Genetic Parameters of Stem and Wood Traits in Full-Sib Silver Birch Families
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Descriptive Statistics, Heritability and Variability of Traits
3.2. Phenotypic and Genotypic Correlations between Traits
3.3. Correlations between Sites and Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Parental Crosses | Mother Unique ID Code | |||||||
|---|---|---|---|---|---|---|---|---|
| 01 | 02 | 03 | 04 | 05 | 66 | 07 | ||
| Father unique ID code | 10 | X | X | |||||
| 11 | X | X | ||||||
| 12 | X | X | ||||||
| 13 | X | X | X | |||||
| 14 | X | X | X | |||||
| 15 | X | X | X | |||||
| 16 | X | X | ||||||
| 66 | X | |||||||
| 17 | X | X | ||||||
| 18 | X | X | ||||||
References
- SLU. Forest Statistics 2019; Swedish University of Agricultural Sciences: Umeå, Sweden, 2019; p. 138. [Google Scholar]
- Woxblom, L.; Nylinder, M. Industrial utilization of hardwood in Sweden. Ecol. Bull. 2010, 53, 43–50. [Google Scholar]
- Götmark, F.; Fridman, J.; Kempe, G.; Norden, B. Broadleaved tree species in conifer-dominated forestry: Regeneration and limitation of saplings in southern Sweden. For. Ecol. Manag. 2005, 214, 142–157. [Google Scholar] [CrossRef]
- Holmström, E.; Karlsson, M.; Nilsson, U. Modeling birch seed supply and seedling establishment during forest regeneration. Ecol. Model. 2017, 352, 31–39. [Google Scholar] [CrossRef]
- Hynynen, J.; Niemistö, P.; Viherä-Aarnio, A.; Brunner, A.; Hein, S.; Velling, P. Silviculture of birch (Betula pendula Roth and Betula pubescens Ehrh.) in northern Europe. For. Int. J. For. Res. 2010, 83, 103–119. [Google Scholar] [CrossRef]
- Cameron, A.D.; Dunham, R.A.; Petty, J.A. The effects of heavy thinning on stem quality and timber properties of silver birch (Betula pendula Roth). For. Int. J. For. Res. 1995, 68, 275–286. [Google Scholar] [CrossRef]
- Heräjärvi, H.; Verkasalo, E. Timber grade distribution and relative stumpage value of mature Finnish Betula pendula and B. pubescens when applying different bucking principles. For. Prod. J. 2002, 52, 40–51. [Google Scholar]
- Heräjärvi, H. Technical properties of mature birch (Betula pendula and B. pubescens) for saw milling in Finland. Silva Fenn. 2001, 35, 469–485. [Google Scholar] [CrossRef] [Green Version]
- Heräjärvi, H. Variation of basic density and Brinell hardness within mature Finnish Betula pendula and B. pubescens stems. Wood Fiber Sci. 2004, 36, 216–227. [Google Scholar]
- Stener, L.-G.; Hedenberg, Ö. Genetic parameters of wood, fibre, stem quality and growth traits in a clone test with Betula pendula. Scand. J. For. Res. 2003, 18, 103–110. [Google Scholar] [CrossRef]
- Rosvall, O. Review of the Swedish Tree Breeding Program; Skogforsk: Uppsala, Sweden, 2011. [Google Scholar]
- Zeltiņš, P.; Matisons, R.; Gailis, A.; Jansons, J.; Katrevičs, J.; Jansons, Ā. Genetic Parameters of Growth Traits and Stem Quality of Silver Birch in a Low-Density Clonal Plantation. Forests 2018, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Rosvall, O.; Jansson, G.; Andersson, B.; Ericsson, T.; Karlsson, B.; Sonesson, J.; Stener, L.-G. Genetiska Vinster i Nuvarande Och Framtida fröPlantager Och Klonbladningar; Uppsala BioCentre: Uppsala, Sweden, 2001. [Google Scholar]
