Abstract
The charcoal disease agents, Biscogniauxia mediterranea and Obolarina persica are two latent, ascomycetous oak pathogens in the Middle Eastern Zagros forests, where they have devastating effects, particularly during drought. Under greenhouse conditions, we investigated the effects of the two charcoal disease agents individually and in combination with drought on survival, growth, foliar gas-exchange, pigment content, oxidative stress and the antioxidant response of Quercus infectoria and Q. libani, two of the dominant tree species in this region. Commonly, the strongest negative effects emerged in the drought–pathogen interaction treatments. Q. infectoria showed less severe lesions, higher survival, more growth, and less leaf loss than Q. libani under combined biotic and abiotic stress. In both oak species, the combination of pathogen infection and drought resulted in more than 50% reduction in foliar gas-exchange parameters with partial recovery over time in Q. infectoria suggesting a superior defense system. Indeed, enhanced foliar anthocyanin, total soluble protein and glutathione concentrations imply an upregulation of the antioxidant defense system in Q. infectoria under stress while none of these parameters showed a significant treatment response in Q. libani. Consequently, Q. infectoria foliage showed no significant increase in superoxide, lower lipoxygenase activity, and less electrolyte leakage compared to the highly elevated levels seen in Q. libani indicating oxidative damage. Our findings indicate greater drought tolerance and pathogen resilience in Q. infectoria compared to Q. libani. Under future climate scenarios, we therefore expect changes in forest community structure driven by a decline in Q. libani and closely associated organisms.
1. Introduction
Under natural conditions, plants are often challenged by a combination of two or more stress factors simultaneously or in quick succession [1,2]. In these cases, abiotic stress may exacerbate the impacts of concurrent biotic stress by direct physiological interactive effects or it may even trigger a plant disease [3]. Fungal pathogens represent a major biotic stress factor, especially under prolonged or harsh drought periods that may promote the outbreak of disease [4]. Regardless of the underlying causes, drought stress may affect the intensity as well as the spatio-temporal distribution patterns of plant diseases [5]. Globally, decline episodes of woody plants have been associated with climatic extremes, pests, pathogens, and the complex interactions between those factors and with the ecosystem operating at various temporal scales [6,7,8,9]. In the oak-dominated forests in the Zagros region in western Iran, there has been a considerable increase in charcoal disease of oak associated with drought-driven tree mortality over the past decades, highlighting the role of drought stress in amplifying fungal disease occurrence. Recently, these oak decline events have been linked to the charcoal canker-causing fungi Biscogniauxia mediterranea (De Not.) Kuntze and Obolarina persica Mirabolfathy, Ju, Hsieh, and Rogers [10,11]. Although considered secondary stress agents, these disease-causing fungi can directly lead to tree dieback and death, especially when previous biotic damage preceded the infection [12,13].
Biscogniauxia mediterranea has been reported to be the most virulent agent of charcoal disease in the Zagros forests; however, in some regions, macroscopic charcoal disease symptoms could be ascribed to the ascomycete fungus O. persica [10,14]. Biscogniauxia mediterranea and O. persica are both members of the ascomycete family Xylariaceae and considered endophytes that usually survive in a latent phase in healthy host tissues for a long time, but may become pathogenic during periods of environmental stress and can rapidly spread from several infection points during their pathogenic phase [6,10,15,16,17]. The growth of fungal stromata in trees under drought stress induces extensive necrosis of the phloem and cambium and also canker formation [6,18].
Charcoal disease of oak is widespread in all types of forests in the Zagros region [10,19]. This is cause for great concern as oaks (Quercus brantii Lindl., Q. libani Oliv., Q. infectoria Oliv.) are typically the dominant, canopy-forming tree species in these uniquely water-limited forests of western Iran, covering a total area of about 5 million hectares [20].
Severe drought impairs plant cell functioning, which may result in a diminished defense potential and increased disease susceptibility against pests and pathogens through changes in the dynamics of primary and secondary metabolism [21,22]. Previous studies have shown that infection by fungal pathogens under drought stress resulted in stronger reductions in dry weight, leaf area, height growth, and survival compared to drought-stressed but uninfected plants [4,22,23]. Many studies have reported marked physiological changes in response to a combination of pathogen infection and drought, especially decreases in stomatal conductance, photosynthetic rate, chlorophyll concentration, relative water content (RWC) and water use efficiency [23,24,25]. Among the most notable biochemical and metabolic changes in plants exposed to drought and pathogen infection are the decrease in pigment content and an increase in reactive oxygen species (ROS) and non-enzymatic metabolites [26,27,28]. Increases in ROS such as the superoxide radical (O2•–) under stressful conditions may cause significant oxidative damage to membrane lipids resulting in electrolyte leakage and ultimately in cell death [29,30].
Climate change increases the likelihood of the occurrence of drought stress and will probably lead to an expansion of the host range of numerous pathogens in the future [31,32,33]. Hence, periods of combined biotic and abiotic stress, which already happen on a regular basis in the Zagros forests, are likely to occur even more frequently in the future. The identification of oak genotypes that are tolerant to drought and pathogens as a starting point for resistance breeding is therefore an important element of disease control.
The research presented here is part of a series of studies aimed at combating the charcoal disease currently ravaging the oak woodlands in the Zagros region. Previously, we have investigated the effects of drought and charcoal agents on growth, physiological and biochemical properties of the locally dominant oak species Quercus brantii [23,34]. We found that drought stress combined with charcoal agent attack induced greater reductions in growth and foliar gas-exchange and larger increases in antioxidant activity in Q. brantii compared to the individual drought and pathogen-related effects [23,34]. In a complimentary study, using the same experimental setup and trees as in the present study, we investigated biomass allocation patterns, leaf water potential, quantum yield of photosystem II, osmotically active compounds (proline, soluble sugars), oxidative stress markers (malondialdehyde, hydrogen peroxide), resistance enzymes (chitinase, β-1,3-glucanase, phenylalanine ammonia lyase), antioxidant enzyme activities, and the level of total phenols, flavonoids, and tannin in the two subdominant oak species Quercus infectoria and Q. libani [35]. We observed stronger reductions in biomass, more negative leaf water potentials and larger increases in proline, chitinase, total phenols, tannin, and flavonoids in Q. infectoria and Q. libani, when exposed to the combined impacts of drought and pathogen infection, suggesting that drought stress greatly intensifies the effects of charcoal disease [35]. Also, higher activities of key antioxidant enzymes in Q. infectoria suggest a more robust response to combined biotic and abiotic stress compared to Q. libani [35]. The current study examines the charcoal disease and drought issue from a different angle focusing on survival, growth, gas-exchange, relative water content (RWC), pigments, oxidative damage, and non-enzymatic antioxidant levels of Q. infectoria and Q. libani exposed to charcoal agents (B. mediterranea and O. persica), drought, and their interaction.
The main purpose of this study was to answer the following questions: (1) Does drought stress increase the susceptibility of oak seedlings to canker disease? (2) How do charcoal disease-causing pathogens affect growth, physiological and biochemical traits of oak seedlings? (3) Which of the two oak species is more vulnerable to pathogen attack and drought stress?
2. Materials and Methods
2.1. Plant Material and Growth Conditions
In this study, we used uniform two-year-old potted seedlings of two oak species (Quercus infectoria and Q. libani), grown in a forest nursery located in the Zagros region in the western Iranian state of Marivan (35° 48’ N; 46° 45’ E, 1320 m a.s.l.). The experiments were conducted in the greenhouse of the Faculty of Agriculture of Tarbiat Modares University (Tehran, Iran, 35° 44’ N; 51° 9’ E, 1278 m a.s.l.). The Q. infectoria and Q. libani seedlings were transferred to the greenhouse in late February 2015 and transplanted into 6 L pots containing sandy loam soil (53% sand, 32% silt, 15% clay, 1.35% organic matter, 0.45% N, 14.56 mg kg−1 available phosphorus, 184 mg kg−1 available potassium, pH 7.29). Plants were allowed to acclimatize for two months under greenhouse conditions (temperature of 25 ± 3 °C, photoperiod of 13 h/11 h (light/dark), relative humidity of 56 ± 3%, photosynthetically active radiation (PAR) of 1000 µmol m−2 s−1 and daily irrigation according to the targeted field capacity).
2.2. Fungal Isolates and Artificial Inoculation
Obolarina persica and B. mediterranea fungal isolates were provided from the Iranian Plant Protection Research Institute fungal collection, which had been isolated from symptomatic tissues of Quercus brantii trees from the Ilam forests within the Zagros mountains and their pathogenicity test found positive on Q. infectoria and Q. libani seedlings in a previous greenhouse trial.
Inoculations were conducted as described by Linaldeddu et al. [36], with minor modifications. The artificial inoculation of seedlings was performed through a small, circular wound in the stem of about 5 mm diameter made with a sterile scalpel 10–20 cm above the root collar. For inoculated seedlings, a single 5 mm diameter agar plug taken from the margins of a B. mediterranea (strain IR B 102) and O. persica (strain Ob 206) colony, actively growing on potato dextrose agar (PDA, Difco—BD), was placed mycelium-side down onto the wound. The pathogenicity of these cultures had been verified in a previous greenhouse trial on Q. infectoria and Q. libani seedlings. All inoculations were then wrapped in Parafilm M® (Bemis NA) to minimize contamination and desiccation. Similarly, a plug consisting of sterile PDA medium was placed on the wound in all non-inoculated seedlings (control seedlings). To keep the inoculation site moist, a piece of cotton was soaked in sterile water and placed on the wounded stem for a week.
2.3. Experimental Design and Greenhouse Experiment
The greenhouse study consisted of a randomized complete block design with two experimental factors applied to Q. infectoria and Q. libani seedlings: watering (well-watered and drought-stressed) and inoculation (non-inoculated, Obolarina persica inoculated and Biscogniauxia mediterranea inoculated). Thus, six different treatment combinations arranged in three blocks were considered for each species: (1) 100%-field capacity watering without fungal inoculation (C); (2) 100%-field capacity watering and inoculated with O. persica (Op); (3) 100%-field capacity watering and inoculated with B. mediterranea (Bm); (4) 20%-field capacity watering without fungal inoculation (D); (5) 20%-field capacity watering and inoculated with O. persica (D × Op); and (6) 20%-field capacity watering and inoculated with B. mediterranea (D × Bm). Eight seedlings of each species were randomly allocated to each treatment combination (288 seedlings in total for the two Quercus species).
After two months of acclimation under greenhouse conditions (described above), seedlings were subjected to the experimental treatments. Q. infectoria and Q. libani seedlings were inoculated with either B. mediterranea or O. persica and 96 non-inoculated seedlings were used as control seedlings. The drought treatments were imposed one month after the fungal inoculation, by weighing pots daily and maintaining them at the desired soil moisture content. To measure field capacity (FC), pots were weighed and watered. Water was allowed to evaporate naturally until the weight of the soil was constant. The FC was determined by obtaining the difference between the soil wet weight and the soil dry weight. At 100% FC, the mean gravimetric water content of the soil was 25%. Water loss through transpiration was measured gravimetrically by weighing all the pots and calculating the weight loss between watering. The pots under the 100% and 20% FC water supply regimes were subjected to compensatory irrigation after weighing at 06:00 pm daily during the experiment to maintain the soil moisture levels at 25% and 5%, respectively. Approximately, each pot received either 1250 mL (well watering) and 250 mL (drought stress) of water once every 3 days. Half of the seedlings for each inoculation treatment were randomly selected and allocated to the well-watered or drought treatment. The experiment lasted 10 months, from early May 2015 to early March 2016.
