Impact of Ex-Closure in above and below Ground Carbon Stock Biomass
Abstract
:1. Introduction
2. Materials and Methodology
3. Issues Exacerbating Carbon Emission
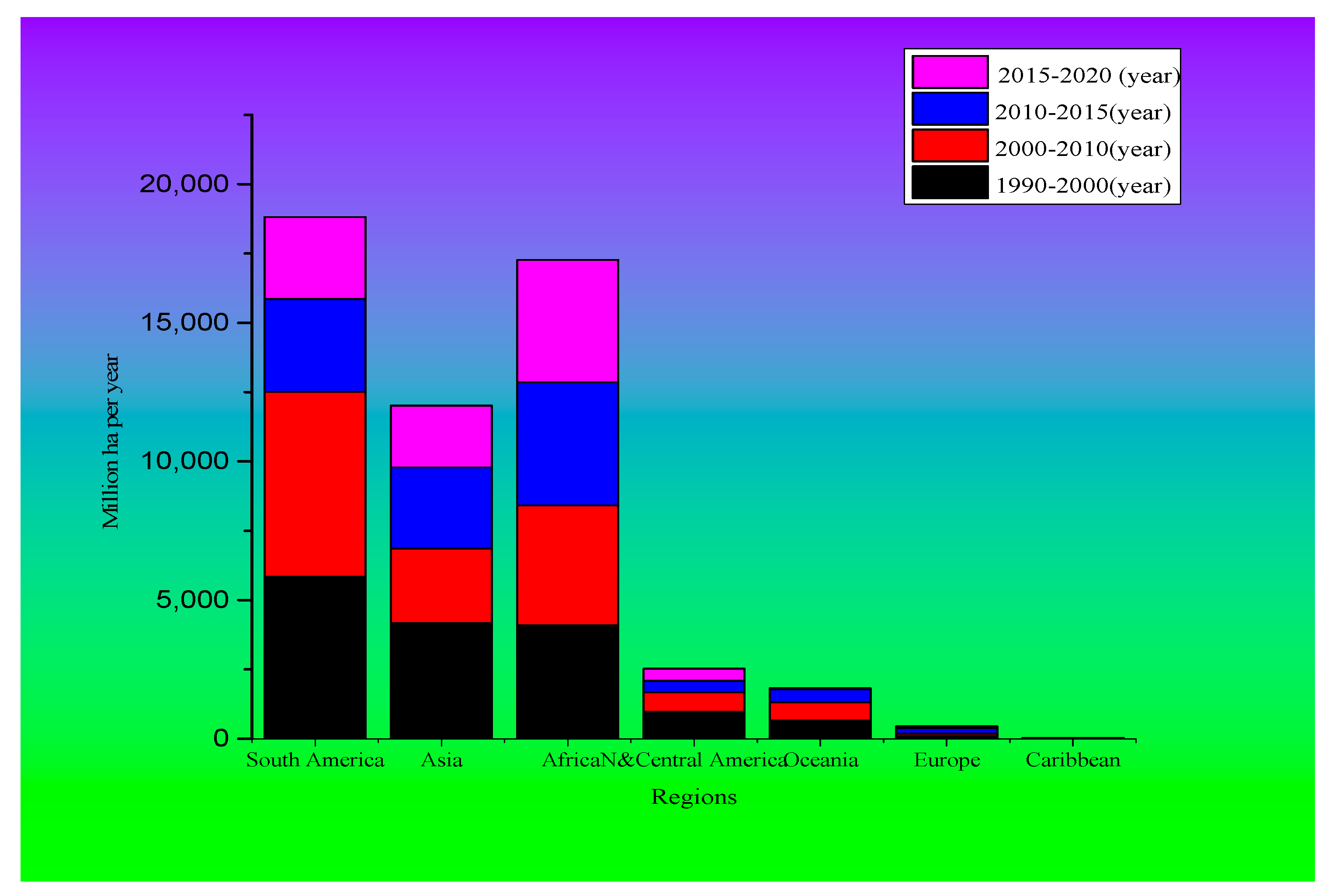
3.1. Deforestation
3.2. Land Degradation
3.3. Overgrazing
The Impacts of Overgrazing on the Characteristics of Vegetation and SOC
4. Mitigation Measures to Reduce the Sources of Carbon Emissions
4.1. Exclosure in Land Rehabilitation and Restoration Forest
4.2. Exclosures on Climate Change Mitigation
4.3. Carbon Stock and Tropical Regions
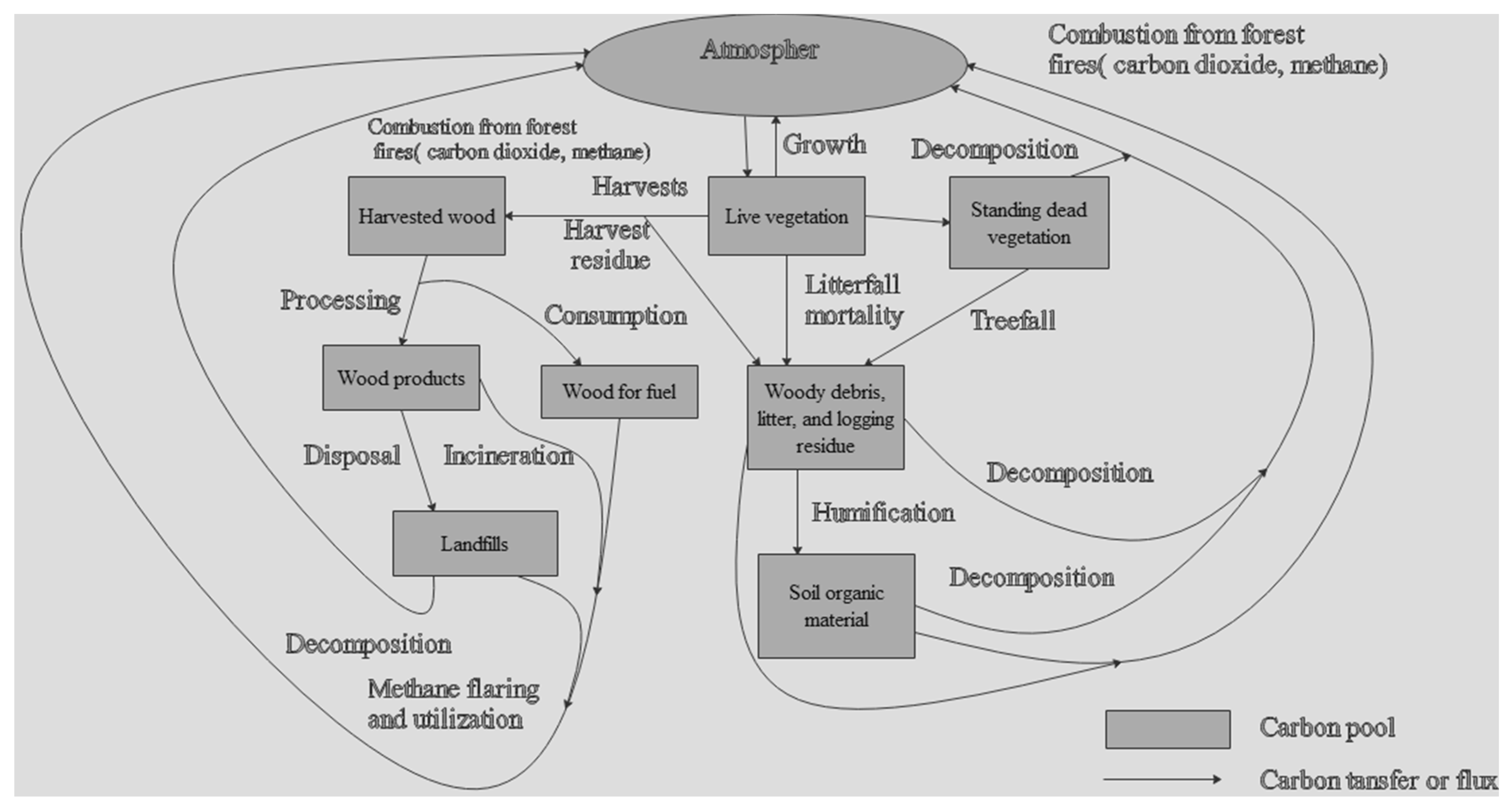
4.4. Carbon Stock Pools and Their Biomass Development
4.4.1. Aboveground Biomass Carbon Stock
4.4.2. Belowground Carbon Stock
4.4.3. Soil Carbon Stock
4.4.4. Carbon Stock in Soils and Tree Biomass
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Global Forest Resources Assessment; Main report; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; p. 184. [Google Scholar]
- Justine, M.F.; Yang, W.; Wu, F.; Tan, B.; Khan, M.N.; Zhao, Y. Biomass stock and carbon sequestration in a chronosequence of Pinus massoniana plantations in the upper reaches of the Yangtze River. Forests 2015, 6, 3665–3682. [Google Scholar] [CrossRef] [Green Version]
- Brown, S. Measuring carbon in forests: Current status and future challenges. Environ. Pollut. 2002, 116, 363–372. [Google Scholar] [CrossRef]
- Keenan, R.J. Climate change impacts and adaptation in forest management: A review. Ann. For. Sci. 2015, 72, 145–167. [Google Scholar] [CrossRef] [Green Version]
- Leithwood, K.; Louis, K.S.; Anderson, S.; Wahlstrom, K. How Leadership Influences Student Learning; Review of Research; Wallace Foundation, Center for Applied Research and Educational Improvement, Minnesota University: Minneapolis, MN, USA, 2004. [Google Scholar]
- Tashi, S.; Keitel, C.; Singh, B.; Adams, M. Allometric equations for biomass and carbon stocks of forests along an altitudinal gradient in the eastern Himalayas. For. Int. J. For. Res. 2017, 90, 445–454. [Google Scholar] [CrossRef]
- MacDicken, K.G. Global forest resources assessment 2015: What, why and how? Ecol. Manag. 2015, 352, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Pandey, D. Carbon Stocks of World Heritage Forest Sites; UNESCO, World Heritage Centre: Paris, France, 2012. [Google Scholar]
- Achard, F.; Boschetti, L.; Brown, S.; Brady, M.; DeFries, R.; Grassi, G.; Herold, M.; Mollicone, D.; Mora, B.; Pandey, D.; et al. A Sourcebook of Methods and Procedures for Monitoring and Reporting Anthropogenic Greenhouse Gas Emissions and Removals Associated with Deforestation, Gains and Losses of Carbon Stocks in Forests Remaining Forests, and Forestation; GOFC-GOLD: Land Cover Project Office, Wageningen University: Wageningen, The Netherlands, 2014. [Google Scholar]
- Nadakavukaren, A.; Caravanos, J. Our Global Environment: A Health Perspective; Waveland Press: Long Grove, IL, USA, 2020. [Google Scholar]
- Manaye, A. Contribution of Exclosures for Restoration of Woody Species Diversity and Regulating Ecosystem Services in Ethiopia; Environment and Forest Research Center: Mekele, Ethiopia, 2017. [Google Scholar]
- Ordoñez, J.C.; Van Bodegom, P.M.; Witte, J.P.M.; Wright, I.J.; Reich, P.B.; Aerts, R. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Glob. Ecol. Biogeogr. 2009, 18, 137–149. [Google Scholar] [CrossRef]
- Hailu, T.A. The contribution of grazing enclosures for sustainable management and enhancing restoration of degraded range lands in Ethiopia: Lessons and forward. J. Environ. Earth Sci. 2016, 6, 112–126. [Google Scholar]
- Birhane, E.; Mengistu, T.; Seyoum, Y.; Hagazi, N.; Putzel, L.; Rannestad, M.M.; Kassa, H. Exclosures as forest and landscape restoration tools: Lessons from Tigray Region, Ethiopia. Int. For. Rev. 2017, 19, 37–50. [Google Scholar] [CrossRef]