- Eriksson, G. Betula Pendula and Betula Pubescens: Recent Genetic Research; Uppsala BioCentre, Department of Plant Biology and Forest Genetics: Uppsala, Sweden, 2011. [Google Scholar]
- Hong, Z.; Fries, A.; Wu, H.X. High negative genetic correlations between growth traits and wood properties suggest incorporating multiple traits selection including economic weights for the future Scots pine breeding programs. Ann. For. Sci. 2014, 71, 463–472. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.X.; Sanchez, L. Effect of selection method on genetic correlation and gain in a two-trait selection scheme. Aust. For. 2011, 74, 36–42. [Google Scholar] [CrossRef]
- Kennedy, S.G.; Cameron, A.D.; Lee, S.J. Genetic relationships between wood quality traits and diameter growth of juvenile core wood in Sitka spruce. Can. J. For. Res. 2013, 43, 1–6. [Google Scholar] [CrossRef]
- Dunham, R.; Cameron, A.; Petty, J. The effect of growth rate on the strength properties of sawn beams of silver birch (Betula pendula Roth). Scand. J. For. Res. 1999, 14, 18–26. [Google Scholar] [CrossRef]
- Liepiòð, K.; Rieksts-Riekstiòð, J. Stemwood density of juvenile silver birch trees (Betula pendula Roth) from plantations on former farmlands. Balt. For. 2013, 19, 179–186. [Google Scholar]
- Bhat, K. Variation in structure and selected properties of Finnish birch wood. 1: Interrelationships of some structural features, basic density and shrinkage [Betula pendula, B. pubescens]. Silva Fenn. 1980, 14, 384–396. [Google Scholar] [CrossRef]
- Johansson, M.; Säll, H.; Lundqvist, S.-O. Properties of Materials from Birch–Variations and Relationships: Part 2. Mechanical and Physical Properties; Linnaeus University: Växjö, Sweden, 2013. [Google Scholar]
- Apiolaza, L. Very early selection for solid wood quality: Screening for early winners. Ann. For. Sci. 2009, 66, 601. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, S.; Aggarwal, P. Segregation of Eucalyptus tereticornis Sm. clones for properties relevant to solid wood products. Ann. For. Sci. 2011, 68, 511. [Google Scholar] [CrossRef] [Green Version]
- Kien, N.D.; Jansson, G.; Harwood, C.; Almqvist, C.; Thinh, H.H. Genetic variation in wood basic density and Pilodyn penetration and their relationships with growth, stem straightness, and branch size for Eucalyptus urophylla in Vietnam. N. Z. J. For. Sci. 2008, 38, 160–175. [Google Scholar]
- Fundova, I.; Funda, T.; Wu, H.X. Non-destructive wood density assessment of Scots pine (Pinus sylvestris L.) using Resistograph and Pilodyn. PLoS ONE 2018, 13, e0204518. [Google Scholar] [CrossRef] [PubMed]