2.4. Survival and Plant Growth
At the end of the experiment, seedling survival, radial growth and height were measured along with leaf area, which was determined using a leaf area meter (Model LI-3000, Li-Cor, Lincoln, NE, USA).
2.5. Gas-Exchange, RWC, and WUEi
Three months after treatment initiation, relative water content (RWC) and leaf gas- exchange of 40 plants were measured at intervals of 3 months (August, November and February). Net photosynthesis (A), transpiration (E), stomatal conductance (gs) and intercellular CO2 concentration (Ci) were determined using a portable photosynthesis system (Li-6400XT, Li-Cor, Ll, USA). Fully developed leaves from the lower part of the seedlings were selected for gas-exchange measurements [37]. The intrinsic water use efficiency was calculated by dividing the photosynthesis rate by stomatal conductance (A/gs). Gas-exchange measurements were conducted at midday under stable leaf chamber CO2 concentration of 350 ppm, relative humidity set to 65 % (65 ± 5 %, mean ± standard error (SE)), leaf temperature set to 30 °C (30 ± 2 °C, mean ± SE) and ambient light conditions [38,39].
Fully developed leaves were sampled in order to assess the relative water content (RWC). Tissue fresh weight (FW) was recorded and leaves were saturated in distilled water under dark conditions at 4 °C for 24 h. After turgor weight was recorded, the leaves were placed into a drying oven at 80 °C for 72 h, followed by dry weight determination. The RWC was calculated according to equation (1) [40]:
where FW = fresh weight, TW = turgor weight, DW = dry weight.
RWC (%) = [(FW − DW)/(TW − DW)] × 100
2.6. Pigment Content
At the end of the experiment, fully developed leaves were collected for spectrophotometric (Cintra GBC, Dandenong, Victoria, Australia) determination of total chlorophyll extracted with acetone (80% v/v). The amount of total chlorophyll (a + b) and carotenoid content were calculated according to Arnon [41].
Anthocyanin content was extracted from leaves using the method of Wagner [42]. Leaves were sampled at the end of the study and ground to a powder in liquid nitrogen before extraction in 10 mL of acidified methanol (methanol: HCL 99:1 v/v) and kept at 25 °C for 24 h in the dark. The extracts were then centrifuged at 4000× g for 10 min and the absorption rate of the supernatant was read by the spectrophotometer at 550 nm. The amount of anthocyanin was calculated using an extraction co-efficient of 33,000 mol−1 cm−1 and expressed as mg g−1 FW.
2.7. Electrolyte Leakage, LOX, and ROS Determination
At the end of the study, leaves were harvested for electrolyte leakage (EL), lipoxygenase (LOX), and reactive oxygen species (ROS) determination. Cell membrane stability was estimated by measuring electrolyte leakage; 100 mg fresh leaf samples were cut into 5 mm width and 8 mm length placed in test tubes containing 10 mL distilled deionized water. The tubes were placed in a water bath maintained at a constant temperature of 32 °C. After 2 h the initial electrical conductivity of the medium (EC1) was measured using an electrical conductivity meter (Model 644, Metrohm, AG Herisau, Switzerland). Then, the samples were put in an oven at 120 °C for 120 min. Samples were then cooled to 25 °C and the final electrical conductivity (EC2) was measured. The electrolyte leakage (EL) was calculated following the equation (2) [43]:
EL (%) = (EC1/EC2) × 100
Lipoxygenase (LOX) activity was measured according to the method of Axelrod et al. [44]. One milligram leaf material was ground in 1 mL homogenizing buffer containing 50 mM K-phosphate buffer, pH 6.5, 10% polyvinyl pyrrolidone (PVP), 0.25% Triton X-100, 1 mM polymethyl sulfonyl fluoride. After thoroughly mixing, the 1.0 mL reaction contained 50 mMTris–HCl buffer (pH 6.5), 0.4 mM linoleic acid (LA) and 10 μL crude extract. The absorbance was recorded at 234 nm and the results were expressed in µmol min−1 mg −1 of protein using an extinction coefficient of 25 mM−1 cm−1.
Determination of tissue superoxide radical content (ROS) was performed according to Bai et al. [45]. Briefly, 1 g of fresh leaf was homogenized with 4 mL 65 mM phosphate buffer (pH 7.8) and centrifuged at 5000× g for 10 min. One mL of the supernatant was mixed with 0.1 mL of 10 mM hydroxylamine chloride and 0.9 mL of 65 mM phosphate buffer (pH 7.8) for 20 min at 25 °C in a water bath. Subsequently, 1 mL of this mixture was mixed with 1 mL 17 mM sulfanilic acid and 1 mL 7 mM α-naphthylamine for 20 min at 25 °C. Absorbance was spectrophotometrically determined at 530 nm. The nitrogen dioxide radical was used as a standard.
2.8. Soluble Protein and Non-Enzymatic Antioxidants Content
Leaf material for the assessment of soluble protein and non-enzymatic antioxidant content was collected at the end of the experiment. The content of the total soluble protein (TSP) in leaf extracts was determined following the method by Bradford [46] using bovine serum albumin (BSA) for the standard curve.
Glutathione (GSH) content was measured following the method of De Vos et al. [47] with some modifications. Fresh leaf samples of 0.5 g were homogenized in 6% m-phosphoric acid (pH 2.8) containing 1 mM EDTA. The buffer was mixed with 630 μL of 0.5 M K2HPO4 and 25 μL of mM 5, 5′–dithiobis (2-nitrobenzoic acid) (final pH 7.2). Absorbance of the solution was measured at 412 nm. Ascorbic acid (AA) content was determined according to Singh et al. [48] with few modifications. Briefly, one gram of fresh leaf sample was extracted with 3 mL of 5% (w/v) trichloroacetic acid (TCA) and centrifuged for 15 min at 18,000× g. AA was measured in a reaction mixture consisting of 0.2 mL of supernatant, 0.5 mL of 150 mm phosphate buffer (pH 7.4, containing 5 mM EDTA) and 0.2 mL of deionized water. Color was developed in reaction mixtures by the addition of 0.4 mL of 10% (w/v) TCA, 0.4 mL of 44% (v/v) phosphoric acid, 0.4 mL of α,α- dipyridyl in 70% (v/v) ethanol and 0.2 mL of 3% (w/v) FeCl3. The reaction mixtures were incubated at 37 °C for 45 min and absorbance of the solution was recorded at 532 nm.
2.9. Statistical Analysis
All data were first checked for normality using the Shapiro–Wilk’s test and for homogeneity of variance using the Levene’s test. Then, a two-way analysis of variance (two-way ANOVA) was applied to evaluate the effects of drought and inoculation treatments and their interaction on growth, physiological, and biochemical parameters. Comparison among group means was done by applying Tukey’s post-hoc test at the α = 0.05 level of significance. All statistical analyses were accomplished using SPSS (version 22.0, SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Visible Symptoms
At the end of this study, the severity of charcoal disease symptoms, such as stem lesions, discoloration of leaves and bark together with wilting caused by charcoal disease pathogens and their combination with drought differed greatly across treatments and also between the two oak species (Figure 1 and Figure 2).

Figure 1.
Longitudinal cross section of Quercus libani and Q. infectoria seedling shoots subjected to charcoal disease pathogens, drought and their interaction. Op: Obolarina persica, Bm: Biscogniauxia mediterranea, D × Op: Drought × O. persica and D × Bm: Drought × B. mediterranea.
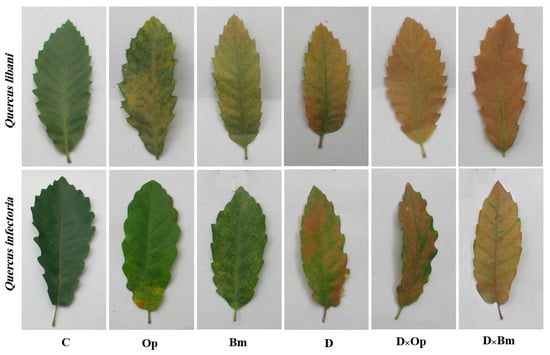
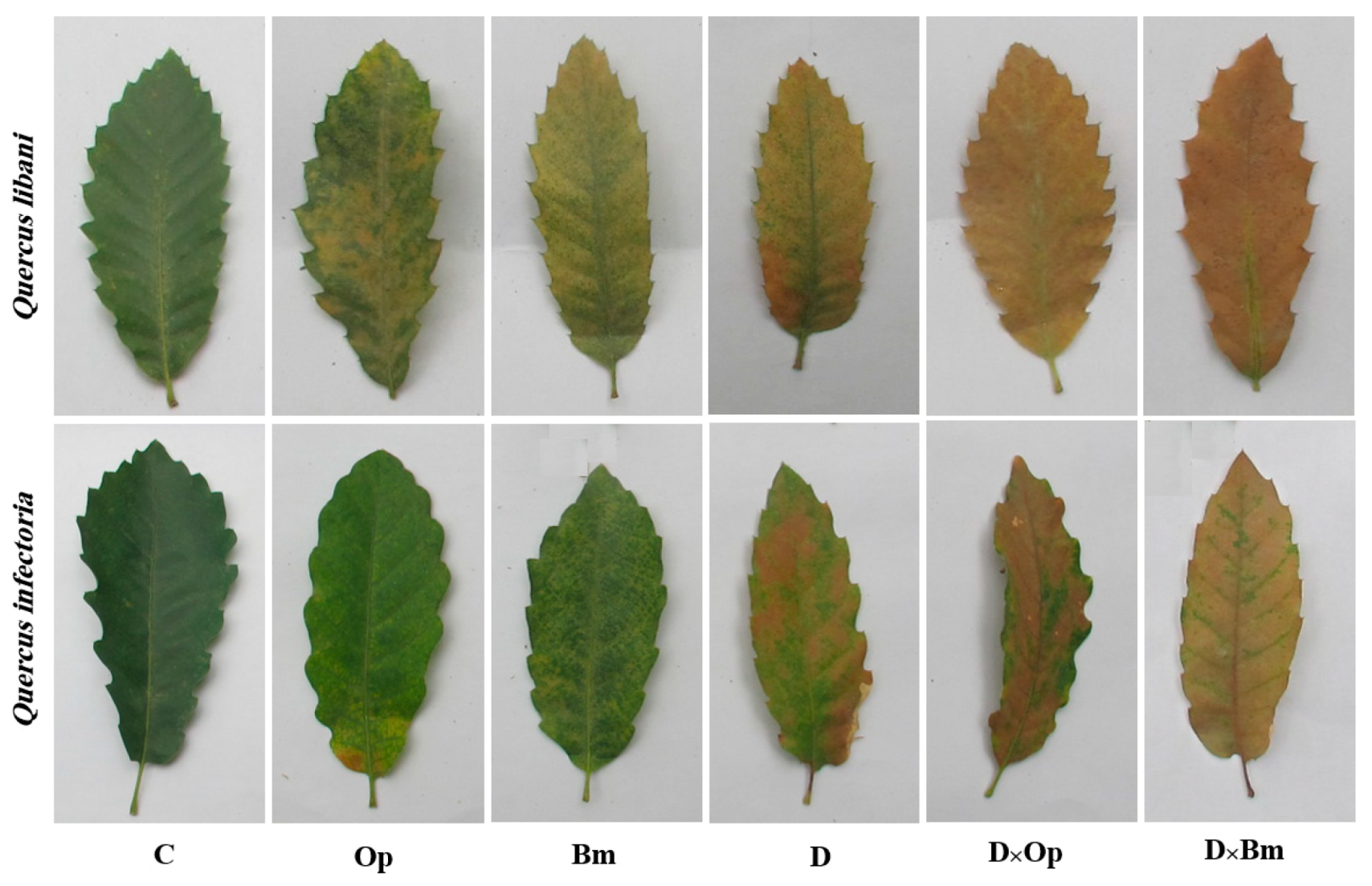
Figure 2.