- Lemenih, M.; Kassa, H. Re-greening Ethiopia: History, challenges and lessons. Forests 2014, 5, 1896–1909. [Google Scholar] [CrossRef]
- Berihu, T.; Girmay, G.; Sebhatleab, M.; Berhane, E.; Zenebe, A.; Sigua, G.C. Soil carbon and nitrogen losses following deforestation in Ethiopia. Agron. Sustain. Dev. 2017, 37, 1. [Google Scholar] [CrossRef] [Green Version]
- Seyoum, Y.; Birhane, E.; Hagazi, N.; Esmael, N.; Mengistu, T.; Kassa, H. Enhancing the Role of Forestry in Building Climate Resilient Green Economy in Ethiopia; State Ministry of Environment, Forest and climate Change, FDRE: Addis Ababa, Ethiopia, 2015. [Google Scholar]
- Mekuria, W.; Aynekulu, E. Exclosure land management for restoration of the soils in degraded communal grazing lands in northern Ethiopia. Land Degrad. Dev. 2013, 24, 528–538. [Google Scholar] [CrossRef]
- Shimelse, S.; Bekele, T.; Nemomissa, S. Effect of Exclosure Age on Carbon Sequestration Potential of Restorations in Tigray Region, N. Ethiopia. Am. J. Biol. Environ. Stat. 2017, 3, 65–80. [Google Scholar] [CrossRef]
- Abril, A.; Bucher, E. The effects of overgrazing on soil microbial community and fertility in the Chaco dry savannas of Argentina. Appl. Soil Ecol. 1999, 12, 159–167. [Google Scholar] [CrossRef]
- Bauer, G. Reproductive strategy of the freshwater pearl mussel Margaritifera margaritifera. J. Anim. Ecol. 1987, 56, 691–704. [Google Scholar] [CrossRef]
- Chuluun, T.; Ojima, D. Climate and grazing sensitivity of the Mongolian rangeland ecosystem. In Proceedings of the VI International Rangeland Congress on “People and Rangelands: Building the Future”, Townsville, Australia, 19–23 July 1999. [Google Scholar]
- Cui, D.; Tian, F.; Ozkan, C.S.; Wang, M.; Gao, H. Effect of single wall carbon nanotubes on human HEK293 cells. Toxicol. Lett. 2005, 155, 73–85. [Google Scholar] [CrossRef]
- Dong, X.Y.; Wang, Y.M.; Yuan, C.; Zou, X.T. The ontogeny of nutrient transporter and digestive enzyme gene expression in domestic pigeon (Columba livia) intestine and yolk sac membrane during pre-and posthatch development. Poult. Sci. 2012, 91, 1974–1982. [Google Scholar] [CrossRef]
- Spear, F.S. Metamorphic Phase Equilibria and Pressure-Temperature-Time Paths; Mineralogical Society of America: Washington, DC, USA, 1995. [Google Scholar]
- Franzluebbers, A.; Stuedemann, J. Soil-profile organic carbon and total nitrogen during 12 years of pasture management in the Southern Piedmont USA. Agric. Ecosyst. Environ. 2009, 129, 28–36. [Google Scholar] [CrossRef]
- Ganjegunte, G.K.; Vance, G.F.; Preston, C.M.; Schuman, G.E.; Ingram, L.J.; Stahl, P.D.; Welker, J.M. Soil organic carbon composition in a northern mixed-grass prairie: Effects of grazing. Soil Sci. Soc. Am. J. 2005, 69, 1746–1756. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.E.; Handley, J.F.; Ennos, A.R.; Pauleit, S. Adapting cities for climate change: The role of the green infrastructure. Built Environ. 2007, 33, 115–133. [Google Scholar] [CrossRef] [Green Version]
- Hafner, J. From Hamiltonians to Phase Diagrams: The Electronic and Statistical-Mechanical Theory of Sp-Bonded Metals and Alloys; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 70. [Google Scholar]
- Hiltbrunner, D.; Schulze, S.; Hagedorn, F.; Schmidt, M.W.; Zimmmermann, S. Cattle trampling alters soil properties and changes soil microbial communities in a Swiss sub-alpine pasture. Geoderma 2012, 170, 369–377. [Google Scholar] [CrossRef]
- Ingram, L.; Mohan, D.; Bricka, M.; Steele, P.; Strobel, D.; Crocker, D.; Mitchell, B.; Mohammed, J.; Cantrell, K.; Pittman, C.U., Jr. Pyrolysis of wood and bark in an auger reactor: Physical properties and chemical analysis of the produced bio-oils. Energy Fuels 2008, 22, 614–625. [Google Scholar] [CrossRef]