- Fundova, I.; Funda, T.; Wu, H.X. Non-destructive assessment of wood stiffness in Scots pine (Pinus sylvestris L.) and its use in forest tree improvement. Forests 2019, 10, 491. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.-Q.; Karlsson, B.; Lundqvist, S.-O.; García Gil, M.R.; Olsson, L.; Wu, H.X. Estimating solid wood properties using Pilodyn and acoustic velocity on standing trees of Norway spruce. Ann. For. Sci. 2015, 72, 499–508. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, D.; Hamilton, M.; Williams, D.; Harwood, C.; Potts, B. Acoustic wave velocity as a selection trait in Eucalyptus nitens. Forests 2014, 5, 744. [Google Scholar] [CrossRef] [Green Version]
- Warren, E.; Smith, R.G.B.; Apiolaza, L.A.; Walker, J.C.F. Effect of stocking on juvenile wood stiffness for three Eucalyptus species. New For. 2009, 37, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Fundova, I.; Hallingbäck, H.; Jansson, G.; Wu, H. Genetic Improvement of Sawn-Board Stiffness and Strength in Scots Pine (Pinus sylvestris L.). Sensors 2020, 20, 1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallingbäck, H.R.; Jansson, G.; Hannrup, B.; Fries, A. Which annual rings to assess grain angles in breeding of Scots pine for improved shape stability of sawn timber. Silv Fenn. 2010, 44, 275–288. [Google Scholar] [CrossRef] [Green Version]
- Hallingbäck, H.R.; Jansson, G.; Hannrup, B. Genetic parameters for grain angle in 28-year-old Norway spruce progeny trials and their parent seed orchard. Ann. For. Sci. 2008, 65, 1. [Google Scholar] [CrossRef] [Green Version]
- Toulmin, M.J.; Raymond, C. Developing a sampling strategy for measuring acoustic velocity in standing Pinus radiata using the TreeTap time of flight tool. N. Z. J. For. Sci. 2007, 37, 96. [Google Scholar]
- Wu, H.X.; Powell, M.B.; Yang, J.L.; Ivković, M.; McRae, T.A. Efficiency of early selection for rotation-aged wood quality traits in radiata pine. Ann. For. Sci. 2007, 64, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Jayawickrama, K.J.S. Breeding radiata pine for wood stiffness: Review and analysis. Aust. For. 2001, 64, 51–56. [Google Scholar] [CrossRef]
- Gao, S.; Wang, X.; Wiemann, M.C.; Brashaw, B.K.; Ross, R.J.; Wang, L. A critical analysis of methods for rapid and nondestructive determination of wood density in standing trees. Ann. For. Sci. 2017, 74, 27. [Google Scholar] [CrossRef] [Green Version]
- Llana, D.F.; Short, I.; Harte, A.M. Use of non-destructive test methods on Irish hardwood standing trees and small-diameter round timber for prediction of mechanical properties. Ann. For. Sci. 2020, 77, 1–13. [Google Scholar] [CrossRef]
- Wessels, C.; Malan, F.S.; Rypstra, T. A review of measurement methods used on standing trees for the prediction of some mechanical properties of timber. Eur. J. For. Res. 2011, 130, 881–893. [Google Scholar] [CrossRef]
- Jacquin, P.; Longuetaud, F.; Leban, J.-M.; Mothe, F. X-ray microdensitometry of wood: A review of existing principles and devices. Dendrochronologia 2017, 42, 42–50. [Google Scholar] [CrossRef]
- Raymond, C.A.; MacDonald, A.C. Where to shoot your pilodyn: Within tree variation in basic density in plantation Eucalyptus globulus and E. nitens in Tasmania. New For. 1998, 15, 205–221. [Google Scholar] [CrossRef]