Discoloration of Quercus libani and Q. infectoria leaves affected by charcoal disease, drought and their interaction. Op: Obolarina persica, Bm: Biscogniauxia mediterranea, D: Drought, D × Op: Drought × O. persica and D × Bm: Drought × B. mediterranea.
As expected, the two fungal agents of charcoal disease, O. persica and B. mediterranea, caused dark brown to black-coloured necroses of inner bark and vascular tissues of Q. libani and Q. infectoria seedlings, which were diagnosed in longitudinal shoot sections. The lesions caused by the charcoal disease pathogens were observable around the inoculation points of all inoculated seedlings, whereas no signs of lesion formation were found in control seedlings. In both oak species, the severity of stem lesions was more pronounced when pathogen-inoculated seedlings were simultaneously subjected to drought (Figure 1). Similarly, other typical disease symptoms such as chlorosis and wilting were more prominent in seedlings exposed to the combined effects of fungal pathogen attack and drought compared to the effects of the individual treatments (Figure 2).
3.2. Survival and Plant Growth
At the end of the experiment, Q. libani seedlings only suffered significant mortality in the combined pathogen and drought treatment in comparison with the non-inoculated and well-watered seedlings (D × Op: 25.9% decrease, D × Bm: 29.3% decrease), whereas survival of Q. infectoria seedlings remained unaffected across treatments (Table 1, Figure 3a).

Table 1.
Two-way ANOVA results for parameters measured in Quercus libani and Q. infectoria seedlings inoculated with charcoal disease agents (O. persica or B. mediterranea) and subjected to drought stress (100% of field capacity or 20% of field capacity). Degrees of freedom (df) for drought stress = 1, pathogen = 2 and pathogen × drought stress = 2. Although all the photosynthesis parameters, WUE and RWC were statistically analyzed for each of the three measurement campaigns, only the results of the final assessment (February 2016) are presented here. The reported figures represent F-values of ANOVA models.
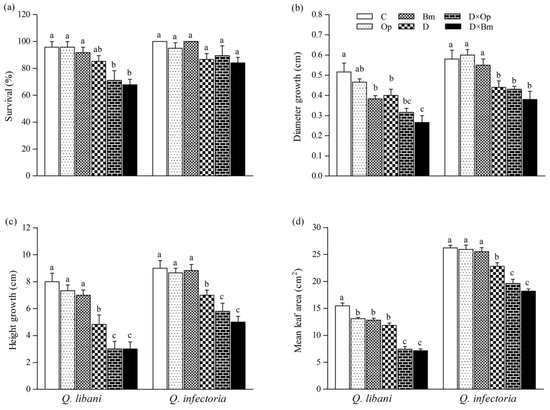
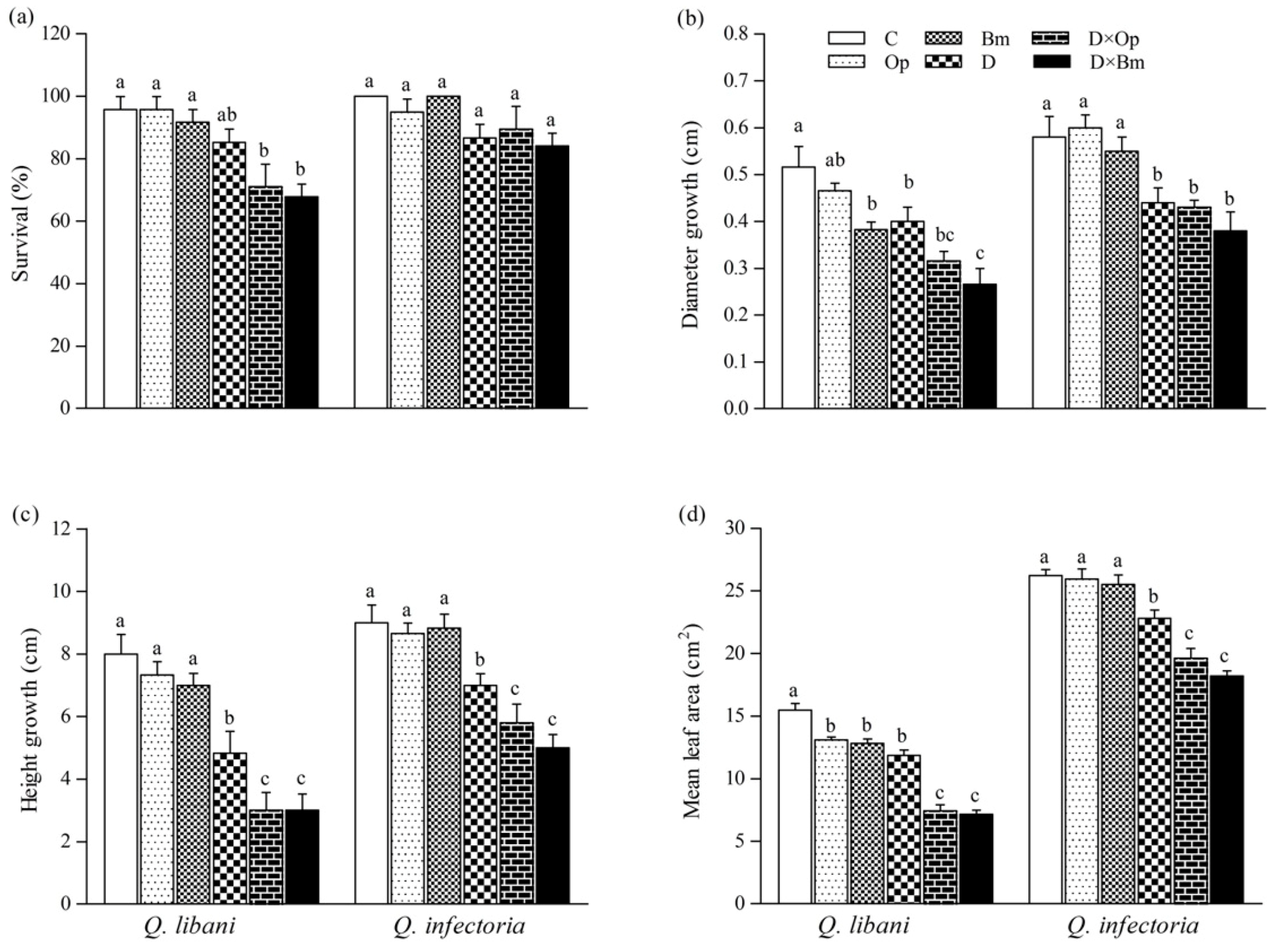
Figure 3.
(a) Survival, (b) diameter growth, (c) height growth, and (d) average leaf area of Quercus libani and Q. infectoria seedlings affected by charcoal disease and drought (Op: Obolarina persica, Bm: Biscogniauxia mediterranea, D: Drought, D × Op: Drought × O. persica and D × Bm: Drought × B. mediterranea). Bars represent means + standard error, n = 6. Different lower-case letters indicate statistically significant differences between the treatments at α = 0.05 based on Tukey’s multiple comparison test.
Drought stress alone and combined with fungal pathogen attack significantly decreased stem diameter and height growth of Q. libani and Q. infectoria seedlings compared to the non-inoculated, undroughted control seedlings (22.5%–48.4% decrease in Q. libani and 24.1%–38.4% decrease in Q. infectoria). Under well-watered conditions, B. mediterranea infection only caused a significant decrease in diameter growth in Q. libani compared with non-inoculated seedlings (Bm: 25.7%, Figure 3b). Also, the mean leaf area of Q. libani seedlings decreased significantly by a similar amount when inoculated with charcoal disease pathogens or exposed to drought (Figure 3d). However, a further significant reduction in leaf area occurred under the combined drought and pathogen treatment amounting roughly to a 50% loss of leaf area relative to the control (non-inoculated and well-watered seedlings). In Q. infectoria seedlings, on the other hand, pathogen inoculation alone had no significant effect on leaf area but drought exposure led to a significant 13% decrease, which grew into a greater loss of leaf area when the drought treatment was combined with pathogen attack (leaf area decreased around 25% and 30% in D × Op and D × Bm compared to the control, respectively, Figure 3d).
3.3. Gas-Exchange, RWC, and WUEi
Throughout the experimental period, all leaf gas-exchange parameters (A, gs and E) were highest in the non-inoculated and well-watered control seedlings regardless of oak species, while they were lowest in the pathogen-inoculated seedlings under drought conditions (Figure 4).
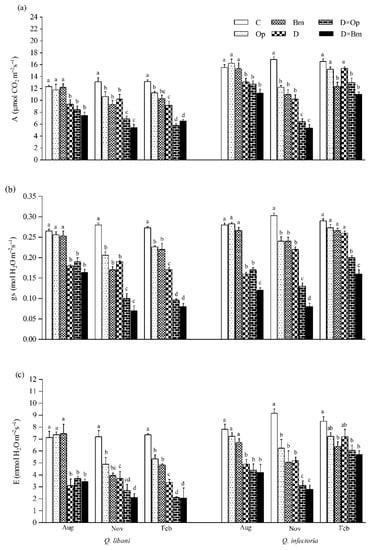
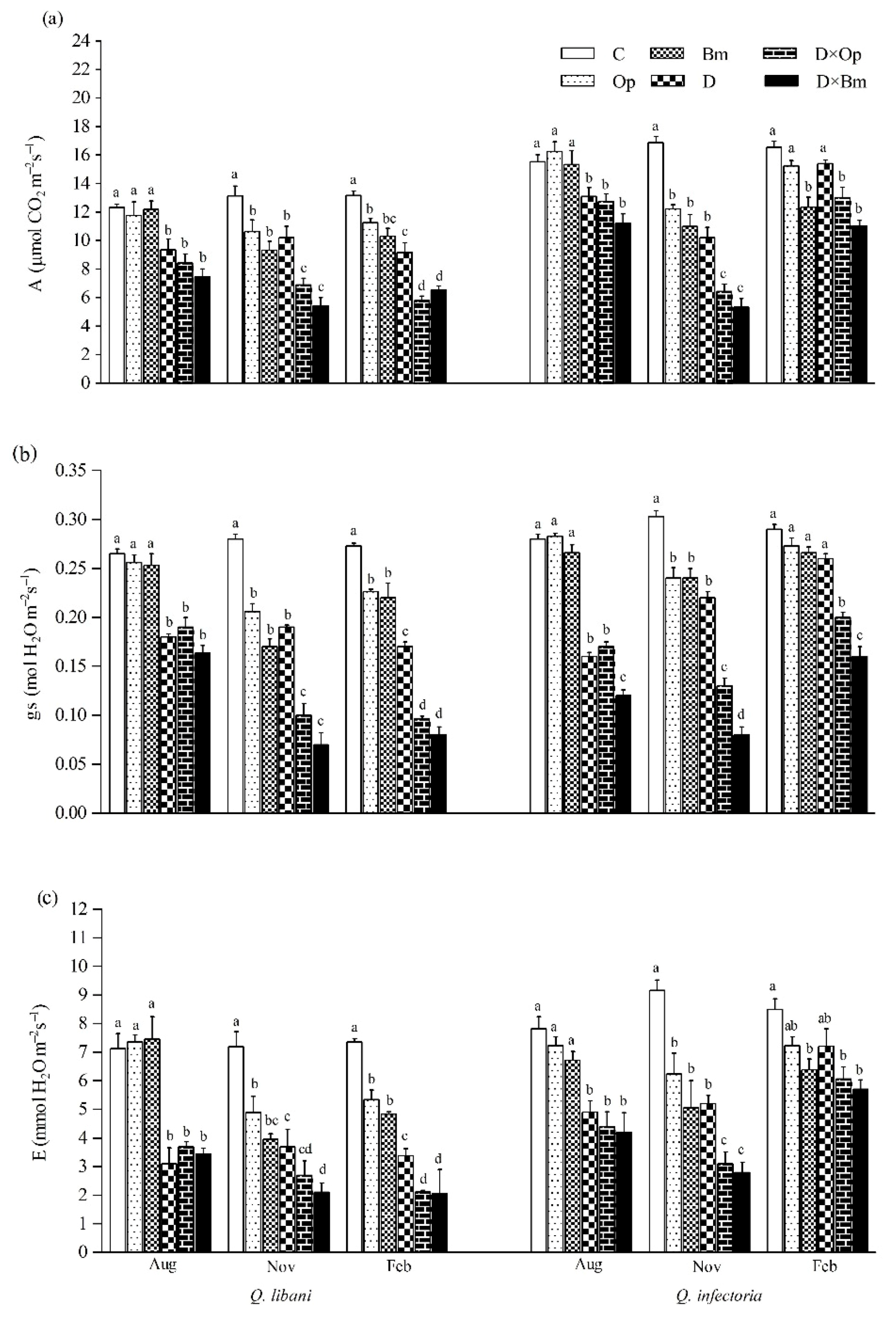
Figure 4.
