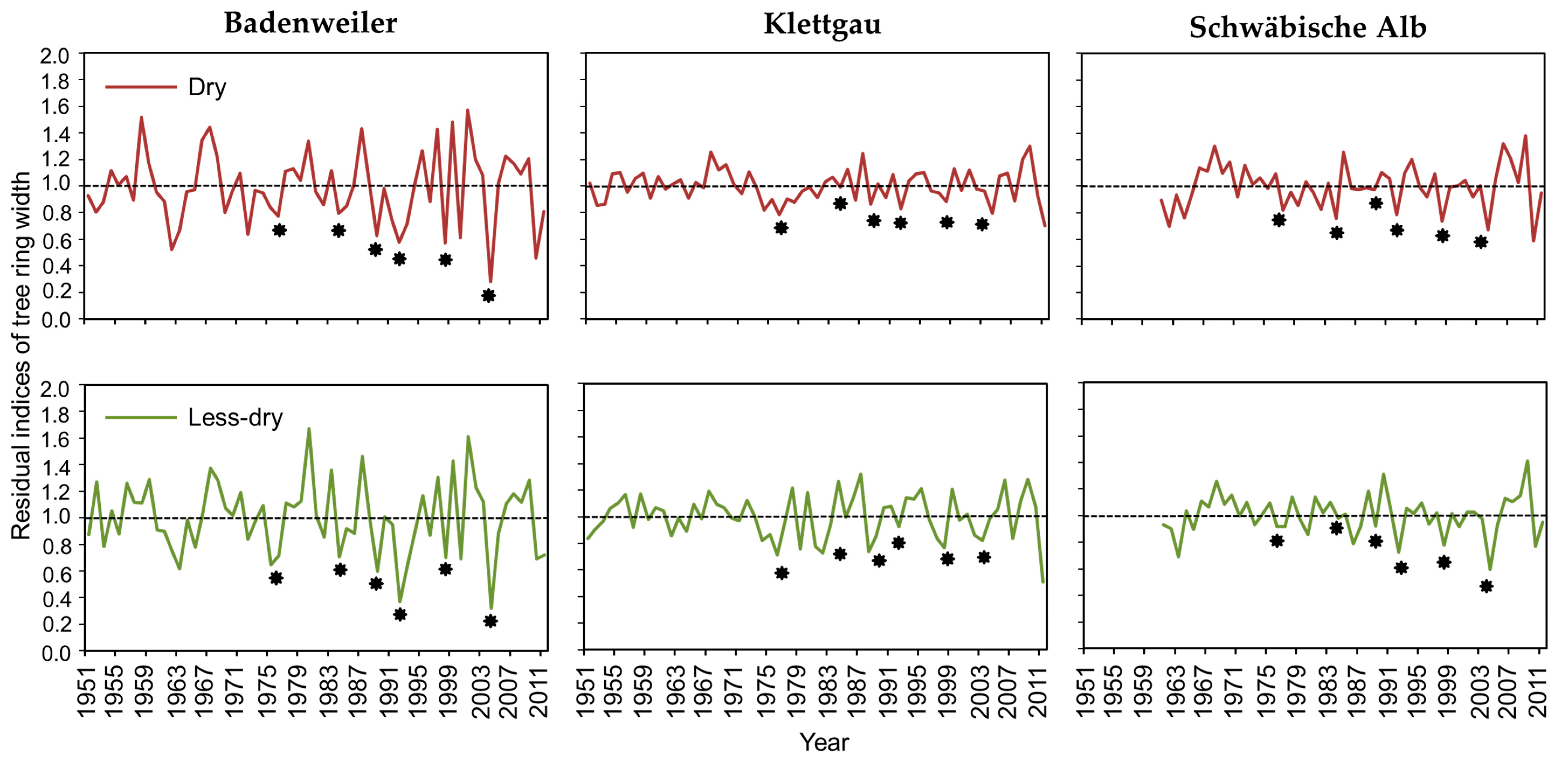
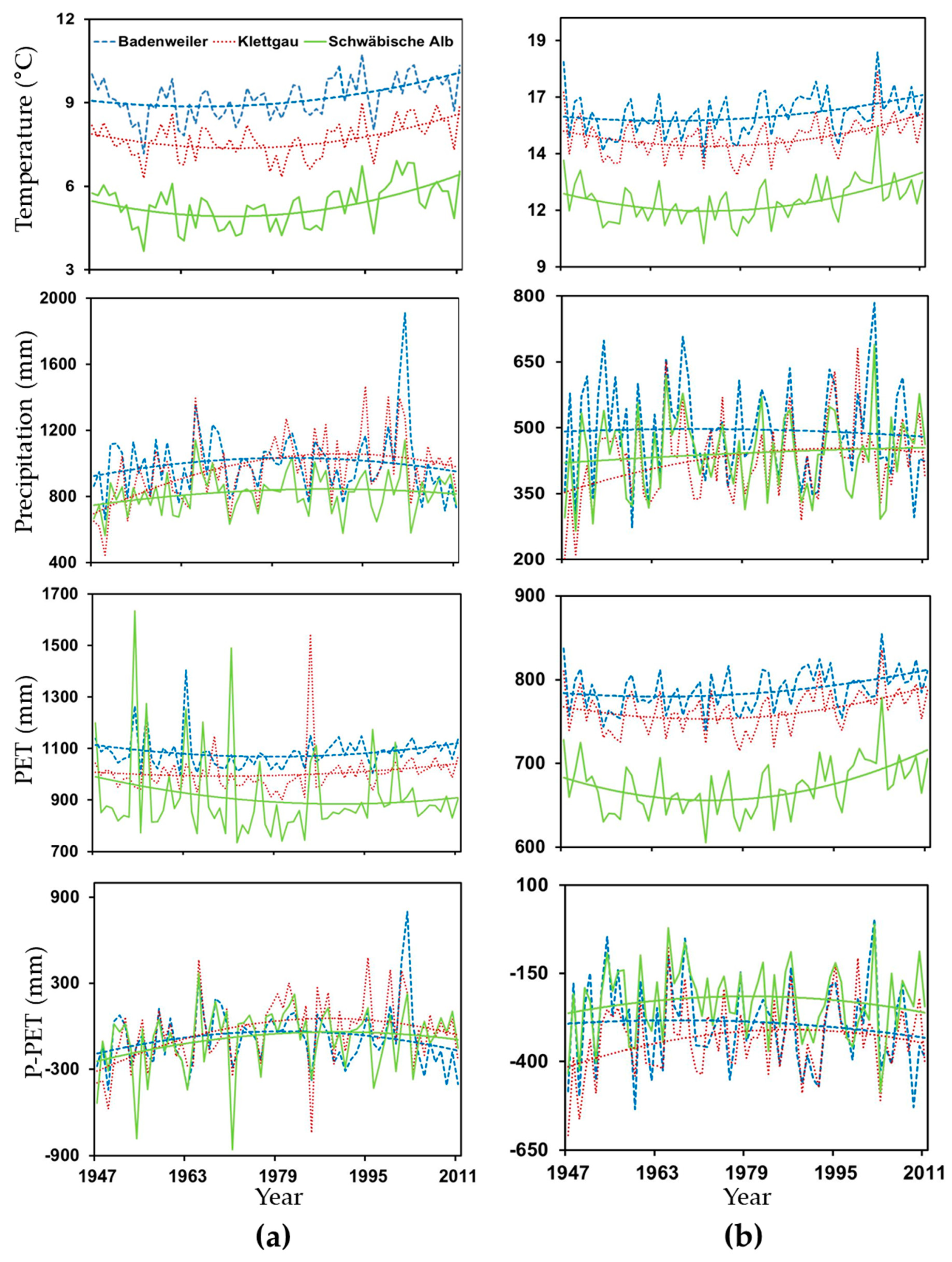
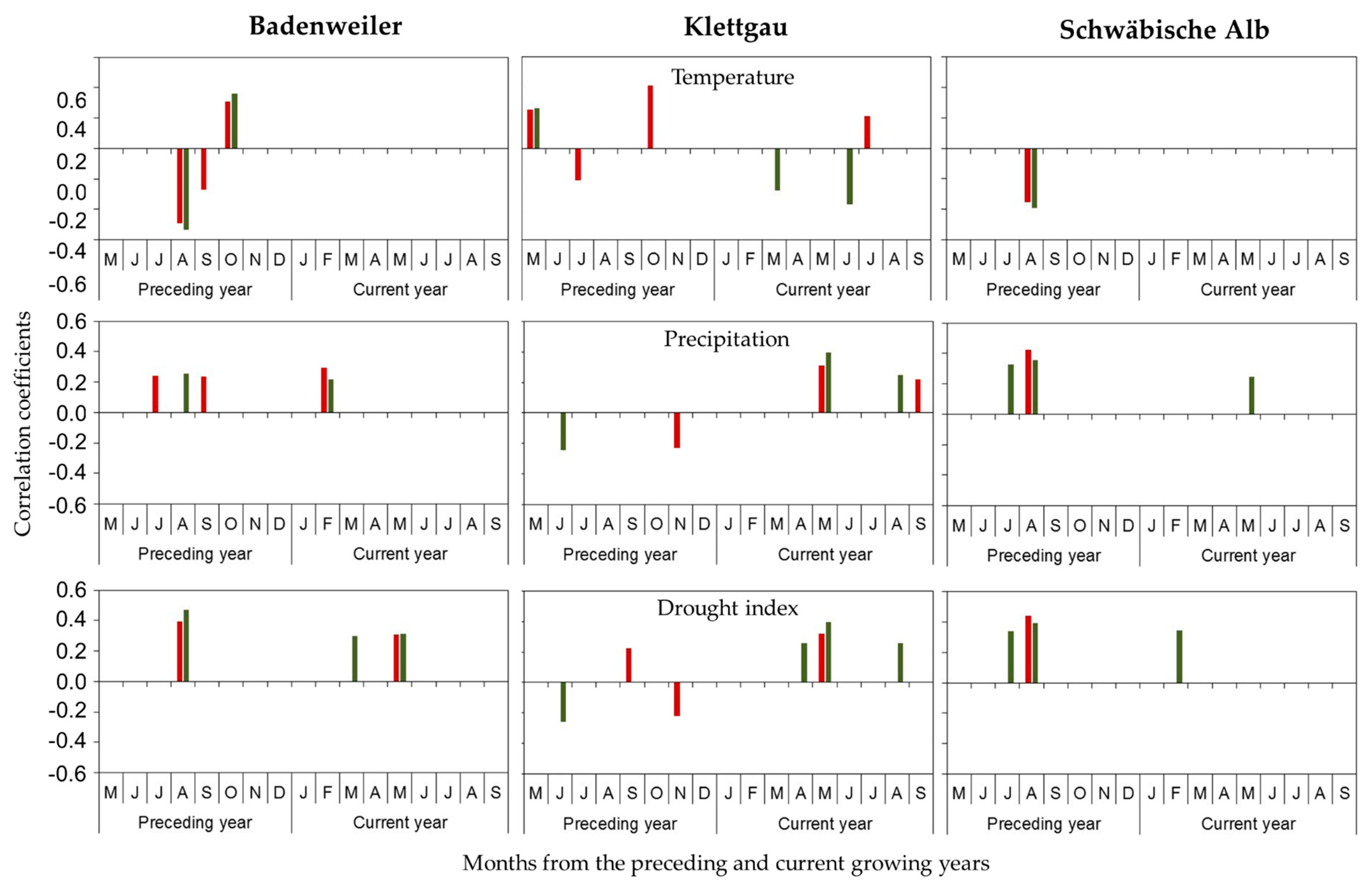

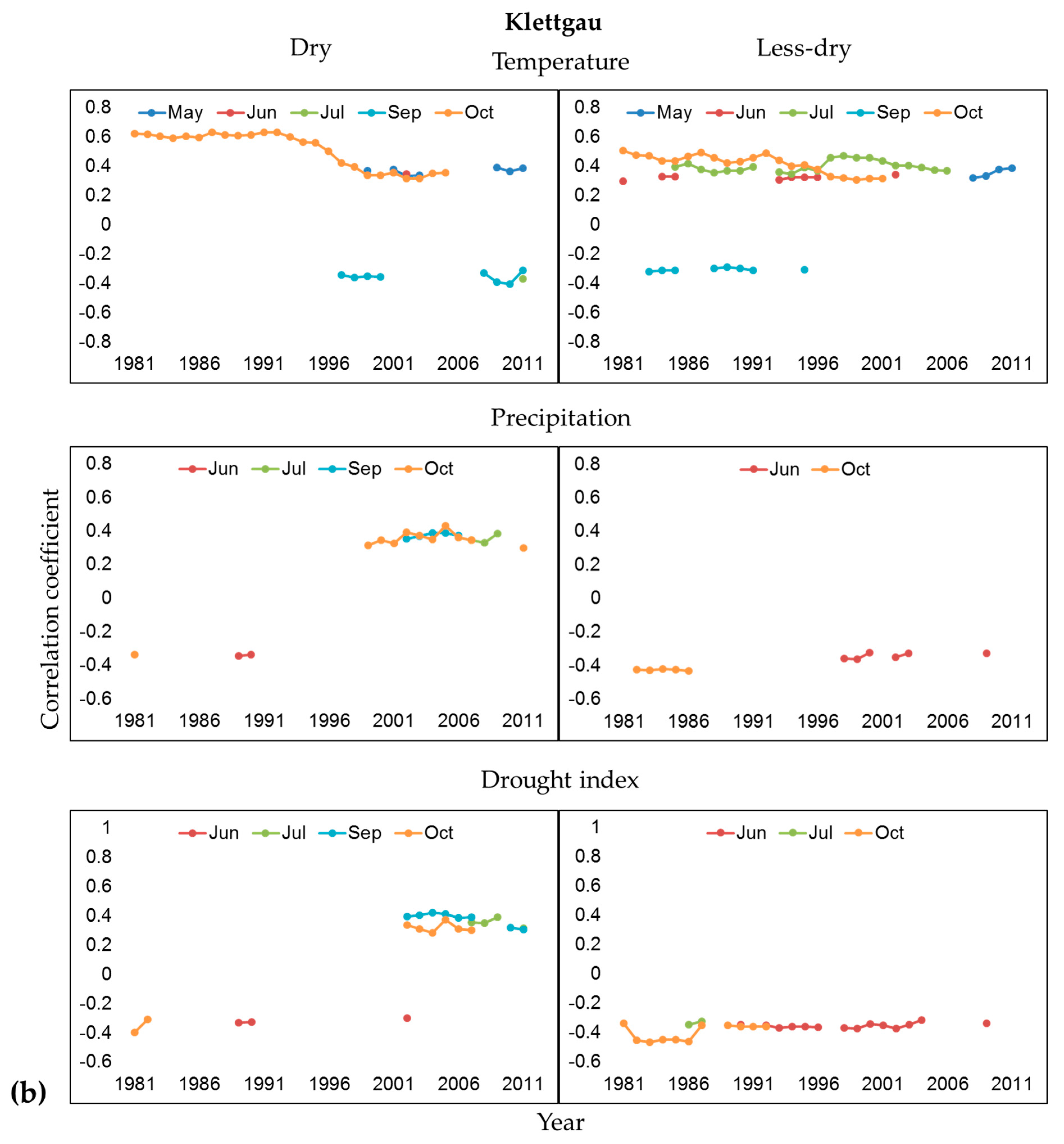
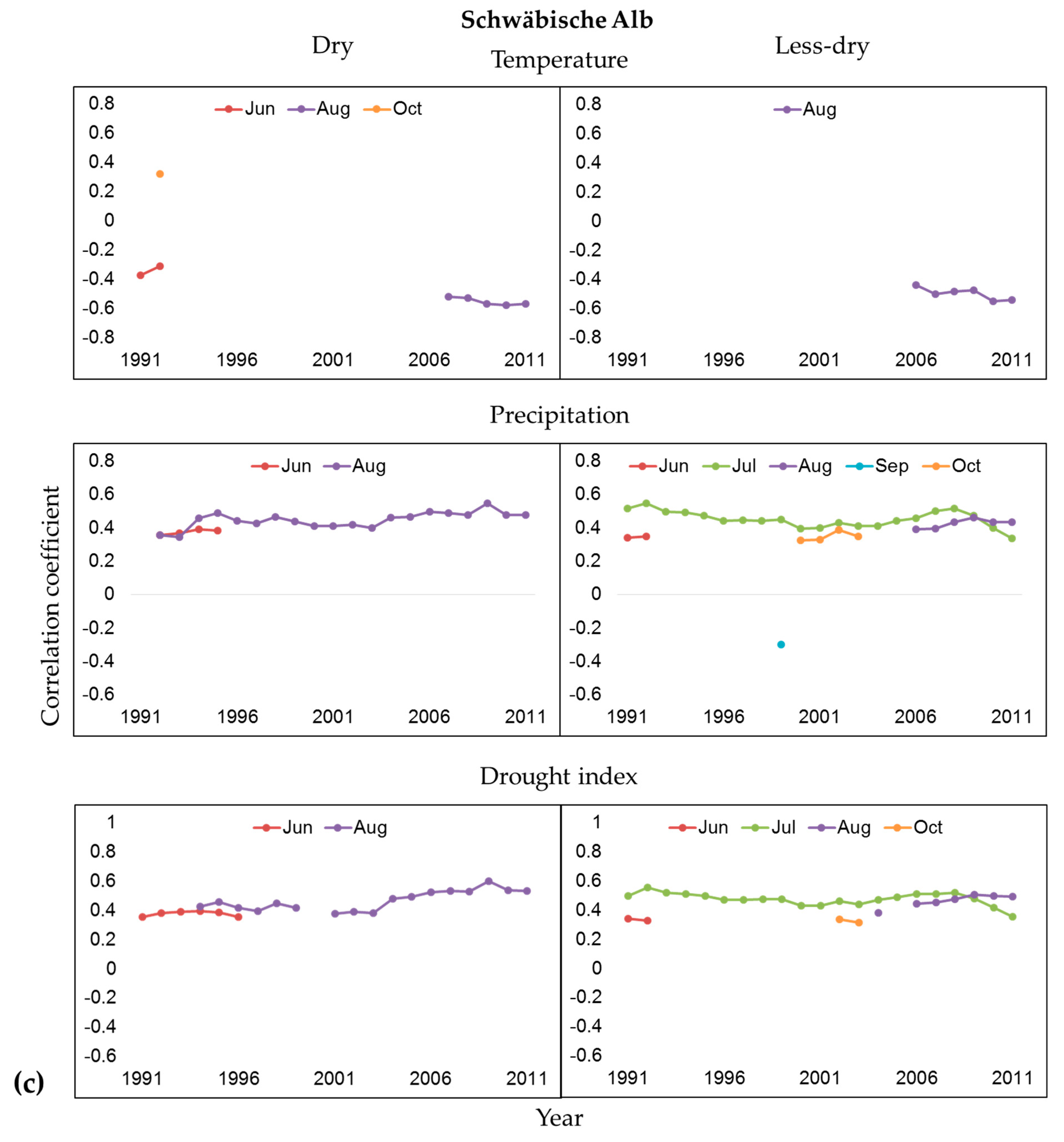

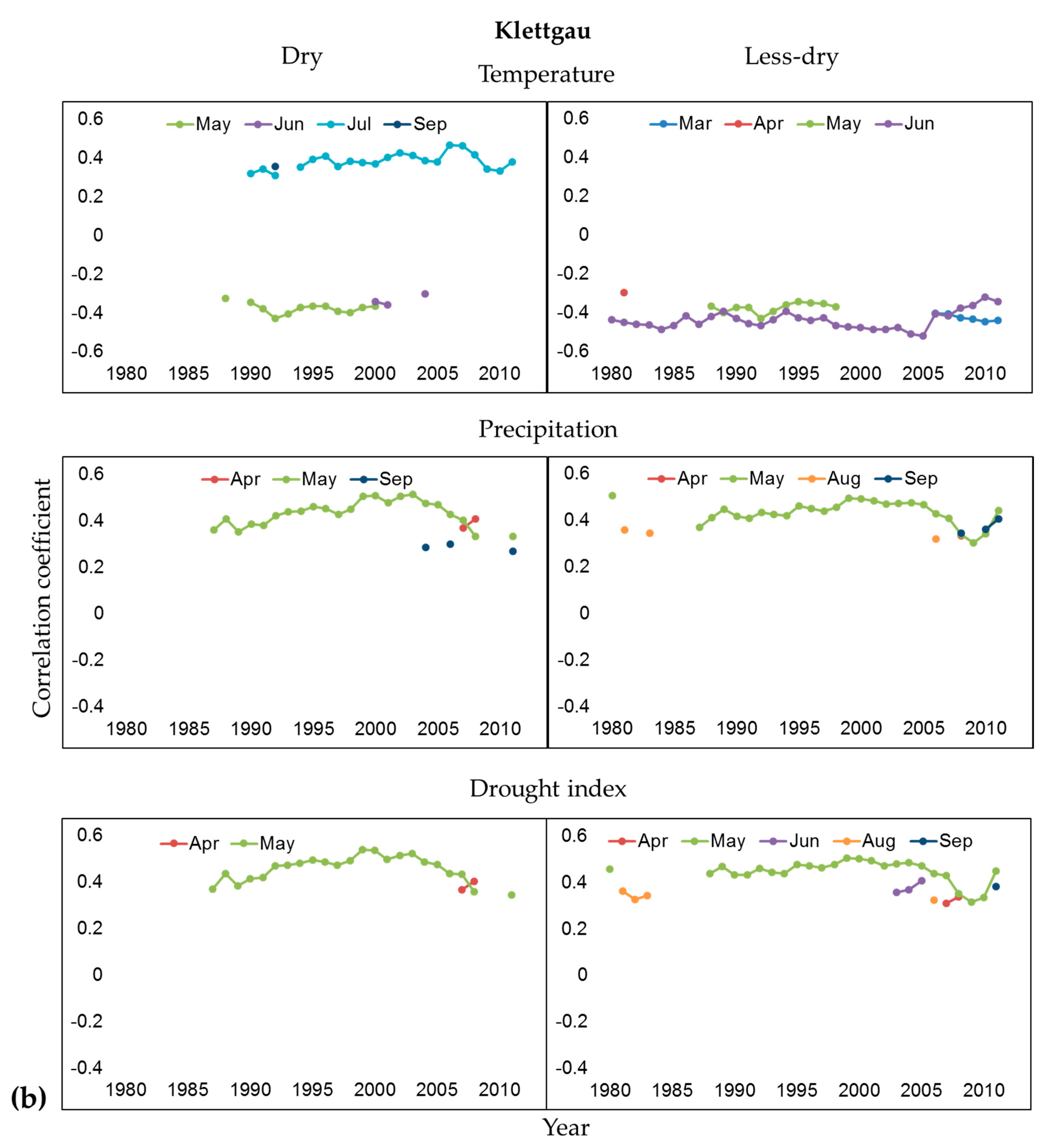
| Stand | Country | Region/Locality | Geographic Location (Long. E, Lat. N) | Elevation (m a.s.l.) | Size (ha) | MAT (°C) 2 | MTAP (mm) 2 | MTAPET (mm) 2 3 | Days > 10 °C 1 | Slope Angle (°) | Aspect (°) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Innerberg | Germany | Badenweiler in Markgräflerland | 7.67, 47.81 | 524 | 0.9 | 9.2 | 1000 | 1085 | 177 | 23 | 155–180 |