- Baliuckienė, A.; Baliuckas, V. Genetic variability of silver birch (Betula pendula L.) wood hardness in progeny testing at juvenile age. Balt. For. 2006, 12, 134–140. [Google Scholar]
- Stackpole, D.J.; Vaillancourt, R.E.; de Aguigar, M.; Potts, B.M. Age trends in genetic parameters for growth and wood density in Eucalyptus globulus. Tree Genet. Genomes 2010, 6, 179–193. [Google Scholar] [CrossRef]
- Raymond, C.A.; Henson, M.; Pelletier, M.-C.; Boyton, S.; Joe, B.; Thomas, D.; Smith, H.; Vanclay, J.K. Improving Dimensional Stability in Plantation-Grown E. pilularis and E. dunnii; FWPA Report PN06.3017; Forest & Wood Products: Melbourne, Australia, 2009. [Google Scholar]
- Cornelius, J. Heritabilities and additive genetic coefficients of variation in forest trees. Can. J. For. Res. 1994, 24, 372–379. [Google Scholar] [CrossRef]
- Mannes, D.; Lehmann, E.; Cherubini, P.; Niemz, P. Neutron imaging versus standard X-ray densitometry as method to measure tree-ring wood density. Trees 2007, 21, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Greaves, B.L.; Borralho, N.M.; Raymond, C.A.; Farrington, A. Use of a Pilodyn for the indirect selection of basic density in Eucalyptus nitens. Can. J. For. Res. 1996, 26, 1643–1650. [Google Scholar] [CrossRef]
- Björklund, J.; von Arx, G.; Nievergelt, D.; Wilson, R.; Van den Bulcke, J.; Günther, B.; Loader, N.; Rydval, M.; Fonti, P.; Scharnweber, T. Scientific merits and analytical challenges of tree-ring densitometry. Rev. Geophys. 2019, 57, 1224–1264. [Google Scholar] [CrossRef] [Green Version]
- Cown, D.; Haslett, A.; Kimberley, M.; McConchie, D. The influence of wood quality on lumber drying distortion. Ann. For. Sci. 1996, 53, 1177–1188. [Google Scholar] [CrossRef] [Green Version]
- Luostarinen, K.; Verkasalo, E. Birch as Sawn Timber and in Mechanical Further Processing in Finland. A Literature Study. Silva Fenn. Monogr. 2000, 1, 40. [Google Scholar]
- Kretschmann, D.E.; Bridwell, J.J.; Nelson, T.C. Effect of changing slope of grain on ash, maple, and yellow birch in bending strength. In Proceedings of the WCTE 2010, World Conference on Timber Engineering, Riva del Garda, Italy, 20–24 June 2010. [Google Scholar]
- Harris, J.M. Non-destructive assessment of spiral grain in standing trees. N. Z. J. For. Sci. 1984, 14, 395–399. [Google Scholar]
- Hannrup, B.; Säll, H.; Jansson, G. Genetic parameters for spiral grain in Scots pine and Norway spruce. Silvae Genetica 2003, 52, 215–220. [Google Scholar]
- Hallingbäck, H.R.; Jansson, G.; Hannrup, B. Genetic correlations between spiral grain and growth and quality traits in Picea abies. Can. J. For. Res. 2010, 40, 173–183. [Google Scholar] [CrossRef]
- Murphy, G.; Cown, D. Stand, stem and log segregation based on wood properties: A review. Scand. J. For. Res. 2015, 30, 757–770. [Google Scholar] [CrossRef]
- Downes, G.M.; Gwinyai Nyakuengama, J.; Evans, R.; Northway, R.; Blakemore, P.; Dickson, R.L.; Lausberg, M. Relationship between wood density, microfibral angle and stiffness in thinned and fertilized Pinus radiata. IAWA J. 2002, 23, 253–265. [Google Scholar] [CrossRef]