(a) Net photosynthesis rate (A); (b) stomatal conductance (gs); and (c) transpiration (E) of Quercus libani and Q. infectoria seedlings affected by charcoal disease, drought and their combination assessed at intervals of three month starting three months after treatment initiation. Op: Obolarina persica, Bm: Biscogniauxia mediterranea, D: Drought, D × Op: Drought × O. persica and D × Bm: Drought × B. mediterranea. Bars represent means + standard error, n = 6. Different lower-case letters indicate statistically significant differences between the treatments at α = 0.05 based on Tukey’s multiple comparison test.
Three months after the start of the experiment, drought alone and in combination with pathogen inoculation significantly decreased photosynthetic rate, stomatal conductance and transpiration rate in Q. libani and Q. infectoria seedlings compared to control seedlings (Q. libani, A: 24.1%–39.6% decrease, gs: 28.3%–38.3% decrease, E: 48%–56.4% decrease; Q. infectoria, A: 15.6%–27.8% decrease, gs: 39.2%–57.1% decrease, E: 37.3%–46.2% decrease) (Table 1). However, pathogen inoculation alone did not significantly affect these parameters (Table 1). Six and nine months after treatment initiation (November and February assessments), all photosynthetic gas-exchange parameters had decreased under the impact of pathogens and drought (alone and in combination with each other) and at the end of the study these reductions were more pronounced in Q. libani seedlings compared to Q. infectoria seedlings (November, Q. libani, A: 18.9%–58.5% decrease, gs: 26.4%–75% decrease, E: 31.8%–70.7% decrease relative to the control; November, Q. infectoria A: 27.4%–68.3% decrease, gs: 20.7%–73.5% decrease, E: 31.9%–69.4% compared to the control; February, A: 14.4%–56% decrease, gs: 17.2%–70.6% decrease, E: 27.4%–71.9% decrease compared to the control, Q. infectoria A: 7.8%–33.4% decrease, gs: 5.8%–44.8% decrease, E: 14.8%–32.8% decrease compared to the control, Figure 4a–c). The effect of B. mediterranea on stomatal conductance of Q. infectoria seedlings in August, November, and February was more severe compared to O. persica (Figure 4b).
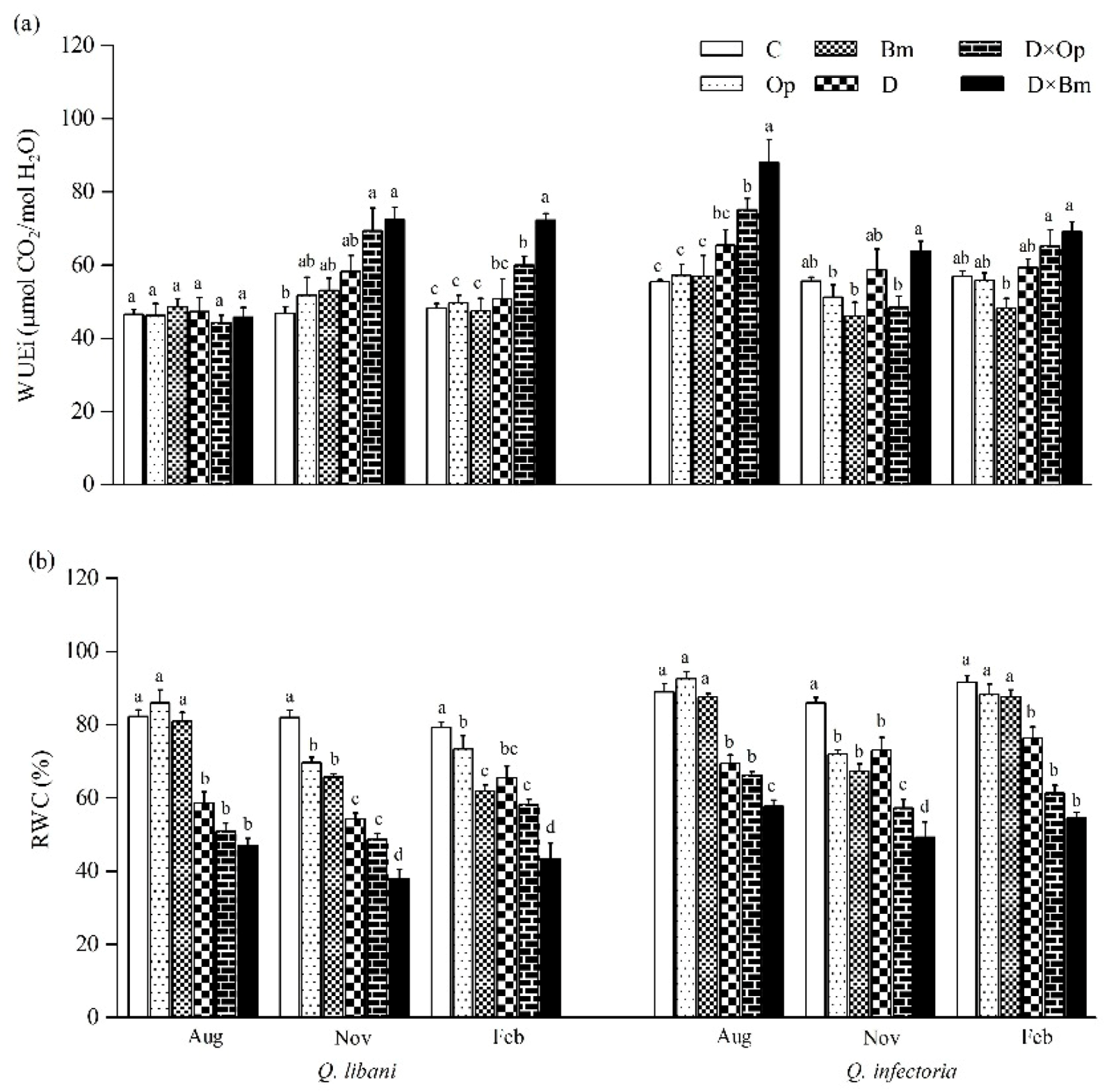
Three months after the start of this study, intrinsic water use efficiency (WUEi) of Q. libani seedlings showed no response to the experimental treatments (August measurements in Figure 5a). However, the data from the following measurement campaigns (November and February) displayed a strikingly similar pattern of significantly enhanced WUEi of seedlings exposed to the combined pathogen and drought treatments (November: D × Op: 48.1% increase, D × Bm: 54.7% increase compared to the control; February: D × Op: 24.5% increase, D × Bm: 50% increase relative to the control) relative to the remaining treatments (including the control), which did not differ significantly from each other (Table 1, Figure 5a). At the first assessment date, Q. infectoria seedlings showed significantly increased WUEi in response to the combined drought and fungal pathogen treatments but not in any other treatment (Figure 5a). In the following November and February recordings, the distinct trend seen before had vanished and no clear pattern was discernible. Relative water content (RWC) of Q. libani and Q. infectoria seedlings was negatively affected by drought alone and in combination with charcoal disease agents throughout the experiment (Figure 5b). At the initial assessment date, none of the two fungal pathogens had an effect on seedling RWC of either oak species, but RWC was significantly reduced as a result of the interaction between pathogen inoculation and drought stress (28.8–42.9% reduction compared to the control, Figure 5b). However, at the two following assessments, RWC had significantly decreased in Q. libani seedlings inoculated with either of the two pathogens alone and in combination with drought stress (November: 15–53.6% decrease, February: 7.5–45.3% decrease relative to the control). In Q. infectoria seedlings, this pathogen-related reduction in RWC was only seen in the November assessment (Ob: 16.2% decrease, Bm: 21.7% decrease than control) but there was no significant difference in RWC between pathogen-inoculated seedlings and control seedlings at the last assessment (Figure 5b). The lowest RWC values of Q. libani and Q. infectoria seedlings were recorded in response to the combination of drought and B. mediterranea (Figure 5b).
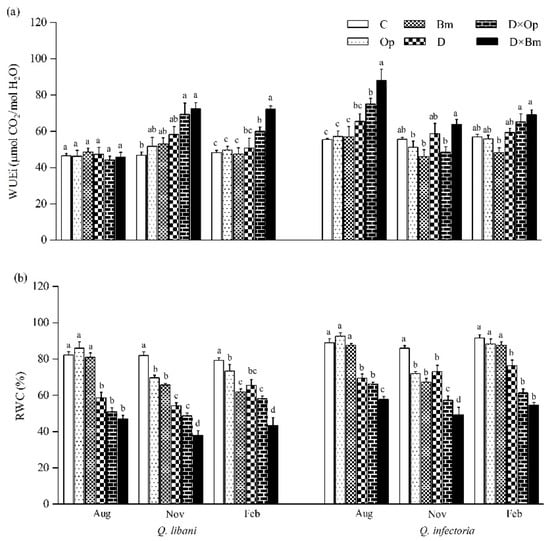
Figure 5.
(a) Intrinsic water use efficiency (WUEi) and (b) relative water content (RWC) of Quercus libani and Q. infectoria seedlings affected by charcoal disease, drought and their combination assessed at intervals of three months (starting three months after treatment initiation). Op: Obolarina persica, Bm: Biscogniauxia mediterranea, D: Drought, D × Op: Drought × O. persica and D × Bm: Drought × B. mediterranea. Bars represent means + standard error, n = 6. Different lower-case letters indicate statistically significant differences between the treatments at α = 0.05 based on Tukey’s multiple comparison test.