- Manley, J.T.; Schuman, G.E.; Reeder, J.D.; Hart, R.H. Rangeland soil carbon and nitrogen responses to grazing. J. Soil Water Conserv. 1995, 50, 294–298. [Google Scholar]
- Martinsen, Ø.L.; Diseth, Å. The assimilator–explorer cognitive styles: Factor structure, personality correlates, and relationship to inventiveness. Creat. Res. J. 2011, 23, 273–283. [Google Scholar] [CrossRef]
- Mchunu, C.; Chaplot, V. Land degradation impact on soil carbon losses through water erosion and CO2 emissions. Geoderma 2012, 177, 72–79. [Google Scholar] [CrossRef]
- Medina-Roldán, E.; Paz-Ferreiro, J.; Bardgett, R.D. Grazing exclusion affects soil and plant communities, but has no impact on soil carbon storage in an upland grassland. Agric. Ecosyst. Environ. 2012, 149, 118–123. [Google Scholar]
- Naeth, M.A.; Bailey, A.W.; Pluth, D.J.; Chanasyk, D.; Hardin, R.T. Grazing impacts on litter and soil organic matter in mixed prairie and fescue grassland ecosystems of Alberta. Rangel. Ecol. Manag. 1991, 44, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Neff, K.D.; Hsieh, Y.; Dejitterat, K. Self-compassion, achievement goals, and coping with academic failure. Self Identity 2005, 4, 263–287. [Google Scholar] [CrossRef]
- Piñeiro, G.; Paruelo, J.M.; Jobbágy, E.G.; Jackson, R.B.; Oesterheld, M. Grazing effects on belowground C and N stocks along a network of cattle exclosures in temperate and subtropical grasslands of South America. Glob. Biogeochem. Cycles 2009, 23, GB2003. [Google Scholar] [CrossRef] [Green Version]
- Potter, D.A.; Skolnik, P. Cell-Permeable Protein Inhibitors of Calpain. Google Patents US6867186B2, 2001. [Google Scholar]
- Raiesi, F.; Asadi, E. Soil microbial activity and litter turnover in native grazed and ungrazed rangelands in a semiarid ecosystem. Biol. Fertil. Soils 2006, 43, 76–82. [Google Scholar] [CrossRef]
- Reeder, J.D.; Schuman, G.E. Influence of livestock grazing on C sequestration in semi-arid mixed-grass and short-grass rangelands. Environ. Pollut. 2002, 116, 457–463. [Google Scholar] [CrossRef]
- Smoliak, S.; Dormaar, J.; Johnson, A. Long-term grazing effects on Stripa-Bouteloua prairie soils. J. Range Manag. Arch. 1972, 25, 246–250. [Google Scholar] [CrossRef]
- Teague, W.R.; Dowhower, S.L.; Baker, S.A.; Haile, N.; DeLaune, P.B.; Conover, D.M. Grazing management impacts on vegetation, soil biota and soil chemical, physical and hydrological properties in tall grass prairie. Agric. Ecosyst. Environ. 2011, 141, 310–322. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Kreyling, O.; Steffens, M.; Schoenbach, P.; Wan, H.; Gierus, M.; Taube, F.; Kölbl, A.; Kögel-Knabner, I. Short-term degradation of semiarid grasslands—results from a controlled-grazing experiment in Northern China. J. Plant Nutr. Soil Sci. 2012, 175, 434–442. [Google Scholar] [CrossRef]
- Wood, M.K.; Blackburn, W.H. Vegetation and soil responses to cattle grazing systems in the Texas rolling plains. J. Range Manag. Arch. 1984, 37, 303–308. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.; Tiessen, H. Effect of land use on soil degradation in alpine grassland soil, China. Soil Sci. Soc. Am. J. 2002, 66, 1648–1655. [Google Scholar] [CrossRef]
- Yong-Zhong, S.; Yu-Lin, L.; Jian-Yuan, C.; Wen-Zhi, Z. Influences of continuous grazing and livestock exclusion on soil properties in a degraded sandy grassland, Inner Mongolia, northern China. Catena 2005, 59, 267–278. [Google Scholar] [CrossRef]
- Tuckett, R. Climate Change and Global Warming: What Can We Do, What Should We Do? Elsevier: Birmingham, UK, 2018. [Google Scholar] [CrossRef]