- Auty, D.; Achim, A. The relationship between standing tree acoustic assessment and timber quality in Scots pine and the practical implications for assessing timber quality from naturally regenerated stands. For. Int. J. For. Res. 2008, 81, 475–487. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ross, R.J.; McClellan, M.; Barbour, R.J.; Erickson, J.R.; Forsman, J.W.; McGinnis, G.D. Strength and Stiffness Assessment of Standing Trees Using a Nondestructive Stress Wave Technique; Research Paper FPL–RP–585; United States Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2000. [Google Scholar]
- Wang, X. Acoustic measurements on trees and logs: A review and analysis. Wood Sci. Technol. 2013, 47, 965–975. [Google Scholar] [CrossRef]
- Armstrong, J.P.; Patterson, D.W.; Sneckenberger, J.E. Comparison of three equations for predicting stress wave velocity as a function of grain angle. Wood Fiber Sci. 2007, 23, 32–43. [Google Scholar]
- Mason, E.G.; Hayes, M.; Pink, N. Validation of ultrasonic velocity estimates of wood properties in discs of radiata pine. N. Z. J. For. Sci. 2017, 47, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Legg, M.; Bradley, S. Measurement of stiffness of standing trees and felled logs using acoustics: A review. J. Acoust. Soc. Am. 2016, 139, 588–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabianowski, M.; Manley, B.; Walker, J. Acoustic measurements on standing trees, logs and green lumber. Wood Sci. Technol. 2006, 40, 205–216. [Google Scholar] [CrossRef]
- Mora, C.R.; Schimleck, L.R.; Isik, F.; Mahon, J.M.; Clark, A.; Daniels, R.F. Relationships between acoustic variables and different measures of stiffness in standing Pinus taeda trees. Can. J. For. Res. 2009, 39, 1421–1429. [Google Scholar] [CrossRef] [Green Version]
- Weather Spark. Available online: https://weatherspark.com/countries/SE (accessed on 5 January 2021).
- Ewertson, M. Resultat från avkommeprövning av plusträd av vårtbjörk (Betula pendula). 2014, Volume 2013, p. 15. Available online: https://stud.epsilon.slu.se/6458/ (accessed on 29 December 2020).
- Russo, D.; Marziliano, P.A.; Macrì, G.; Zimbalatti, G.; Tognetti, R.; Lombardi, F. Tree Growth and Wood Quality in Pure Vs. Mixed-Species Stands of European Beech and Calabrian Pine in Mediterranean Mountain Forests. Forests 2019, 11, 6. [Google Scholar] [CrossRef] [Green Version]
- Hallingbäck, H.R.; Högberg, K.-A.; Säll, H.; Lindeberg, J.; Johansson, M.; Jansson, G. Optimal timing of early genetic selection for sawn timber traits in Picea abies. Eur. J. For. Res. 2018, 137, 553–564. [Google Scholar] [CrossRef] [Green Version]
- Bergsten, U.; Lindeberg, J.; Rindby, A.; Evans, R. Batch measurements of wood density on intact or prepared drill cores using x-ray microdensitometry. Wood Sci. Technol. 2001, 35, 435–452. [Google Scholar] [CrossRef]
- Gilmour, A.; Gogel, B.; Cullis, B.; Thompson, R. ASReml User Guide Release 3.0; VSN International Ltd.: Hemel Hempstead, UK, 2009. [Google Scholar]