3.4. Pigment Content
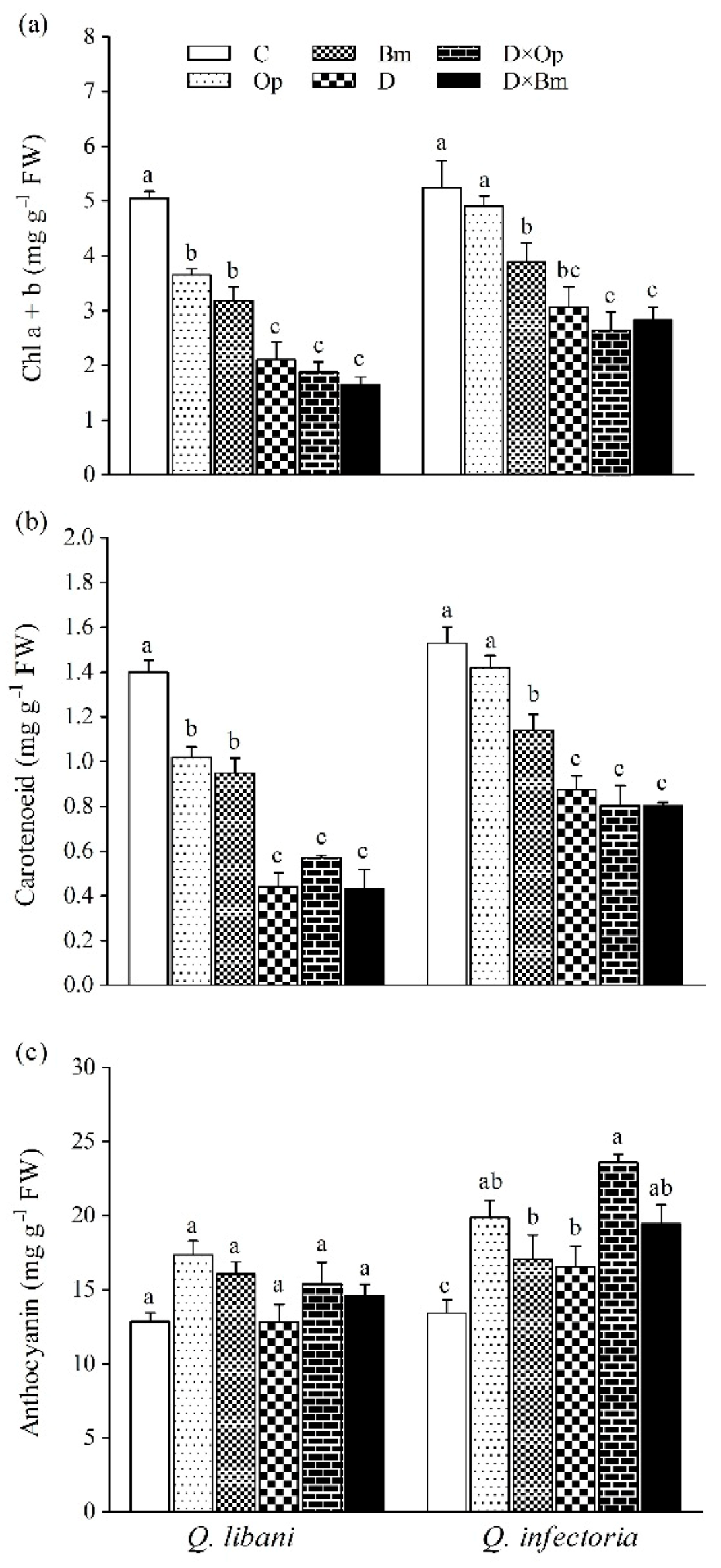
Total chlorophyll (a + b) and carotenoid content in leaves of Q. libani and Q. infectoria seedlings was significantly reduced by all treatments except for O. persica-inoculated Q. infectoria seedlings whose pigment content remained unaffected (Table 1, Figure 6a,b). The largest reductions in chlorophyll and carotenoid content occurred in seedlings exposed to drought and the combination of drought and pathogen inoculation, which were statistically similar. In this regard, seedlings of Q. libani were more affected than Q. infectoria seedlings (Chlorophyll a + b: 27.7%–67.3% decrease in Q. libani, and 6.4%–49.6% decrease in Q. infectoria, carotenoids: 27.1%–69.2% decrease in Q. libani, and 7.1%–47.6% decrease in Q. infectoria compared to the control, Figure 6a,b). Anthocyanin content remained unaffected in Q. libani seedlings but showed significant increases in response to all treatments in Q. infectoria seedlings compared to the control seedlings (Figure 6c).
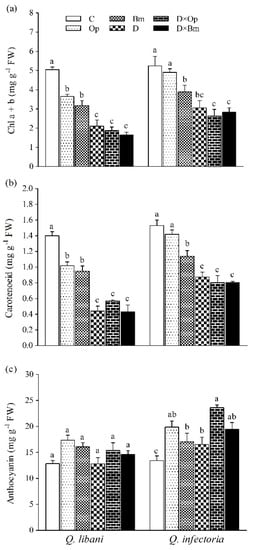
Figure 6.
(a) Chlorophyll a + b, (b) carotenoid, and (c) and anthocyanin content in leaves of Quercus libani and Q. infectoria seedlings affected by charcoal disease, drought, and their combined effects. Op: Obolarina persica, Bm: Biscogniauxia mediterranea, D: Drought, D × Op: Drought × O. persica and D × Bm: Drought × B. mediterranea. Bars represent means + standard error, n = 3. Different lower-case letters indicate statistically significant differences between the treatments at α = 0.05 based on Tukey’s multiple comparison test.
3.5. Electrolyte Leakage, LOX, and ROS Determination
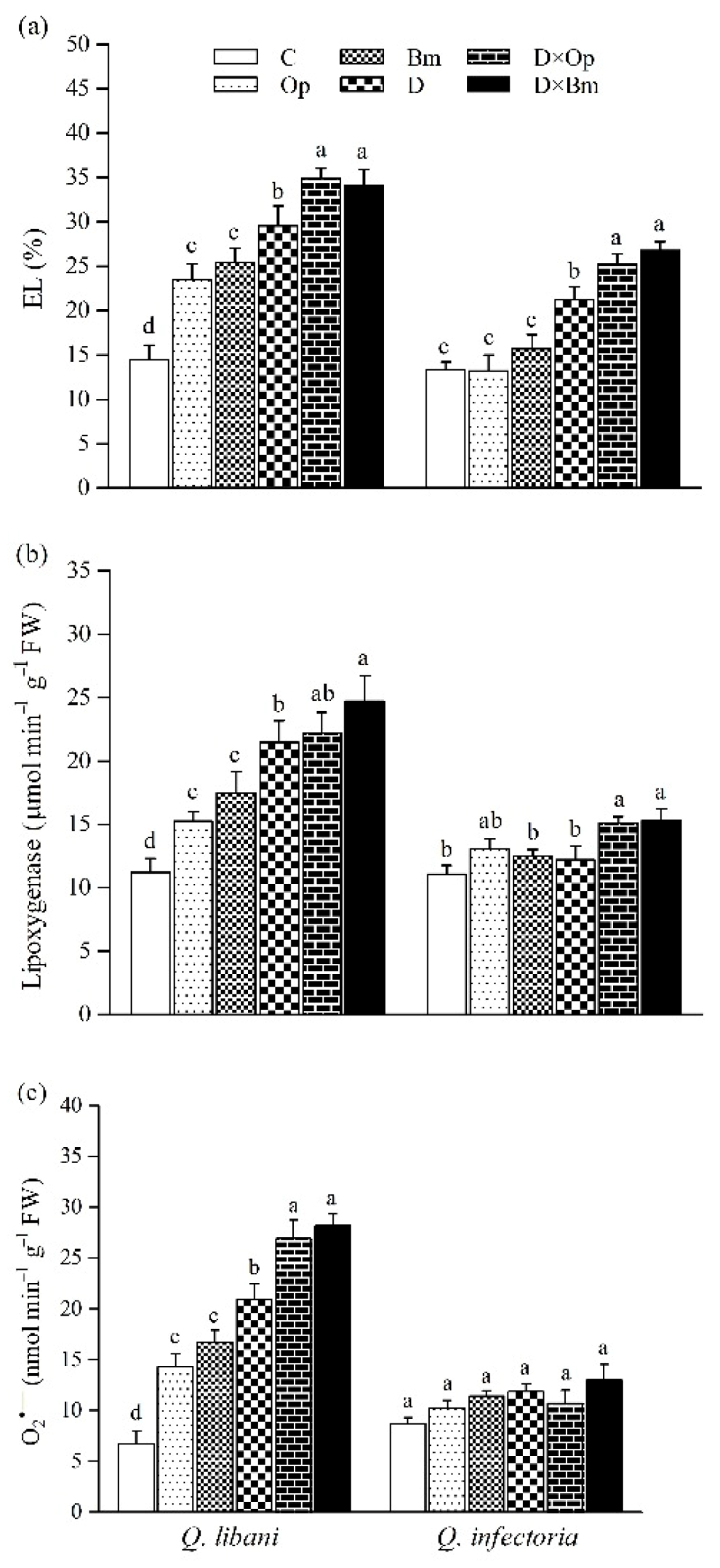
In both oak species, the strongest increase in electrolyte leakage (EL) was recorded in seedlings subjected to the combination of charcoal disease pathogens and drought compared to control seedlings (Q. libani 62.3%–141.5% increase, Q. infectoria 17.6%–100.8% increase compared to the control; Table 1, Figure 7a). In Q. libani, LOX activity significantly increased by similar amounts over the control when seedlings were inoculated with either pathogen species (ca. + 35%) and a further significant increase was observed in non-inoculated, drought-stressed seedlings (+91%; Table 1, Figure 7b). The combination of drought and O. persica inoculation resulted in LOX activity levels similar to the drought stress-only treatment but B. mediterranea inoculation under drought conditions produced a further significant increase in activity (+ 120% than control; Figure 7b). By contrast, in Q. infectoria, LOX activity only showed significant increases in seedlings exposed to the combination of drought and fungal pathogens (Op × D: 36.3% increase, Op × D: 38.1% increase relative to the control), which was, however, not significantly different from the levels seen in well-watered seedlings inoculated with O. persica (Figure 7b). In Q. libani, the significant increase in O2•– content across treatments strongly resembled the pattern of LOX activity, but here both pathogen species significantly increased foliar O2•– content of drought-exposed seedlings by a similar amount compared to the drought stress-only treatment (Table 1, Figure 7c). The combination of pathogen inoculation and drought resulted in a ca. 3.5-fold increase in foliar O2•– content of Q. libani seedlings relative to the control group. Surprisingly, the O2•– content of Q. infectoria seedlings remained unchanged across treatments (Figure 7c).
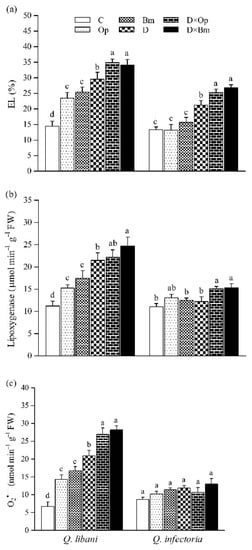
Figure 7.
(a) Electrolyte leakage, (b) lipoxygenase activity, and (c) O2•– content in leaves of Quercus libani and Q. infectoria seedlings affected by charcoal disease, drought and their interaction. Op: Obolarina persica, Bm: Biscogniauxia mediterranea, D: Drought, D × Op: Drought × O. persica and D × Bm: Drought × B. mediterranea. Bars represent means + standard error, n = 3. Different lower-case letters indicate statistically significant differences between the treatments at α = 0.05 based on Tukey’s multiple comparison test.