- Achard, F.; Beuchle, R.; Mayaux, P.; Stibig, H.J.; Bodart, C.; Brink, A.; Carboni, S.; Desclée, B.; Donnay, F.; Eva, H.D.; et al. Determination of tropical deforestation rates and related carbon losses from 1990 to 2010. Glob. Chang. Biol. 2014, 20, 2540–2554. [Google Scholar] [CrossRef]
- Houghton, R.A. Carbon flux to the atmosphere from land-use changes: 1850–2005. In TRENDS: A Compendium of Data on Global Change; Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy: Oak Ridge, TN, USA, 2008. [Google Scholar]
- Baccini, A.G.S.J.; Goetz, S.J.; Walker, W.S.; Laporte, N.T.; Sun, M.; Sulla-Menashe, D.; Hackler, J.; Beck, P.S.A.; Dubayah, R.; Friedl, M.A.; et al. Estimated carbon dioxide emissions from tropical deforestation improved by carbon-density maps. Nat. Clim. Chang. 2012, 2, 182–185. [Google Scholar] [CrossRef]
- Part, B. Climate Change 2014 Impacts, Adaptation, and Vulnerability. In Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2014. [Google Scholar]
- Van der Werf, G.R.; Morton, D.C.; DeFries, R.S.; Olivier, J.G.; Kasibhatla, P.S.; Jackson, R.B.; Collatz, G.J.; Randerson, J.T. CO2 emissions from forest loss. Nat. Geosci. 2009, 2, 737–738. [Google Scholar] [CrossRef]
- Feldpausch, T.R.; Lloyd, J.; Lewis, S.L.; Brienen, R.J.; Gloor, M.; Monteagudo Mendoza, A.; Lopez-Gonzalez, G.; Banin, L.; Abu Salim, K.; Affum-Baffoe, K.; et al. Tree height integrated into pantropical forest biomass estimates. Biogeosciences 2012, 9, 3381–3403. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Sharma, M. Assessment of carbon stocks in forest and its implications on global climate changes. J. Mater. Environ. Sci. 2015, 6, 3548–3564. [Google Scholar]
- Ubuy, M.H.; Eid, T.; Bollandsås, O.M.; Birhane, E. Aboveground biomass models for trees and shrubs of exclosures in the drylands of Tigray, northern Ethiopia. J. Arid Environ. 2018, 156, 9–18. [Google Scholar] [CrossRef]
- Ponce-Hernandez, R.; Koohafkan, P.; Antoine, J. Assessing Carbon Stocks and Modelling Win-Win Scenarios of Carbon Sequestration Through Land-Use Changes; Food & Agriculture Org: Rome, Italy, 2004; Volume 1. [Google Scholar]
- Ogle, K.; Pathikonda, S.; Sartor, K.; Lichstein, J.W.; Osnas, J.L.; Pacala, S.W. A model-based meta-analysis for estimating species-specific wood density and identifying potential sources of variation. J. Ecol. 2014, 102, 194–208. [Google Scholar] [CrossRef] [Green Version]
- Sloan, S.; Sayer, J.A. Forest Resources Assessment of 2015 shows positive global trends but forest loss and degradation persist in poor tropical countries. For. Ecol. Manag. 2015, 352, 134–145. [Google Scholar] [CrossRef] [Green Version]
- Howat, D.H.; Lier, M.; Korhonen, K.T.; Pekkarinen, A.; Garzuglia, M.; Jonsson, Ö. Report of the Expert Consultation on Global Forest Resources Assessment; Towards FRA 2020: Joensuu, Finland, 2017. [Google Scholar]
- Yirdaw, E.; Tigabu, M.; Monge, A. Rehabilitation of degraded dryland ecosystems–review. Silva Fenn. 2017, 51, 1673. [Google Scholar] [CrossRef] [Green Version]
- Gaveau, D.L.; Epting, J.; Lyne, O.; Linkie, M.; Kumara, I.; Kanninen, M.; Leader-Williams, N. Evaluating whether protected areas reduce tropical deforestation in Sumatra. J. Biogeogr. 2009, 36, 2165–2175. [Google Scholar] [CrossRef]
- Birhane, E.; Teketay, D.; Barklund, P. Actual and potential contribution of exclosures to enhance biodiversity of woody species in the drylands of Eastern Tigray. J. Drylands 2006, 1, 134–147. [Google Scholar]
- Fritsche, U.R.; Berndes, B.; Cowie, A.L.; Dale, V.H.; Kline, K.L.; Johnson, F.X.; Langeveld, H.; Sharma, N.; Watson, H.; Woods, J. Energy and Land Use, Sustainable Energy Options and Implications for Land Use. 2017. Available online: https://knowledge.unccd.int/publication/energy-and-land-use (accessed on 22 January 2021).