- Isik, F.; Holland, J.; Maltecca, C. Genetic Data Analysis for Plant and Animal Breeding; Springer International Publishing AG: Cham, Switzerland, 2017. [Google Scholar]
- Skogsstyrelsens. Forestry Statistics Database; Skogsstyrelsens: Jönköping, Sweden, 2019. [Google Scholar]
- Dubois, H.; Verkasalo, E.; Claessens, H. Potential of Birch (Betula pendula Roth and B. pubescens Ehrh.) for Forestry and Forest-Based Industry Sector within the Changing Climatic and Socio-Economic Context of Western Europe. Forests 2020, 11, 336. [Google Scholar] [CrossRef] [Green Version]
- Ekö, P.-M.; Johansson, U.; Petersson, N.; Bergqvist, J.; Elfving, B.; Frisk, J. Current growth differences of Norway spruce (Picea abies), Scots pine (Pinus sylvestris) and birch (Betula pendula and Betula pubescens) in different regions in Sweden. Scand. J. For. Res. 2008, 23, 307–318. [Google Scholar] [CrossRef]
- Bergh, J.; Nilsson, U.; Kjartansson, B.; Karlsson, M. Impact of climate change on the productivity of silver birch, Norway spruce and Scots pine stands in Sweden and economic implications for timber production. Ecol. Bull. 2010, 53, 185–196. [Google Scholar]
- Felton, A.; Gustafsson, L.; Roberge, J.M.; Ranius, T.; Hjältén, J.; Rudolphi, J.; Lindbladh, M.; Weslien, J.; Rist, L.; Brunet, J.; et al. How climate change adaptation and mitigation strategies can threaten or enhance the biodiversity of production forests: Insights from Sweden. Biol. Conserv. 2016, 194, 11–20. [Google Scholar] [CrossRef]
- Li, Y.; Suontama, M.; Burdon, R.D.; Dungey, H.S. Genotype by environment interactions in forest tree breeding: Review of methodology and perspectives on research and application. Tree Genet. Genomes 2017, 13, 60. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, G.; Ekberg, I.; Clapham, D. Genetics Applied to Forestry: An Introduction; Genetic Centre, Department of Plant Biology and Forest Genetics: Uppsala, Sweden, 2013. [Google Scholar]
- Stener, L.-G.; Jansson, G. Improvement of Betula pendula by clonal and progeny testing of phenotypically selected trees. Scand. J. For. Res. 2005, 20, 292–303. [Google Scholar] [CrossRef]
- Gailis, A.; Zeltiņš, P.; Purviņš, A.; Augustovs, J.; Vīndedzis, V.; Zariņa, I.; Jansons, Ā. Genetic parameters of growth and quality traits in open-pollinated silver birch progeny tests. Silva Fenn. 2020, 54, 10220. [Google Scholar] [CrossRef] [Green Version]
- Skrøppa, T.; Solvin, T.M. Genetic variation and inheritance in a 9 × 9 diallel in silver birch (Betula pendula). Scand. J. For. Res. 2019, 34, 178–188. [Google Scholar] [CrossRef]
- Viherä-Aarnio, A.; Velling, P. Growth, wood density and bark thickness of silver birch originating from the Baltic countries and Finland in two Finnish provenance trials. Silva Fenn. 2017, 51, 18. [Google Scholar] [CrossRef] [Green Version]
- Hannrup, B.; Grabner, M.; Karlsson, B.; Mueller, U.; Rosner, S.; Wilhelmsson, L.; Wimmer, R. Genetic parameters for spiral-grain angle in two 19-year-old clonal Norway spruce trials. Ann. For. Sci. 2002, 59, 551–556. [Google Scholar] [CrossRef] [Green Version]