3.6. Soluble Protein and Non-Enzymatic Antioxidants Content
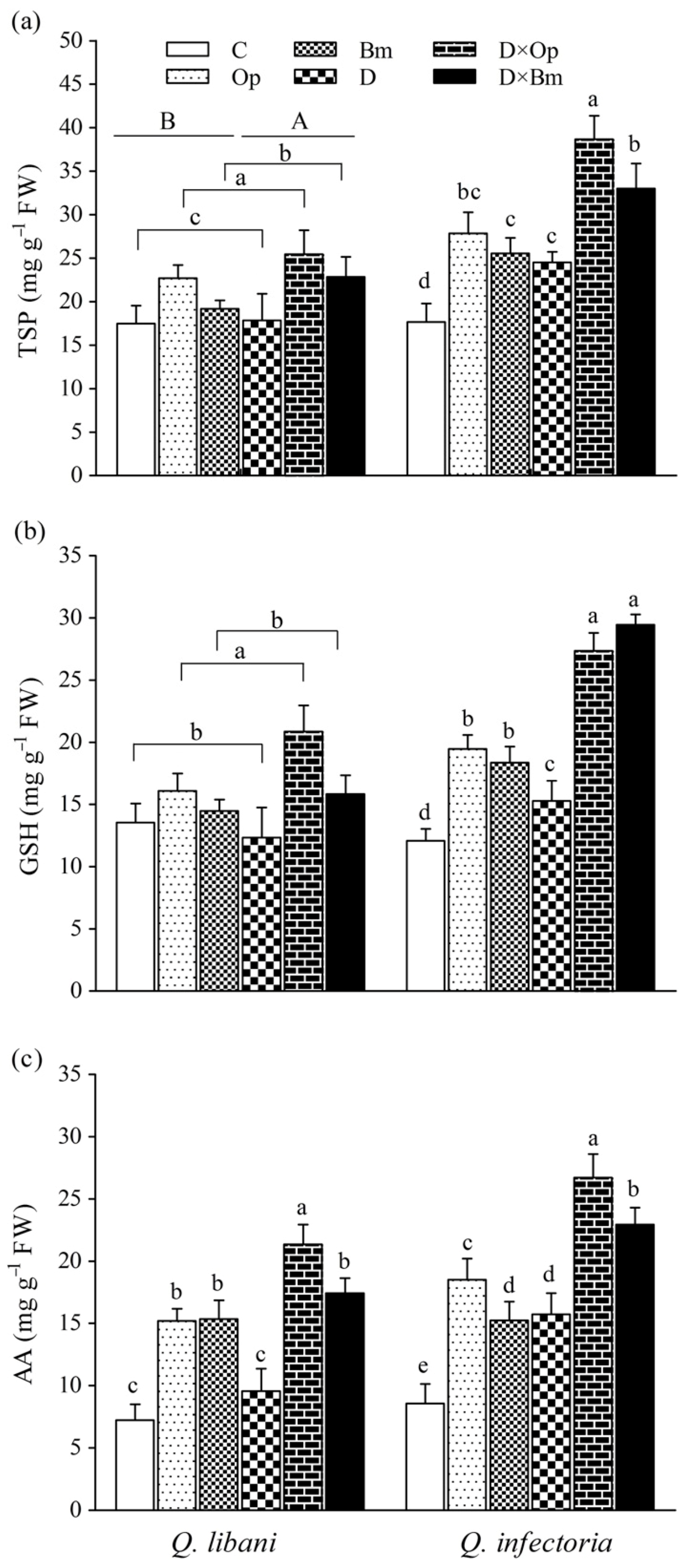
In seedlings of Q. libani, we detected no significant pathogen × drought interaction on total TSP and GSH content but the main effect of pathogen inoculation was statistically significant and driven by O. persica, which produced significantly higher GSH as well as TSP levels. Drought alone also resulted in slightly but significantly enhanced TSP content (Table 1, Figure 8). In Q. infectoria seedlings, however, the amount of TSP content was significantly higher in all treatments compared to the control (1.38 to 2.18-fold higher). The largest amount of TSP occurred in O. persica-inoculated seedlings under drought stress (Figure 8a). GSH concentration was significantly higher in all treatments compared to the control (1.26 to 2.43-fold higher) and reached peak values in the combined drought and pathogen treatments (Op × D: 27.37 mg g−1 FW, Op × D: 29.45 mg g−1 FW, Figure 8b). Pathogen infection alone and in combination with drought significantly increased foliar AA content of Q. libani seedlings but drought alone had no significant effect. Both pathogens roughly doubled AA content, drought in combination with O. persica tripled the AA content and drought combined with B. mediterranea increased AA 2.5-fold (Figure 8c). In Q. infectoria seedlings, all treatments resulted in significantly increased AA levels compared to the control with the highest value seen in seedlings dealing with O. persica infection and drought stress at the same time. Generally, the magnitude of the treatment-related increase in AA content relative to the control was greater in Q. infectoria (+ 78%–211.9%) compared with Q. libani (+ 34.4%–195.7%; Figure 8c).
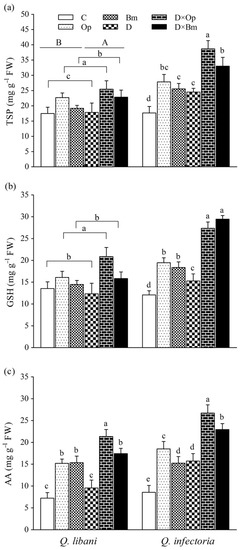
Figure 8.
(a) Total soluble protein (TSP), (b) glutathione (GSH), and (c) ascorbic acid (AA) contents in leaves of Quercus libani and Q. infectoria seedlings affected by charcoal disease and drought (Op: Obolarina persica, Bm: Biscogniauxia mediterranea, D: Drought, D × Op: Drought × O. persica and D × Bm: Drought × B. mediterranea). Bars represent means + standard error, n = 3. Different lower-case letters indicate statistically significant differences between the treatments at α = 0.05 based on Tukey’s multiple comparison test.
4. Discussion
In this study we examined the effects of the two charcoal disease-causing pathogens (Biscogniauxia mediterranea and Obolarina persica) and drought as well as their combination on survival, growth, foliar gas-exchange, pigment content, oxidative stress, and antioxidant response in seedlings of two dominant oak species of the Zagros forests in Iran. In line with our expectations, the combined effects of charcoal disease agents and drought stress were usually more detrimental to survival, growth, and physiological parameters, than the effects of pathogen attack or drought stress alone [49]. Our findings thus imply enhanced susceptibility of oak seedlings to charcoal canker disease under drought conditions. Ghanbary et al. [23] reported that survival of seedlings of Quercus brantii, the most dominant tree species in the Zagros forests, slightly decreased under the combination of drought and B. mediterranea infection but remained unaffected by O. persica. Here, we found mortality only showed a significant increase in Q. libani seedlings simultaneously exposed to drought and pathogen attack, which can be interpreted as an accumulative negative effect of biotic and abiotic stress [50] (Figure 3). This finding implies greater drought-induced predisposition to charcoal disease in Q. libani compared with Q. infectoria, whose lack of significant treatment-related seedling mortality suggests superior resilience.
Diameter and height growth were not significantly affected by charcoal disease agents, except for the reduction in diameter growth in Q. libani caused by B. mediterranea. However, drought stress alone and together with pathogen attack resulted in reduced diameter and height growth as well as reduced mean leaf area in both oak species but that effect was more severe in Q. libani. When looking at the individual effects, drought stress affected growth parameters more strongly than pathogen infection. Our height growth and leaf area data clearly indicate that drought stress may worsen the impact of charcoal disease. Under optimal conditions, plants allocate their energy to growth, cellular maintenance, and reproduction, whereas under drought and pathogen attack plants need to balance energy production and plant defense to safeguard survival [51,52]. Under drought and pathogen attack, energy is often allocated away from growth to defense-related processes and reduced photosynthetic activity is also a common response [4,52,53]. Our findings are consistent with the reduced growth of drought-stressed Quercus ilex infected with Phytophthora cinnamomi and drought-exposed Q. brantii seedlings infected with B. mediterranea and O. persica [23,54]. Similar observations of Phytophthora cinnamomic-related growth reductions under drought were reported for Q. robur in Central Europe [55].
Three months after pathogen inoculation, photosynthetic activity was not affected by charcoal disease agents under well-watered conditions, indicating that pathogen development had not advanced to a stage yet where it could cause measurable growth and physiology impairment [10]. However, 6 months after treatment initiation, charcoal disease agents, drought stress and their combination produced significant reductions in A, gs, and transpiration rate in both oak species. At the end of the study, this effect pattern persisted in Q. libani, whereas Q. infectoria seedlings showed partial and in some treatments even full recovery of foliar gas-exchange parameters. This finding indicates that the plant defense system operated more efficiently in Q. infectoria and that this species seems to be able to adjust osmotically to increase water uptake in response drought which is in line with the high proline levels found in drought-stressed Q. infectoria seedlings in an earlier study [35]. Our results are in agreement with previous observations of reduced photosynthesis in oak species in response to fungal pathogen attack such as Erysiphe alphitoides in Quercus robur [4,56], Ceratocystis fagacearum in Quercus fusiformis [57], B. mediterranea in Quercus brantii, Q. suber and Q. ilex [23,36,58], Botryosphaeria corticola in Q. suber [36], and also O. persica in Q. brantii [23]. Stomatal downregulation of transpiration is a common response to drought in order to maintain favorable plant water potential in drought-avoiding species [59] but reductions in stomatal conductance may also occur under fungal attack as a result of hyphal growth blocking the stomatal opening [60]. The observed reductions in photosynthesis are largely linked to corresponding decreases in stomatal conductance similar to the findings of Hossain et al. [22] investigating the canker disease of Corymbia trees in Australia. However, in the long-term, reductions in photosynthetic activity as a result of disease or drought might also be related to chlorophyll and chloroplast degeneration (see below), altered leaf photochemistry, as well as inhibition of Rubisco and other photosynthetic enzymes [51,61,62,63]. Furthermore, toxins released by fungal pathogens such as B. mediterranea may suppress the host defense response and can reduce leaf photosynthetic area [64]. For instance, B. mediterranea has been reported to reduce the rate of photosynthesis and stomatal conductance in seedlings of Quercus ilex and Q. suber by releasing toxic compounds [36,57].
Leaf RWC is an important indicator of plant water status [65] and reductions might be related to changes in stomatal conductance and declines in cellular expansion [66]. RWC-based indications of strong water deficit in plants often result in metabolic changes such as photosynthetic impairment and increases in respiration [67]. In line with our gas-exchange data, we did not see pathogen-induced changes in RWC in well-watered seedlings of either oak species three months after inoculation, consistent with an early stage of pathogen development (Figure 5). At the end of the experiment, the combination of pathogens and drought stress caused a significant reduction in RWC below 55% in both oak species, which was not statistically different though from the drought-only treatment. Biscogniauxia mediterranea caused a significantly stronger decrease in RWC of drought-stressed Q. libani seedlings than O. persica but both pathogens had similar effects on drought-stressed Q. infectoria seedlings. In light of the stomatal downregulation of transpiration, the strongly reduced RWC suggests impaired cuticle integrity and thus increased non-stomatal water loss which can be explained by the strong increase in chlorotic leaf spots in the combined drought and pathogen treatments. This is consistent with the findings of Hutchinson [68] who demonstrated that chlorotic leaves lost water much more rapidly than green leaves and that the water loss was proportional to the degree of chlorosis across a range of woody and herbaceous species. This notion is further supported by our data on electrolyte leakage.
Because gs of Q. libani seedlings had decreased more strongly than photosynthesis in response to the combined pathogen and drought treatment at the end of the study, the associated WUEi was significantly higher than in the control. By contrast, the rather strong combined treatment effects on gs in Q. infectoria seen at the first two assessments, had partially recovered at the end of the experiment resulting in WUEi values that were not significantly different from the control.