- Houghton, R.; Hackler, J. Sources and sinks of carbon from land-use change in China. Glob. Biogeochem. Cycles 2003, 17, 1034. [Google Scholar] [CrossRef]
- Mortimore, M. Roots in the African Dust: Sustaining the Sub-Saharan Drylands; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Hunter, M.D.; Linnen, C.R.; Reynolds, B.C. Effects of endemic densities of canopy herbivores on nutrient dynamics along a gradient in elevation in the southern Appalachians. Pedobiologia 2003, 47, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [Green Version]
- Briggs, M.K.; Lozano-Cavazos, E.A.; Poulos, H.M.; Ochoa-Espinoza, J.; Rodriguez-Pineda, J.A. The Chihuahuan Desert: A Binational Conservation Response to Protect a Global Treasure; Reference Module in Earth Systems and Environmental Sciences. 2019. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, H.M.; Treydte, A.C.; Sauerborn, J. Managing semi-arid rangelands for carbon storage: Grazing and woody encroachment effects on soil carbon and nitrogen. PLoS ONE 2015, 10, e0109063. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, M.A.; Bravo-Oviedo, A.; Bravo, F.; Ruiz-Peinado, R. Aboveground biomass equations for sustainable production of fuelwood in a native dry tropical afro-montane forest of Ethiopia. Ann. Sci. 2016, 73, 411–423. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Pandey, P.C.; Singh, B.K.; Katiyar, S.; Mandal, V.P.; Rani, M.; Tomar, V.; Patairiya, S. Estimation of accumulated soil organic carbon stock in tropical forest using geospatial strategy. Egypt. J. Remote Sens. Space Sci. 2016, 19, 109–123. [Google Scholar] [CrossRef] [Green Version]
- Welemariam, M.; Kebede, F.; Bedadi, B.; Birhane, E. Exclosures backed up with community-based soil and water conservation practices increased soil organic carbon stock and microbial biomass carbon distribution, in the northern highlands of Ethiopia. Chem. Biol. Technol. Agric. 2018, 5, 12. [Google Scholar] [CrossRef]
- Ceccarelli, T.; Bajocco, S.; Salvati, L.; Perini, L. Investigating syndromes of agricultural land degradation through past trajectories and future scenarios. Soil Sci. Plant Nutr. 2014, 60, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Petrosillo, I.; Müller, F.; Jones, K.B.; Zurlini, G.; Krauze, K.; Victorov, S.; Li, B.-L.; Kepner, W.G. Contributions of Landscape Sciences to The Development of Environmental Security. In Use of Landscape Sciences for the Assessment of Environmental Security; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–17. [Google Scholar]
- Acharya, K.; Dangi, R.; Acharya, M. Understanding forest degradation in Nepal. Unasylva 2011, 62, 238. [Google Scholar]
- Andela, N.; Morton, D.C.; Giglio, L.; Chen, Y.; Van Der Werf, G.R.; Kasibhatla, P.S.; DeFries, R.S.; Collatz, G.J.; Hantson, S.; Kloster, S.; et al. A human-driven decline in global burned area. Science 2017, 356, 1356–1362. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Yao, Q. Logistics system design for biomass-to-bioenergy industry with multiple types of feedstocks. Bioresour. Technol. 2011, 102, 10936–10945. [Google Scholar] [CrossRef]
- Novák, J.; Jankowski, K.; Sosnowski, J.; Malinowska, E.; Wiśniewska-Kadżajan, B. Influence of Plant Species and Grasslands Quality on Sequestration of Soil Organic Carbon. Ekológia 2020, 39, 289–300. [Google Scholar]
- Ubuy, M.H.; Gebrehiwot, K.; Raj, A.J. Biomass estimation of exclosure in the Debrekidan watershed, Tigray region, northern Ethiopia. Int. J. Agric. 2014, 4, 88–93. [Google Scholar]
- Wang, C.; He, N.; Zhang, J.; Lv, Y.; Wang, L. Long-term grazing exclusion improves the composition and stability of soil organic matter in Inner Mongolian grasslands. PLoS ONE 2015, 10, e0128837. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.P.; Su, J.S.; Wu, G.L.; Gillet, F. Long-term effects of grazing exclusion on aboveground and belowground plant species diversity in a steppe of the Loess Plateau, China. Plant. Ecol. Evol. 2011, 144, 313–320. [Google Scholar] [CrossRef]
- Kölbl, A.; Steffens, M.; Wiesmeier, M.; Hoffmann, C.; Funk, R.; Krümmelbein, J.; Reszkowska, A.; Zhao, Y.; Peth, S.; Horn, R. Grazing changes topography-controlled topsoil properties and their interaction on different spatial scales in a semi-arid grassland of Inner Mongolia, PR China. Plant. Soil 2011, 340, 35–58. [Google Scholar] [CrossRef]
- Steffens, M.; Kölbl, A.; Kögel-Knabner, I. Alteration of soil organic matter pools and aggregation in semi-arid steppe topsoils as driven by organic matter input. Eur. J. Soil Sci. 2009, 60, 198–212. [Google Scholar] [CrossRef]
- Tanentzap, A.J.; Coomes, D.A. Carbon storage in terrestrial ecosystems: Do browsing and grazing herbivores matter? Biol. Rev. 2012, 87, 72–94. [Google Scholar]
- Qiu, L.; Wei, X.; Zhang, X.; Cheng, J. Ecosystem carbon and nitrogen accumulation after grazing exclusion in semiarid grassland. PLoS ONE 2013, 8, e55433. [Google Scholar]
- Wiesmeier, M.; Steffens, M.; Kölbl, A.; Kögel-Knabner, I. Degradation and small-scale spatial homogenization of topsoils in intensively-grazed steppes of Northern China. Soil Tillage Res. 2009, 104, 299–310. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Zhao, X.; Awada, T.; Shang, W.; Han, J. Effects of grazing exclusion on soil properties and on ecosystem carbon and nitrogen storage in a sandy rangeland of Inner Mongolia, Northern China. Environ. Manag. 2012, 50, 622–632. [Google Scholar]