| Schönberg | Germany | Schwäbische Alb | 9.12, 48.42 | 781 | 0.8 | 5.3 | 831 | 912 | 143 | 31 | 205–243 |
| Kätzler | Germany | Klettgau | 8.43, 47.62 | 566 | 0.3 | 7.7 | 966 | 1006 | 164 | 30 | 185–228 |
| Steimüri | Switzerland | Klettgau | 8.50, 47.65 | 584 | 0.5 | 7.7 | 966 | 992 | 164 | 24 | 251–280 |
| Steinbruch | Switzerland | Klettgau | 8.50, 47.67 | 643 | 0.4 | 7.7 | 966 | 1004 | 164 | 16 | 210–220 |
| Parameter | Badenweiler | Klettgau | Schwäbische Alb | |||
|---|---|---|---|---|---|---|
| Dry Plots | Less-Dry Plots | Dry Plots | Less-Dry Plots | Dry Plots | Less-Dry Plots | |
| Number of trees (N) | 9 | 9 | 12 | 9 | 9 | 8 |
| Mean DBH (cm) | 7.82 | 8.97 | 7.76 | 9.98 | 7.03 | 7.5 |
| St. dev. of DBH | 3.1 | 3.22 | 2.2 | 2.95 | 2.7 | 3.59 |
| Mean age (years) | 52 | 56 | 81 | 78 | 46 | 47 |
| St. Dev. Of age | 9 | 16 | 21 | 15 | 10 | 7 |
| Mean height (m) | 8.3 | 9.78 | 7.3 | 8.89 | 6.8 | 7.25 |
| St. dev. of height | 1.9 | 2.11 | 2.3 | 2.65 | 2.3 | 1.25 |
| Total timespan | 1948–2011 | 1939–2011 | 1908–2011 | 1910–2011 | 1958–2011 | 1959–2011 |
| Reliable timespan | 1951–2011 | 1951–2011 | 1951–2011 | 1951–2011 | 1961–2011 | 1961–2011 |