- Thinley, C.; Palmer, G.; Vanclay, J.K.; Henson, M. Spiral and interlocking grain in Eucalyptus dunnii. Holz als Roh-und Werkst. 2005, 63, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Liang, D.; Zhang, X.; Wang, C.; Wang, X.; Li, K.; Liu, G.; Zhao, X.; Qu, G.-Z. Evaluation of Betula platyphylla Families Based on Growth and Wood Property Traits. For. Sci. 2018, 64, 663–670. [Google Scholar] [CrossRef]
- Chen, Z.-Q.; Hai, H.N.T.; Helmersson, A.; Liziniewicz, M.; Hallingbäck, H.R.; Fries, A.; Berlin, M.; Wu, H.X. Advantage of clonal deployment in Norway spruce (Picea abies (L.) H. Karst). Ann. For. Sci. 2020, 77, 14. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Lee, J. Age-age correlations and early selection for end-of-rotation wood density in radiata pine. For. Genet. 2002, 9, 323–330. [Google Scholar]
- Giagli, K.; Vavrcik, H.; Fajstavr, M.; Černý, J.; Novosadová, K.; Martiník, A. Stand factors affecting the wood density of naturally regenerated young silver birch growing at the lower altitude of the Czech Republic region. Wood Res. 2019, 64, 1011–1022. [Google Scholar]
- Velling, P. Wood density in two Betula pendula Roth progeny trials. Folia For. 1979, 416, 1–24. [Google Scholar]
- Dobrowolska, E.; Wroniszewska, P.; Jankowska, A. Density Distribution in Wood of European Birch (Betula pendula Roth.). Forests 2020, 11, 445. [Google Scholar] [CrossRef] [Green Version]

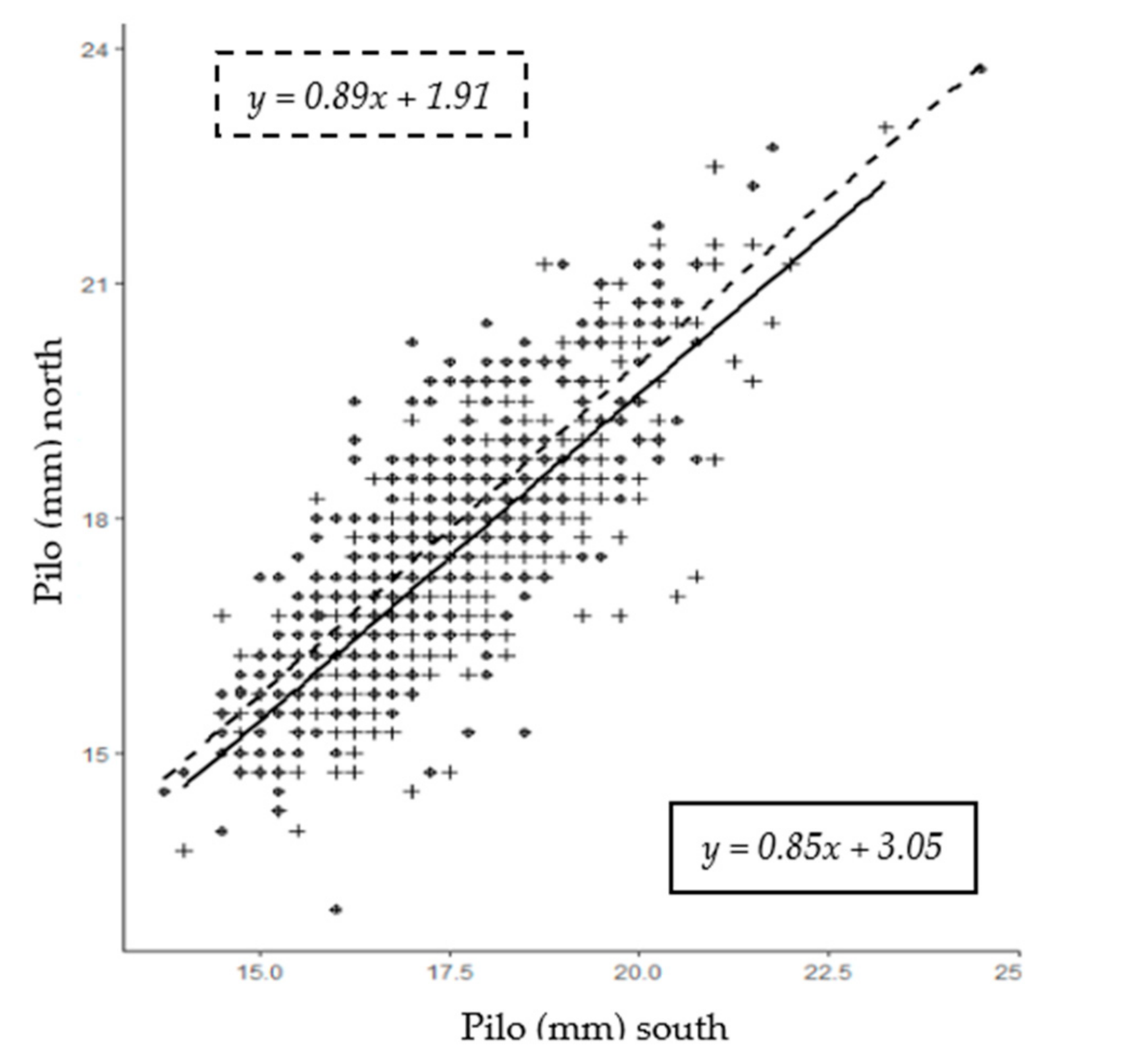
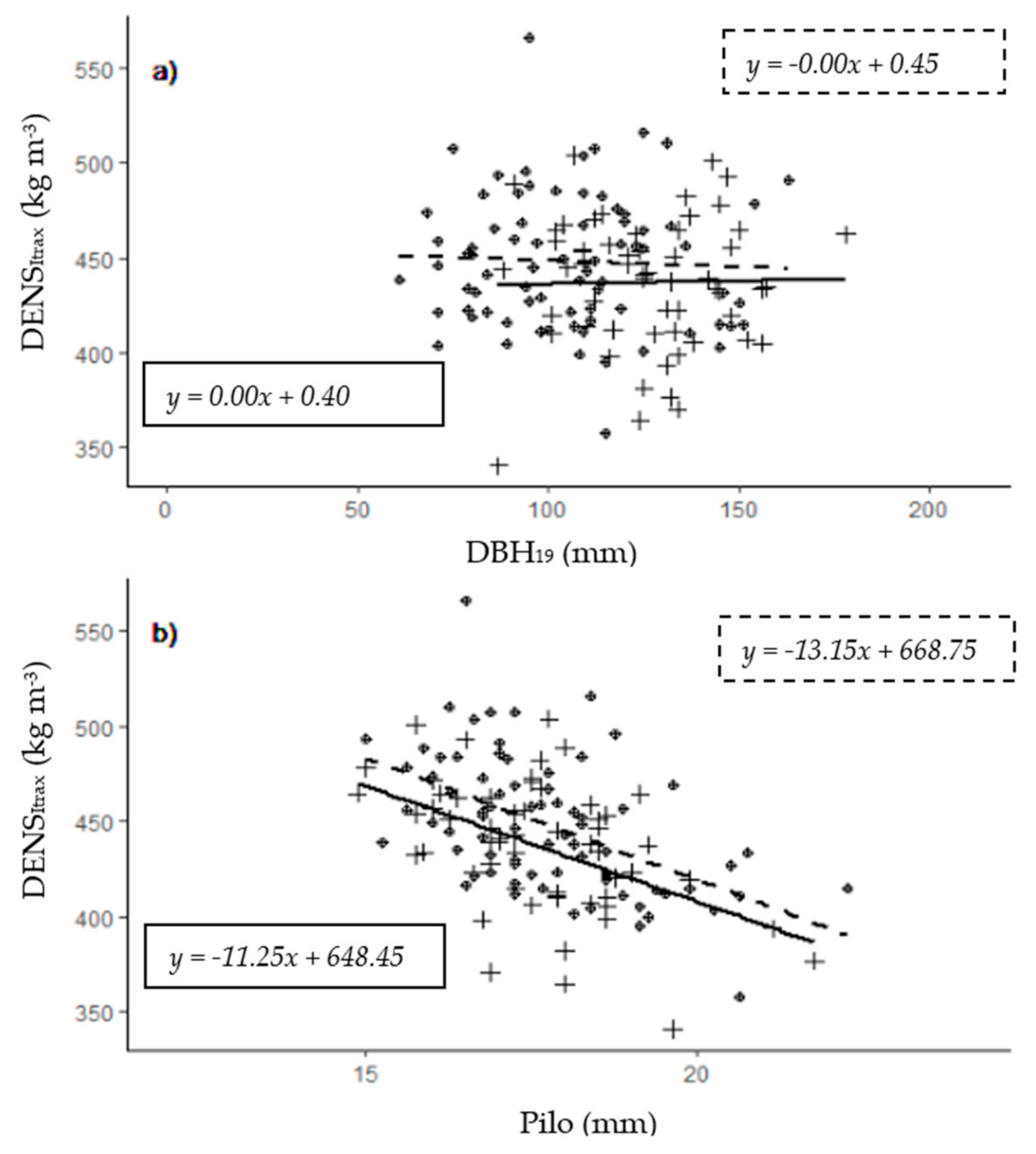
| Measured Traits | Code | Description (units) |
|---|---|---|
| Size traits | ||
| Height age 6 | H6 | Length of main stem (cm) |
| Diameter age 6 | DBH6 | Stem diameter at 1.3 m height (mm) age 6 |
| Diameter age 12 | DBH12 | Stem diameter at 1.3 m height (mm) age 12 |
| Diameter age 19 | DBH19 | Stem diameter at 1.3 m height (mm) age 19 |
| Stem form traits | ||
| Straightness | STR | Straightness score for stem below 8 m high (1–9) |
| Bark height | BH | Height at which smooth bark dominates (cm) |
| Wood quality traits | ||
| Grain angle | GA | Average grain angle measurement for north and south face at 1.3 m (°) |
| Pilodyn penetration | Pilo | Penetration depth by Pilodyn at 1.3 m height (mm) |
| Acoustic velocity | AV | 1,000,000 divided by Fakopp (ToF) value (μs to travel 1 m) is AV (m s−1) |
| Itrax benchmark wood density | DENSItrax | 1.3 m core average wood density from Itrax densitometer and LignoVisionTM (kg m−3) |
| Traits | N | Mean | Min | Max | PCV (%) | h2 | SE (%) | GCV (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | H | B | H | B | H | B | H | B | H | B | H | B | H | B | H | B |
| H6 (mm) | 3360 | 2279 | 71 | 47 | 27 | 8 | 105 | 80 | 15 | 28 | 0.2 | 0.07 | 0.02 | 0.01 | 6.3 | 6.3 |
| DBH6 (mm) | 3360 | 2262 | 56 | 36 | 11 | 2 | 111 | 82 | 27 | 42 | 0.19 | 0.04 | 0.02 | 0.01 | 11.1 | 7.8 |
| DBH12 (mm) | 3320 | 2230 | 83 | 65 | 17 | 7 | 157 | 163 | 25 | 32 | 0.28 | 0.05 | 0.02 | 0.01 | 13.5 | 7.6 |