- Wiesmeier, M.; Steffens, M.; Mueller, C.W.; Kölbl, A.; Reszkowska, A.; Peth, S.; Horn, R.; Kögel-Knabner, I. Aggregate stability and physical protection of soil organic carbon in semi-arid steppe soils. Eur. J. Soil Sci. 2012, 63, 22–31. [Google Scholar] [CrossRef]
- West, N.E.; Provenza, F.D.; Johnson, P.S.; Owens, M.K. Vegetation change after 13 years of live-stock grazing exclusion on sagebrush semidesert in west central Utah. Rangel. Ecol. Manag. J. Range Manag. Arch. 1984, 37, 262–264. [Google Scholar]
- Lü, X.T.; Freschet, G.T.; Kazakou, E.; Wang, Z.W.; Zhou, L.S.; Han, X.G. Contrasting responses in leaf nutrient-use strategies of two dominant grass species along a 30-yr temperate steppe grazing exclusion chronosequence. Plant Soil 2015, 387, 69–79. [Google Scholar] [CrossRef]
- Martinsen, V.; Mulder, J.; Austrheim, G.; Mysterud, A. Carbon storage in low-alpine grassland soils: Effects of different grazing intensities of shee. Eur. J. Soil Sci. 2011, 62, 822–833. [Google Scholar] [CrossRef]
- Bilotta, G.; Brazier, R. Haygarth, The impacts of grazing animals on the quality of soils, vegetation, and surface waters in intensively managed grasslands. Adv. Agron. 2007, 94, 237–280. [Google Scholar]
- Seyler, W.R.; Olson, J.W.; Maier, R.J. Superoxide Dismutase-Deficient Mutants ofHelicobacter pylori Are Hypersensitive to Oxidative Stress and Defective in Host Colonization. Infect. Immun. 2001, 69, 4034–4040. [Google Scholar] [CrossRef] [Green Version]
- Dlamini, P.; Chivenge, P.; Chaplot, V. Overgrazing decreases soil organic carbon stocks the most under dry climates and low soil pH: A meta-analysis shows. Agric. Ecosyst. Environ. 2016, 221, 258–269. [Google Scholar] [CrossRef]
- Preger, A.C.; Kösters, R.; Du Preez, C.C.; Brodowski, S.; Amelung, W. Carbon sequestration in secondary pasture soils: A chronosequence study in the South African Highveld. Eur. J. Soil Sci. 2010, 61, 551–562. [Google Scholar] [CrossRef]
- Teshager, Z.; Argaw, M.; Eshete, A. Variations in Forest Carbon Stocks along Environmental Gradients in Weiramba Forest of Amhara Region, Ethiopia: Implications of Managing Forests for Climate Change Mitigation. Int. J. Sci. Eng. Res. 2018, 9, 13. [Google Scholar] [CrossRef]
- Mekuria, W. Changes in regulating ecosystem services following establishing exclosures on communal grazing lands in Ethiopia: A synthesis. J. Ecosyst. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Roni, P.; Quimby, E. Monitoring Stream and Watershed Restoration; CABI: Wallingford, UK, 2005. [Google Scholar] [CrossRef]
- Koskei, E.; Kitetu, J. The Factors Impeding Clean Development Mechanism (CDM) Implementation and Carbon Emissions Reductions and Energy Management in Relation to Climate Change and Sustainable Development in Africa; Kabarak University: Nakuru, Kenya, 2019. [Google Scholar]
- Liu, S.; Daka, J.P.; Kobayashi, S.; Lund, H.G. Definitions and Methodological Options to Inventory Emissions from 3 Direct Human-induced Degradation of Forests and Devegetation of Other Vegetation Types. 2003. Available online: https://www.ipcc.ch/site/assets/uploads/2018/03/Degradation.pdf (accessed on 22 January 2021).
- Sisay, K.; Thurnher, C.; Belay, B.; Belete, W.; Teklehaymanot, T.; Habte, K.; Abera, S.; Kahesay, H.; Lindner, G.; Hasenauer, H. Estimation of aboveground volume, carbon stocks and NPP using terrestrial and satellite data of Amhara region, Ethiopia. In Proceedings of the Conference on International Research on Food Security, Washington, DC, USA, 6–8 December 2016. [Google Scholar]
- Skutsch, M.; McCall, M. The role of community forest management in REDD+. Unasylva 2012, 239, 51–56. [Google Scholar]
- Shahidul, M.I.; Malcolm, M.L.; Hashmi, M.S.; Alhaji, M.H. Waste Resources Recycling in Achieving Economic and Environmental Sustainability: Review on Wood Waste Industry. Ref. Modul. Mater. Sci. Mater. Eng. 2018, 1–10. [Google Scholar] [CrossRef]
- Chen, G.; Powers, R.P.; de Carvalho, L.M.; Mora, B. Spatiotemporal patterns of tropical deforestation and forest degradation in response to the operation of the Tucuruí hydroelectric dam in the Amazon basin. Appl. Geogr. 2015, 63, 1–8. [Google Scholar] [CrossRef]
- Solomon, N.; Pabi, O.; Annang, T.; Asante, I.K.; Birhane, E. The effects of land cover change on carbon stock dynamics in a dry Afromontane forest in northern Ethiopia. Carbon Balance Manag. 2018, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Solomon, N.; Birhane, E.; Tadesse, T.; Treydte, A.C.; Meles, K. Carbon stocks and sequestration potential of dry forests under community management in Tigray, Ethiopia. Ecol. Process. 2017, 6, 20. [Google Scholar] [CrossRef]
- Gaudel, G.; Hui, Z.W.; Hung, D.Q.; Hien, L.T. The Global Terrestrial Carbon Stocks, Status of Carbon in Forest and Shrub Land of Nepal, and Relationship between Carbon Stock and Diversity. 2016. Available online: http://internationaljournalofresearch.org/ (accessed on 22 January 2021).
- Saatchi, S.S.; Harris, N.L.; Brown, S.; Lefsky, M.; Mitchard, E.T.; Salas, W.; Zutta, B.R.; Buermann, W.; Lewis, S.L.; Hagen, S. Benchmark map of forest carbon stocks in tropical regions across three continents. Proc. Natl. Acad. Sci. USA 2011, 108, 9899–9904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penman, J.; Gytarsky, M.; Hiraishi, T.; Krug, T.; Kruger, D.; Pipatti, R.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K.; et al. Good Practice Guidance for Land Use, Land-Use Change and Forestry. 2003. Available online: https://www.ipcc-nggip.iges.or.jp/public/gpglulucf/gpglulucf_contents.html (accessed on 22 January 2021).