| Mean BAI (mm2) | 71.10 | 93.31 | 54.38 | 87.34 | 65.82 | 86.62 |
| St. dev. of BAI | 52.89 | 44.79 | 14.90 | 28.03 | 33.71 | 37.52 |
| Mean sensitivity | 0.491 | 0.496 | 0.277 | 0.313 | 0.337 | 0.353 |
| Mean rbar | 0.396 | 0.414 | 0.353 | 0.427 | 0.466 | 0.416 |
| EPS | 0.86 | 0.86 | 0.0.87 | 0.87 | 0.89 | 0.85 |
| Mean AWC (mm) | 49.6 | 73.14 | 42.23 | 75.83 | 48.62 | 101.54 |
| St. dev. of AWC | 11 | 12.7 | 10.05 | 13.06 | 11.89 | 37.09 |
| Locations | Mean Tree Species Richness | Basal Area (m2/ha) | Stem Density/ha | Basal Area Proportion (%) of Quercus sp. | Basal Area Proportion (%) of Fagus Sylvatica | Basal Area Proportion (%) of Other Tree Species |
|---|---|---|---|---|---|---|
| Badenweiler | 5 | 47 | 3640 | 87.71 | 5.55 | 6.74 |
| Klettgau | 5 | 42 | 1628 | 43.53 | 37.36 | 19.11 |
| Schwäbische Alb | 8 | 33 | 3594 | 50.65 | 21.17 | 28.18 |
| Explanatory Variables | Coefficient | SE of Coefficient | t Value | p Value | Lower CI | Upper CI | Predictors’ Importance |
|---|---|---|---|---|---|---|---|
| Intercept | 98.87 | 12.68 | 7.79 | 0.0001 | 73.401 | 124.35 | |
| Hegyi’s index | −2.29 | 0.41 | −5.48 | 0.0001 | −3.13 | −1.45 | 0.78 |
| Canopy openness | 2.72 | 1.13 | 2.406 | 0.02 | 0.45 | 5.08 | 0.15 |