| DBH19 (mm) | 2362 | 1409 | 110 | 98 | 17 | 20 | 211 | 194 | 25 | 28 | 0.21 | 0.09 | 0.04 | 0.03 | 10.6 | 7.1 |
| STR (1–9) | 1123 | 1410 | 5 | 4.6 | 1 | 1 | 8 | 8 | 18.1 | 20.1 | 0.23 | 0.22 | 0.03 | 0.03 | 8.4 | 9.4 |
| BH (cm) | 1141 | 1390 | 67 | 46 | 0 | 10 | 280 | 240 | 65 | 74 | 0.44 | 0.39 | 0.04 | 0.03 | 2.4 | 45.4 |
| GA (°) | 440 | 393 | −1.8 | −1.5 | −6.3 | −6.3 | 2.5 | 2.8 | 63 | 74 | 0.20 | 0.21 | 0.03 | 0.03 | 31 | 51.9 |
| Pilo (mm) | 442 | 453 | 17.6 | 17.2 | 13.8 | 8.3 | 23.2 | 24.1 | 7.7 | 10.2 | 0.53 | 0.48 | 0.06 | 0.07 | 5.8 | 7.1 |
| AV (m s−1) | 450 | 453 | 4184 | 4237 | 6135 | 4717 | 3636 | 3165 | 4.8 | 5.5 | 0.05 | 0.3 | 0.04 | 0.08 | 0.6 | 1.6 |
| DENSItrax (kg m−3) | 115 | 151 | 431 | 444 | 329 | 358 | 520 | 566 | 8 | 7.1 | NA | NA | NA | NA | NA | NA |
| Traits | H6 | DBH6 | DBH12 | DBH19 | BH | GA | Pilo | AV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | H | B | H | B | H | B | H | B | H | B | H | B | H | B | H | B |
| H6 | 1 | 0.84 | 0.93 | 0.80 | 0.82 | 0.70 | 0.76 | 0.32 | 0.38 | −0.04 | −0.01 | −0.04 | −0.01 | −0.18 | −0.27 | |
| DBH6 | 0.68 | 0.67 | 1 | 0.92 | 0.90 | 0.84 | 0.83 | 0.54 | 0.51 | 0.03 | −0.03 | 0.17 | 0.02 | 0.10 | −0.10 | |
| DBH12 | 0.71 | 0.55 | 0.95 | 0.90 | 1 | 0.94 | 0.94 | 0.51 | 0.51 | −0.03 | −0.04 | 0.12 | 0.12 | 0.03 | −0.07 | |
| DBH19 | 0.73 | 0.61 | 0.90 | 0.99 | 0.98 | 0.99 | 1 | 0.53 | 0.51 | −0.16 | −0.12 | 0.08 | 0.18 | 0.00 | −0.11 | |
| BH | 0.08 | −0.11 | 0.5 | 0.35 | 0.42 | 0.23 | 0.37 | 0.25 | 1 | −0.15 | −0.07 | 0.04 | −0.12 | 0.25 | 0.09 | |
| GA | 0.67 | 0.28 | 0.85 | 0.24 | 0.97 | 0.45 | 0.99 | 0.49 | 0.29 | 0.05 | 1 | 0.15 | 0.06 | −0.16 | −0.12 | |
| Pilo | −0.22 | −0.19 | −0.03 | −0.19 | −0.15 | −0.07 | −0.34 | 0.02 | −0.17 | −0.36 | −0.18 | −0.04 | 1 | 0.16 | 0.09 | |
| AV | 0.37 | −0.61 | −0.16 | −0.25 | −0.06 | −0.11 | −0.11 | 0.04 | −0.11 | 0.29 | −0.46 | −0.07 | −0.12 | −0.10 | 1 | |
| Trait | Genetic Correlation between Hyssna and Brunsberg | SE |
|---|---|---|
| H6 | 0.757 | 0.104 |
| DBH6 | 0.444 | 0.149 |
| DBH12 | 0.466 | 0.127 |
| DBH19 | 0.350 | 0.13 |
| STR | 0.824 | 0.113 |
| BH | 0.673 | 0.069 |
| GA | 0.956 | 0.117 |
| Pilo | 0.815 | 0.098 |
| AV | 0.998 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, G.; Liziniewicz, M.; Adamopoulos, S.; Lindeberg, J. Genetic Parameters of Stem and Wood Traits in Full-Sib Silver Birch Families. Forests 2021, 12, 159. https://doi.org/10.3390/f12020159
Jones G, Liziniewicz M, Adamopoulos S, Lindeberg J. Genetic Parameters of Stem and Wood Traits in Full-Sib Silver Birch Families. Forests. 2021; 12(2):159. https://doi.org/10.3390/f12020159
Chicago/Turabian StyleJones, Grace, Mateusz Liziniewicz, Stergios Adamopoulos, and Johan Lindeberg. 2021. "Genetic Parameters of Stem and Wood Traits in Full-Sib Silver Birch Families" Forests 12, no. 2: 159. https://doi.org/10.3390/f12020159
APA StyleJones, G., Liziniewicz, M., Adamopoulos, S., & Lindeberg, J. (2021). Genetic Parameters of Stem and Wood Traits in Full-Sib Silver Birch Families. Forests, 12(2), 159. https://doi.org/10.3390/f12020159