- Malhi, Y.; Meir, P.; Brown, S. Forests, carbon and global climate. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2002, 360, 1567–1591. [Google Scholar] [CrossRef]
- Aber, J.; Christensen, N.; Fernandez, I.; Franklin, J.; Hidinger, L.; Hunter, M.; McMahon, J.; Mladenhoff, D.; Pastor, J.; Perry, D. pplying Ecological Principles to Management of the U.S. National Forest. 2003. Available online: https://www.esa.org/wp-content/uploads/2013/03/issue61.pdf (accessed on 22 January 2021).
- Seger, J. Vehicle Integration for US EPA 2010 Emissions and Lowest Cost of Ownership; SAE Technical Paper: Warrendale, PA, USA, 2010. [Google Scholar] [CrossRef]
- Toledo, R.M.; Santos, R.F.; Baeten, L.; Perring, M.P.; Verheyen, K. Soil properties and neighbouring forest cover affect above-ground biomass and functional composition during tropical forest restoration. Appl. Veg. Sci. 2018, 21, 179–189. [Google Scholar] [CrossRef]
- Ravindranath, N.H.; Srivastava, N.; Murthy, I.K.; Malaviya, S.; Munsi, M.; Sharma, N. Deforestation and forest degradation in India–implications for REDD+. Curr. Sci. 2012, 1117–1125. [Google Scholar]
- Mulugeta, G.; Achenef, A. Socio-economic challenges of area exclosure practices: A case of Gonder Zuria Woreda, Amhara region, Ethiopia. J. Nat. Sci. Res. 2015, 5, 123–132. [Google Scholar]
- Pasquali, A.; Jacobsen, H.K. Construction of Energy Savings Cost Curves: An Application for Denmark. 2019. Available online: https://mpra.ub.uni-muenchen.de/93076/1/MPRA_paper_93076.pdf (accessed on 22 January 2021).
- Eshete, A.; Mamo, D.A.N.B.N. Area Closures: A Climate Smart Approach to Rehabilitate Degraded Lands and to Improve Livelihoods. 2016. Available online: https://iiste.org/Journals/index.php/JNSR/article/view/34737 (accessed on 22 January 2021).
- Forsyth, D.M.; Scroggie, M.P.; Arthur, A.D.; Lindeman, M.; Ramsey, D.S.; McPhee, S.R.; Bloomfield, T.; Stuart, I.G. Density-dependent effects of a widespread invasive herbivore on tree survival and biomass during reforestation. Ecosphere 2015, 6, 1–17. [Google Scholar] [CrossRef]
- Beets, P.N.; Pearce, S.H.; Oliver, G.R.; Clinton, P.W. Root/shoot ratios for deriving below-ground biomass of Pinus radiata stands. N. Z. J. Sci. 2007, 37, 267. [Google Scholar]
- Kassahun, K.; Soromessa, T.; Belliethathan, S. Forest Carbon Stock in Woody Plants of Ades Forest, Western Hararghe Zone of Ethiopia and its variation along environmental factors: Implication for climate change mitigation. Forest 2015, 5, 21. [Google Scholar]
- Gibbs, H.K.; Brown, S.; Niles, J.O.; Foley, J.A. Monitoring and estimating tropical forest carbon stocks: Making REDD a reality. Environ. Res. Lett. 2007, 2, 5023. [Google Scholar] [CrossRef]
- Baishya, R.; Barik, S.K. Estimation of tree biomass, carbon pool and net primary production of an old-growth Pinus kesiya Royle ex. Gordon forest in north-eastern India. Ann. Sci. 2011, 68, 727–736. [Google Scholar] [CrossRef] [Green Version]
- Gedefaw, M. Estimation of Above and Belowground Carbon Stocks of Forests: Implications for Sustainable Forest Management and Climate Change Mitigation: A Case Study of Tara Gedam Forest, Ethiopia. J. Earth Sci. Clim. Chang. 2005. [Google Scholar] [CrossRef]
- Birhan, E. Actual and Potential Contributions of Enclosure to Enhance Biodiversity in Drylands of Eastern Tigray with Particular Emphasis on Woody Plants; VDM Velag: Saarbrücken, Germany, 2002. [Google Scholar]
- Eggleston, S.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. (Eds.) IPCC 2006 Guidelines for National Greenhouse Gas Inventories; Prepared by the National Greenhouse Gas Inventories Programme; IGES: Hayama, Japan, 2006. [Google Scholar]
- Sedjo, R.; Sohngen, B. Carbon sequestration in forests and soils. Annu. Rev. Resour. Econ. 2012, 4, 127–144. [Google Scholar] [CrossRef]




| Reference | Country | Location | N | t | Map (mm) | amt (°C) | z (m) | Clay (%) | Socd-nd (kg Cm3 ) | Socd-d |
|---|---|---|---|---|---|---|---|---|---|---|
| Abril and Bucher (1999) [20] | Argentina | Salta | 3 | 0.2 | 550 | 22.7 | 217 | 17.6 | 41 | 27.5 |
| Bauer et al. (1987) [21]. | USA | North Dakota | 2 | 0.46 | 538 | 3.4 | 670 | 26.5 | 2.8 | 2.4 |
| Chuluun et al. (1999) [22] | China | Mongolia | 8 | 0.06 | 307 | 2.6 | 1165 | 10.3 | 88.3 | 8.3 |
| Cui et al. (2005) [23] | China | Inner Mongolia | 21 | 0.3 | 350 | 0.2 | 1255 | 21 | 17.6 | 11 |
| Dong et al. (2012) [24] | China | Qinghai-Tibetan | 6 | 0.13 | 570 | 0.6 | 4200 | 20 | 137.8 | 13.3 |
| Frank et al. (1995) [25] | USA | Mandan, N. D | 59 | 0.19 | 404 | 4.4 | 573 | 10 | 48.7 | 15.4 |