| AWC Class Dry vs. Less dry | −14.33 | 9.34 | −1.53 | 0.131 | −33.1 | 4.43 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakraborty, T.; Reif, A.; Matzarakis, A.; Saha, S. How Does Radial Growth of Water-Stressed Populations of European Beech (Fagus sylvatica L.) Trees Vary under Multiple Drought Events? Forests 2021, 12, 129. https://doi.org/10.3390/f12020129
Chakraborty T, Reif A, Matzarakis A, Saha S. How Does Radial Growth of Water-Stressed Populations of European Beech (Fagus sylvatica L.) Trees Vary under Multiple Drought Events? Forests. 2021; 12(2):129. https://doi.org/10.3390/f12020129
Chicago/Turabian StyleChakraborty, Tamalika, Albert Reif, Andreas Matzarakis, and Somidh Saha. 2021. "How Does Radial Growth of Water-Stressed Populations of European Beech (Fagus sylvatica L.) Trees Vary under Multiple Drought Events?" Forests 12, no. 2: 129. https://doi.org/10.3390/f12020129
APA StyleChakraborty, T., Reif, A., Matzarakis, A., & Saha, S. (2021). How Does Radial Growth of Water-Stressed Populations of European Beech (Fagus sylvatica L.) Trees Vary under Multiple Drought Events? Forests, 12(2), 129. https://doi.org/10.3390/f12020129