Leaf pigment content including chlorophyll and carotenoids is an important indicator of plant physiological status that can be used to evaluate plant photosynthetic activity [69]. Abiotic and biotic stress often results in reduced chloroplast numbers and the breakdown of chlorophyll followed by chlorosis and necrosis in leaves, which was also observed in the current study [26,27,35,70]. Chlorophyll and carotenoid content of both oak species declined significantly in response to pathogen attack, drought stress and their interaction, except for well-watered Q. infectoria seedlings whose pigment content remained unaffected by infection with O. persica. In contrast to RWC and some of the gas-exchange parameters, drought stress did not intensify the negative effects of charcoal disease pathogens on foliar pigment content beyond the reductions seen in the drought-only treatment indicating no synergistic effects of drought and pathogen attack. Usually, leaf chlorophyll declines following biotic or abiotic stresses because of photo-oxidation, chlorophyll degradation, or impaired chlorophyll biosynthesis [71] and our data suggest that these processes have occurred more frequently in Q. libani. Anthocyanin production is induced by environmental stressors such as high light, drought stress, as well as pests and pathogens and has been linked to ROS scavenging, photoprotection, and stress signal transduction [72,73,74]. All treatments resulted in significantly higher amounts of foliar anthocyanin in Q. infectoria seedlings compared with the control, while anthocyanin content in Q. libani leaves remained unaffected. This finding implies that anthocyanins are critical to the host defense and drought tolerance of Q. infectoria but not in Q. libani. Anthocyanins may play a major role in the defense against environmental stresses and have been reported to be involved in disease resistance [75,76]. Anthocyanin accumulation has been shown to have a photo-protective function through their role as antioxidants [77,78].
One of the first processes associated with plant defense against both biotic and abiotic stress, is the rapid production of ROS [79]. Reactive oxygen species like superoxide radicals are essential to induce the hypersensitive response, programmed cell death, and strengthening of plant cell walls and while they are toxic for pathogens they also affect cellular functions in the host [80,81]. Oxidative stress and ensuing damage occur when the intracellular ROS concentration escapes the control of the antioxidant system [82]. In our study, the O2•– content in leaves of Q. libani increased significantly to similar levels following inoculation with O. persica and B. mediterranea and to even higher levels under drought stress. A further increase seen in pathogen-infected seedlings under drought indicates a synergistic effect and highlights how abiotic stressors such as drought can shape plant–pathogen relations. Many studies have indicated that under biotic and abiotic stress, ROS are mainly generated in the reaction center of leaf chloroplasts [83,84,85], reflecting the inhibition of photosystem II through blocked electron transfer to plastoquinone. The resulting ROS formation can lead to protein and cell membrane damage [86,87]. Surprisingly, there was no stress-induced change in O2•– content in Q. infectoria leaves, suggesting an upregulation of the production of non-enzymatic antioxidants to scavenge ROS, including the observed increase in anthocyanin, which has been reported to be involved in quenching O2•– [88].
Changes in membrane permeability and integrity are the first detectable signs of disease infestation by pathogens [89]. LOX is responsible for superoxide formation [90] and is also involved in membrane degradation as it catalyzes the deoxygenation of polyunsaturated fatty acids that have cytotoxic properties [91]. Costa et al. [92] also observed that the activation of LOX caused the degradation of chlorophyll. We found that pathogen infection as well as drought significantly increased LOX activity in Q. libani and even stronger increases occurred in infected seedlings under drought stress indicating that drought conditions exacerbate the adverse effects of charcoal disease pathogens (Figure 7). By contrast, pathogen infection and drought applied individually had no significant effect on LOX activity in Q. infectoria and even their combined effects only resulted in a moderate increase, much lower than the corresponding values seen in Q. libani. LOX activity has been shown to increase in response to pathogen infection and drought stress and is likely to be involved in the loss of membrane integrity and other cell damage [93,94,95], which is in accordance with our results. High LOX activity was associated with a significant increase in EL, which indicates lipid peroxidation.
Soluble proteins are synthesized in response to pathogen infection or drought stress and comprise all the enzymatic components of the antioxidant system and also proteins involved in osmotic adjustment [96,97]. Koç et al. [98] reported that the increase in TSP was related to the resistance of plants to fungal infection. In our study, drought and infection of charcoal disease agents led to significant increases in the TSP levels of Q. infectoria seedlings in all treatments, especially in the combined drought and pathogen treatments suggesting that water deficit aggravates the harmful effects of fungal pathogens. The lacking increase in foliar O2•–, LOX activity and in part also the EL data suggest that antioxidant enzymes must have constituted a sizeable portion of the protein pool and the partial recovery of transpiration at the end of the study hints at a role of soluble proteins in osmotic adjustment. Contrary to expectations, the TSP content in Q. libani foliage remained unaffected by the experimental treatments indicating that antioxidant enzymes play a minor role in its defense system, which was corroborated by the strong treatment-related increase in LOX activity, O2•– and EL.
Oxidative damage can be minimized by non-enzymatic antioxidants such as AA and GSH [99,100], which help maintain normal cell metabolism and play an important role in plant stress resistance [101,102,103]. Changes in the non-enzymatic antioxidant pool size are an indirect indicator of drought and disease resistance in plants [104,105]. In the current study, AA content was increased in pathogen-infected and drought-stressed seedlings of both oak species (except for Q. libani in the drought-only treatment), while GSH increases were only observed in Q. infectoria. Obolarina persica induced stronger effects on foliar AA content than B. mediterranea. Again, the highest values of AA and GR were observed in drought-stressed seedlings infected with charcoal disease confirming the pathogen-driven stimulation. Accumulation of AA and GSH may provide protection to cell membranes by scavenging the O2•– and other ROS, thereby limiting the hypersensitive response levels [85,94,106,107], which highlights the importance of non-enzymatic antioxidants in the protection against oxidative stress. Pathogen infection combined with drought stress induced an increase in mitochondrial AA and GSH which was linked to enhance pathogen resistance [108,109,110]. The increase in AA in drought-stressed and pathogen-infected seedlings could not prevent the accumulation of ROS in leaves of Q. libani indicating that the scavenging system became overtaxed.
5. Conclusions
Our findings clearly underline the importance of the interaction between abiotic and biotic stress on oak seedling health and performance, suggesting that the devastating effects of the charcoal disease in the Zagros oak forests are likely to become worse in the future given the projected increase in drought frequency and severity [111] together with the expected range expansion of the two fungal disease agents.
In this context, it is important to establish whether root anastomoses can form intraspecifically or perhaps even among the three oak species co-occurring in this region as this would allow rapid tree-to-tree pathogen transmission through hydraulic connections [112] which could greatly accelerate the spread of charcoal disease.
Our data further indicate that Q. libani seedlings are in many respects more sensitive to pathogen attack, drought and their interaction than young Q. infectoria. In naturally regenerating forest patches, we therefore anticipate a shift in community structure towards a dominance of the more resilient Q. infectoria and Q. brantii [23] resulting in a decline in abundance of insect herbivores, fungi, and microorganisms closely associated with Q. libani.
Our study provides vital information assisting policy-makers, forest owners and practitioners with the decision-making process in forest management and planning issues, especially with regard to future reforestation and afforestation programs in the Zagros forests.
Author Contributions
E.G., M.T.K., and M.Z. conceived and designed research. O.F., S.J., and I.P. conducted experiments. E.G. and O.F. analyzed data. E.G., M.K.-F.B. and G.A.P. wrote the manuscript. M.K.-F.B. reviewed and approved the final manuscript. All authors have read and approved the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
The authors are indebted to Tarbiat Modares University for providing the funding for this research. We are extremely grateful to Mansoureh Mirabolfathy for her valuable assistance with artificial inoculation and technical advice, to Seyed Ali Mohammad Modarres Sanavi for encouragement and some of the measurements and to Saham Mirzaei for his help in the greenhouse.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Holopainen, J.K.; Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef]
- Niinemets, Ü. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance and acclimation. For. Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar] [CrossRef]
- Ramegowda, V.; Senthil-Kumar, M. The interactive effects of simultaneous biotic and abiotic stresses on plants: Mechanistic understanding from drought and pathogen combination. J. Plant Physiol. 2015, 176, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Copolovici, L.; Väärtnõu, F.; Estrada, M.P.; Niinemets, Ü. Oak powdery mildew (Erysiphe alphitoides)-induced volatile emissions scale with the degree of infection in Quercus robur. Tree Physiol. 2014, 34, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Dikilitas, M.; Karakas, S.; Hashem, A.; Allah, E.A.; Ahmad, P. Oxidative stress and plant responses to pathogens under drought conditions. In Water Stress and Crop Plants; Wiley: Hoboken, NJ, USA, 2016; pp. 102–123. [Google Scholar]
- Vannini, A.; Lucero, G.; Anselmi, N.; Vettraino, A. Response of endophytic Biscogniauxia mediterranea to variation in leaf water potential of Quercus cerris. For. Pathol. 2009, 39, 8–14. [Google Scholar] [CrossRef]
- Cobb, R.C.; Ruthrof, K.X.; Breshears, D.D.; Lloret, F.; Aakala, T.; Adams, H.D.; Anderegg, W.R.L.; Ewers, B.E.; Galiano, L.; Grünzweig, J.M.; et al. Ecosystem dynamics and management after forest die-off: A global synthesis with conceptual state-and-transition models. Ecosphere 2017, 8, e02034. [Google Scholar] [CrossRef]
- Wood, J.D.; Knapp, B.O.; Muzika, R.-M.; Stambaugh, M.C.; Gu, L. The importance of drought–pathogen interactions in driving oak mortality events in the Ozark Border Region. Environ. Res. Lett. 2018, 13, 015004. [Google Scholar] [CrossRef]