| Franzluebbers and Stuedmann (2009) [26] | USA | Georgia | 34 | 0.15 | 1250 | 16.5 | 153 | 10 | 32 | 6.1 |
| Ganjegunte et al. (2005) [27] | USA | Cheyenne | 8 | 0.05 | 384 | 15 | 1930 | 35 | 21.6 | 21.8 |
| Gill (2007) [28] | USA | Utah | 5 | 0.15 | 932 | 1.3 | 1600 | 24.5 | 50.3 | 21.7 |
| Hafner et al. (2012) [29] | China | Qinghai-Tibetan | 1 | 0.17 | 582 | 1.7 | 3440 | 25 | 41 | 11.3 |
| Hiltbrunner et al. (2012) [30] | Switzerland | Fribourg | 63 | 0.15 | 1250 | 6 | 1600 | 52.9 | 32 | 7.2 |
| Ingram et al. (2008) [31] | USA | Cheyenne | 2 | 0.1 | 425 | 15 | 1930 | 10 | 21.6 | 11.1 |
| Manley et al. (1995) [32] | USA | Cheyenne | 3 | 0.3 | 384 | 13 | 1930 | 10 | 18.9 | 19 |
| Martinsen et al. (2011) [33] | Norway | Burskerud County | 4 | 0.05 | 1000 | 1.5 | 1211 | 3 | 13.8 | 10.2 |
| Mchunu and Chaplot (2012) [34] | South Africa | Bergville | 3 | 0.02 | 684 | 13 | 1300 | 16.6 | 15 | 5.5 |
| Medina-Roldán et al. (2012) [35] | England | Yorkshire Dales | 7 | 0.2 | 1840 | 2.8 | 400 | 10 | 29.5 | 31.2 |
| Naeth et al. (1991) [36] | Canada | Alberta | 14 | 0.1 | 355 | 4 | 745 | 15.8 | 55 | 40 |
| Neff et al. (2005) [37] | USA | Utah | 31 | 0.1 | 207 | 11.7 | 1500 | 4.8 | 5 | 1.5 |
| Piñeiro et al. (2009) [38] | Uraguay | Rio de la Plata | 10 | 0.3 | 1100 | 17.3 | 110 | 25.5 | 36.3 | 23 |
| Potter et al. (2001) [39] | USA | Oklahoma | 4 | 0.33 | 842 | 17 | 2438 | 23.3 | 13.8 | 3.7 |
| Raiesi and Asadi (2006) [40] | Iran | Shahrekord | 4 | 0.3 | 860 | 6.7 | 2500 | 50 | 26.7 | 22.2 |
| Reeder and Schuman (2002) [41] | USA | Cheyenne | 3 | 0.30 | 343 | 15.0 | 1930 | 10.0 | 19.4 | 12.5 |
| Smoliak et al. (1972) [42] | Canada | Alberta | 26 | 0.10 | 550 | 1.3 | 926 | 15.8 | 14.0 | 14.4 |
| Teague et al. (2011) [43] | Texas | USA | 9 | 0.23 | 820 | 18.1 | 315 | 30.0 | 50.6 | 26.0 |
| Wiesmeier et al. (2012) [44] | China | Inner Mongolia | 1 | 0.10 | 350 | 0.7 | 1260 | 20.4 | 20.0 | 18.1 |
| Wood and Blackburn (1984) [45] | USA | Texas | 14 | 0.03 | 624 | 17.0 | 316 | 30.0 | 55.9 | 46.8 |
| Wu and Tiessen (2002) [46] | China | Tianzhu | 21 | 0.15 | 416 | 0.3 | 2940 | 27.3 | 57.8 | 27.0 |
| Yong-Zhong et al. (2005) [47] | China | Naiman County | 48 | 0.15 | 366 | 6.5 | 360 | 2.5 | 3.7 | 2.9 |
| Region | Forest Area (Million ha) | AGB Carbon Density (tons/ha) | Total Biomass Carbon Density (tons C/ha) |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Africa | |||
| Tropical Rain Forest | 252.9 | 107 ± 51 | 135 ± 64 |
| Tropical Moist Deciduous Forest | 110.6 | 38 ± 18 | 53 ± 32 |
| Tropical Shrub Land | 1.6 | 41 ± 25 | 49 ± 23 |
| Tropical Dry Forest | 36.1 | 38 ± 18 | 82 ± 49 |
| Tropical Mountain System | 22.7 | 64 ± 39 | 49 ± 19 |
| Sub-tropical Humid Forest | 1.5 | 38 ± 15 | 41 ± 21 |
| Sub-tropical Dry Forest | 0.7 | 31 ± 16 | 45 ± 14 |
| Sub-tropical Mountain System | 1.1 | 34 ± 11 | 45 ± 14 |
| Africa Total | 427.2 | 80 ± 78 | 102 ± 98 |
| Latin America | |||
| Tropical Rain Forest | 587.1 | 115 ± 34 | 146 ± 42 |
| Tropical Moist Deciduous Forest | 179.3 | 54 ± 42 | 69 ±53 |
| Tropical Shrub Land | 0.9 | 55 ± 41 | 71 ± 51 |
| Tropical Dry Forest | 47.6 | 27 ± 23 | 36 ± 19 |
| Tropical Mountain System | 71.8 | 86 ± 50 | 110 ± 62 |
| Sub-tropical Humid Forest | 20.4 | 51 ± 38 | 66 ± 48 |
| Sub-tropical Dry Forest | 5.3 | 55 ± 51 | 71 ± 64 |
| Sub-tropical Mountain System | 7.2 | 21 ± 23 | 27 ± 29 |
| Latin America Total | 919.8 | 94 ± 110 | 119 ± 138 |
| Southeast Asia | |||
| Tropical Rain Forest | 261.6 | 121 ± 50 | 153 ± 62 |
| Tropical Moist Deciduous Forest | 55.6 | 105 ± 49 | 133 ± 61 |
| Tropical Shrub Land | 2.5 | 64 ± 39 | 82 ± 49 |
| Tropical Dry Forest | 17.6 | 83 ± 50 | 106 ± 63 |
| Tropical Mountain System | 53.6 | 128 ± 34 | 162 ± 42 |
| Sub-tropical Humid Forest | 0.8 | 88 ± 34 | 112 ± 42 |
| Sub-tropical Mountain System | 7.7 | 101 ± 41 | 128 ± 52 |
| Southeast Asia Total | 399.5 | 118 ± 114 | 149 ± 142 |
| All Total | 1746,5 | 94 ± 110 | 122 ± 221 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsegay, G.; Meng, X.-Z. Impact of Ex-Closure in above and below Ground Carbon Stock Biomass. Forests 2021, 12, 130. https://doi.org/10.3390/f12020130
Tsegay G, Meng X-Z. Impact of Ex-Closure in above and below Ground Carbon Stock Biomass. Forests. 2021; 12(2):130. https://doi.org/10.3390/f12020130
Chicago/Turabian StyleTsegay, Gedion, and Xiang-Zhou Meng. 2021. "Impact of Ex-Closure in above and below Ground Carbon Stock Biomass" Forests 12, no. 2: 130. https://doi.org/10.3390/f12020130