- Freeman, A.J.; Hammond, W.M.; Dee, J.R.; Cobb, R.C.; Marek, S.M.; Adams, H.D. The effect of prescribed fire on Biscogniauxia infection and δ13C in an upland oak-pine forest. Forest Ecol. Manag. 2019, 451, 117525. [Google Scholar] [CrossRef]
- Mirabolfathy, M.; Ju, Y.-M.; Hsieh, H.-M.; Rogers, J.D. Obolarina persica sp. nov., associated with dying Quercus in Iran. Mycoscience 2013, 54, 315–320. [Google Scholar] [CrossRef]
- Safaee, D.; Khodaparast, S.A.; Mirabolfathy, M.; Mousanejad, S. A multiplex PCR-based technique for identification of Biscogniauxia mediterranea and Obolarina persica causing charcoal disease of oak trees in Zagros forests. For. Pathol. 2017, 47, e12330. [Google Scholar] [CrossRef]
- Capretti, P.; Battisti, A. Water stress and insect defoliation promote the colonization of Quercus cerris by the fungus Biscogniauxia mediterranea. For. Pathol. 2007, 37, 129–135. [Google Scholar] [CrossRef]
- Olson, J. Biscogniauxia (Hypoxylon) canker and dieback of trees. In Oklahoma Cooperative Ex-Tenison Service EPP-7620; O.S. University: Stillwater, OK, USA, 2013. [Google Scholar]
- Alidadi, A.; Kowsari, M.; Javan-Nikkhah, M.; Salehi Jouzani, G.R.; Ebrahimi Rastaghi, M. New pathogenic and endophytic fungal species associated with Persian oak in Iran. Eur. J. Plant Pathol. 2019, 155, 1017–1032. [Google Scholar] [CrossRef]
- Mazzaglia, A.; Anselmi, N.; Gasbarri, A.; Vannini, A. Development of a Polymerase Chain Reaction (PCR) assay for the specific detection of Biscogniauxia mediterranea living as an endophyte in oak tissues. Mycol. Res. 2001, 105, 952–956. [Google Scholar] [CrossRef]
- Collado, J.; Platas, G.; Pelaez, F. Identification of an endophytic Nodulisporium sp. from Quercus ilex in central Spain as the anamorph of Biscogniauxia mediterranea by rDNA sequence analysis and effect of different ecological factors on distribution of the fungus. Mycologia 2001, 93, 875–886. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Sirca, C.; Spano, D.; Franceschini, A. Variation of endophytic cork oak-associated fungal communities in relation to plant health and water stress. For. Pathol. 2010, 41, 193–201. [Google Scholar] [CrossRef][Green Version]
- Guo, R.; Wang, Z.; Zhou, C.; Huang, Y.; Haijuan, F.; Wang, Y.; Zhihua, L. Biocontrol potential of Trichoderma asperellum mutants T39 and T45 and their growth promotion of poplar seedlings. J. For. Res. 2020, 31, 1035–1043. [Google Scholar] [CrossRef]
- Safaee, D.; Khodaparast, S.A.; Mirabolfathy, M.; Sheikholeslami, M. Some aspects of biology and host range of Biscogniauxia mediterranea, one of the causal agent of oak charcoal disease. Mycol. Iran. 2017, 4, 121–129. [Google Scholar]
- Nouri, A.; Kiani, B.; Hakimi, M.H.; Mokhtari, H.M. Estimating oak forest parameters in the western mountains of Iran using satellite-based vegetation indices. J. For. Res. 2020, 31, 541–552. [Google Scholar] [CrossRef]
- Bucci, S.J.; Goldstein, G.; Scholz, F.G.; Meinzer, F.C. Physiological Significance of Hydraulic Segmentation, Nocturnal Transpiration and Capacitance in Tropical Trees: Paradigms Revisited. Tree Physiol. 2016, 6, 205–225. [Google Scholar] [CrossRef]
- Hossain, M.; Veneklaas, E.J.; Hardy, G.E.S.J.; Poot, P. Tree host–pathogen interactions as influenced by drought timing: Linking physiological performance, biochemical defense and disease severity. Tree Physiol. 2019, 39, 6–18. [Google Scholar] [CrossRef]
- Ghanbary, E.; Tabari Kouchaksaraei, M.; Mirabolfathy, M.; Modarres Sanavi, S.A.M.; Rahaei, M. Growth and physiological responses of Quercus brantii seedlings inoculated with Biscogniauxia mediterranea and Obolarina persica under drought stress. For. Pathol. 2017, 47, e12353. [Google Scholar] [CrossRef]
- Hajji, M.; Dreyer, E.; Marçais, B. Impact of Erysiphe alphitoides on transpiration and photosynthesis in Quercus robur leaves. Eur. J. Plant Pathol. 2009, 125, 63–72. [Google Scholar] [CrossRef]
- Karakaya, A.; Dikilitas, M. Biochemical, Physiological and molecular defense mechanisms of tea plants against pathogenic agents under changing climate conditions. In Stress Physiology of Tea in the Face of Climate Change; Springer: Singapore, 2018; pp. 241–268. [Google Scholar]
- Dinis, L.-T.; Peixoto, F.; Zhang, C.; Martins, L.; Costa, R.L.; Gomes-Laranjo, J. Physiological and biochemical changes in resistant and sensitive chestnut (Castanea) plantlets after inoculation with Phytophthora cinnamomi. Physiol. Mol. Plant Pathol. 2011, 75, 146–156. [Google Scholar] [CrossRef]
- Vasques, A.R.; Pinto, G.; Dias, M.C.; Correia, C.M.; Moutinho-Pereira, J.M.; Vallejo, V.R.; Santos, C.; Keizer, J.J. Physiological response to drought in seedlings of Pistacia lentiscus (mastic tree). New For. 2016, 47, 119–130. [Google Scholar] [CrossRef]
- Senthilkumar, P.; Thirugnanasambantham, K.; Mandal, A.K.A. Suppressive Subtractive Hybridization Approach Revealed Differential Expression of Hypersensitive Response and Reactive Oxygen Species Production Genes in Tea (Camellia sinensis (L.) O. Kuntze) Leaves during Pestalotiopsis thea Infection. Appl. Biochem. Biotechnol. 2012, 168, 1917–1927. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993, 125, 27–58. [Google Scholar] [CrossRef]
- Shukla, N.; Awasthi, R.; Rawat, L.; Kumar, J. Biochemical and physiological responses of rice (Oryza sativa L.) as influenced by Trichoderma harzianum under drought stress. Plant Physiol. Biochem. 2012, 54, 78–88. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, art129. [Google Scholar] [CrossRef]
- Garrett, K.A.; Dendy, S.P.; Frank, E.E.; Rouse, M.N.; Travers, S.E. Climate Change Effects on Plant Disease: Genomes to Ecosystems. Annu. Rev. Phytopathol. 2006, 44, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Hubbart, J.A.; Guyette, R.; Muzika, R.-M. More than Drought: Precipitation Variance, Excessive Wetness, Pathogens and the Future of the Western Edge of the Eastern Deciduous Forest. Sci. Total Environ. 2016, 566, 463–467. [Google Scholar] [CrossRef]
- Ghanbary, E.; Kouchaksaraei, M.T.; Guidi, L.; Mirabolfathy, M.; Etemad, V.; Sanavi, S.A.M.M.; Struve, D. Change in biochemical parameters of Persian oak (Quercus brantii Lindl.) seedlings inoculated by pathogens of charcoal disease under water deficit conditions. Trees 2018, 32, 1595–1608. [Google Scholar] [CrossRef]
- Ghanbary, E.; Tabari Kouchaksaraei, M.; Zarafshar, M.; Bader, M.K.-F.; Mirabolfathy, M.; Ziaei, M. Differential physiological and biochemical responses of Quercus infectoria and Q. libani to drought and charcoal disease. Physiol. Plant. 2020, 168, 876–892. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Sirca, C.; Spano, D.; Franceschini, A. Physiological responses of cork oak and holm oak to infection by fungal pathogens involved in oak decline. For. Pathol. 2009, 39, 232–238. [Google Scholar] [CrossRef]
- Oguchi, R.; Hikosaka, K.; Hiura, T.; Hirose, T. Leaf anatomy and light acclimation in woody seedlings after gap formation in a cool-temperate deciduous forest. Oecologia 2006, 149, 571–582. [Google Scholar] [CrossRef]
- Baltzer, J.L.; Thomas, S.C. Determinants of whole-plant light requirements in Bornean rain forest tree saplings. J. Ecol. 2007, 95, 1208–1221. [Google Scholar] [CrossRef]
- Parad, G.A.; Tabari Kouchaksaraei, M.; Striker, G.G.; Sadati, S.E.; Nourmohammadi, K. Growth, morphology and gas exchange responses of two-year-old Quercus castaneifolia seedlings to flooding stress. Scand. J. For. Res. 2015, 35, 458–466. [Google Scholar]
- Almeida, T.; Pinto, G.; Correia, B.; Santos, C.; Gonçalves, S. QsMYB1 expression is modulated in response to heat and drought stresses and during plant recovery in Quercus suber. Plant Physiol. Biochem. 2013, 73, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.J. Content and Vacuole/Extravacuole Distribution of Neutral Sugars, Free Amino Acids, and Anthocyanin in Protoplasts. Plant Physiol. 1979, 64, 88–93. [Google Scholar] [CrossRef]
- Nayyar, H. Accumulation of osmolytes and osmotic adjustment in water-stressed wheat (Triticum aestivum) and maize (Zea mays) as affected by calcium and its antagonists. Environ. Exp. Bot. 2003, 50, 253–264. [Google Scholar] [CrossRef]
- Axelrod, B.; Cheesbrough, T.M.; Laakso, S. Lipoxygenases from soybeans. In Methods in Enzymology; Lowenstein, J.M., Ed.; Academic Press: New York, NY, USA, 1981; pp. 441–451. [Google Scholar]
- Bai, T.; Li, C.; Ma, F.; Feng, F.; Shu, H. Responses of growth and antioxidant system to root-zone hypoxia stress in two Malus species. Plant Soil 2009, 327, 95–105. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- De Vos, C.H.R.; Vonk, M.J.; Vooijs, R.; Schat, H. Glutathione Depletion Due to Copper-Induced Phytochelatin Synthesis Causes Oxidative Stress in Silene cucubalus. Plant Physiol. 1992, 98, 853–858. [Google Scholar] [CrossRef]
- Singh, N.; Lena, Q. India (host institution) Lucknow 226001 Rana Pratap Marg National Botanical Research Institute; Srivastava, M.; Rathinasabapathi, B. Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L. and Pteris ensiformis L. Plant Sci. 2006, 170, 274–282. [Google Scholar] [CrossRef]
- Nowakowska, J.A.; Stocki, M.; Stocka, N.; Ślusarski, S.; Tkaczyk, M.; Caetano, J.M.; Tulik, M.; Hsiang, T.; Oszako, T. Interactions between Phytophthora cactorum, Armillaria gallica and Betula pendula Roth. Seedlings Subjected to Defoliation. Forests 2020, 11, 1107. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, D.D.; Zavala, J.A.; Zhu, J.; Clough, S.L.; Ort, D.R.; De Lucia, E.H. Biotic stress globally down regulates photosynthesis genes. Plant Cell Environ. 2010, 33, 1597–1613. [Google Scholar] [CrossRef] [PubMed]
- Ogaya, R.; Penuelas, J. Comparative field study of Quercus ilex and Phillyrea latifolia: Photosynthetic response to experimental drought conditions. Environ. Exp. Bot. 2003, 50, 137–148. [Google Scholar] [CrossRef]
- Corcobado, T.; Cubera, E.; Juárez, E.; Moreno, G.; Solla, A. Drought events determine performance of Quercus ilex seedlings and increase their susceptibility to Phytophthora cinnamomi. Agric. For. Meteorol. 2014, 192, 1–8. [Google Scholar] [CrossRef]
- Oszako, T.; Orlikowski, L.B. First data on the occurrence of Phytophthora cinnamomi on pedonculate oak in Poland. Sylwan 2005, 149, 47–53. [Google Scholar]
- Oszako, T.; Voitka, D.; Stocki, M.; Stocka, N.; Nowakowska, J.A.; Linkiewicz, A.; Hsiang, T.; Belbahri, L.; Berezovska, D.; Malewski, T. Trichoderma asperellum efficiently protects Quercus robur leaves against Erysiphe alphitoides. Eur. J. Plant Pathol. 2021, 159, 295–308. [Google Scholar] [CrossRef]
- Anderson, L.J.; Harley, P.C.; Monson, R.K.; Jackson, R.B. Reduction of isoprene emissions from live oak (Quercus fusiformis) with oak wilt. Tree Physiol. 2000, 20, 1199–1203. [Google Scholar] [CrossRef]
- Luque, J.; Cohen, M.; Savé, R.; Biel, C.; Álvarez, I.F. Effects of three fungal pathogens on water relations, chlorophyll fluorescence and growth of Quercus suber L. Ann. Sci. For. 1999, 56, 19–26. [Google Scholar] [CrossRef]
- Valladares, F.; Sanchez-Gomez, D. Ecophysiological traits associated with drought in Mediterranean tree seedlings: Individual responses versus interspecific trends in eleven species. Plant Biol. 2006, 8, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gallego, M.; Bader, M.K.-F.; Scott, P.M.; Leuzinger, S.; Williams, N.M. Phytophthora pluvialis Studies on Douglas-fir Require Swiss Needle Cast Suppression. Plant Dis. 2017, 101, 1259–1262. [Google Scholar] [CrossRef]
- Kalmatskaya, O.A.; Karavaev, V.A. The fluorescent indices of bean leaves treated with sodium fluoride. Biophysics 2015, 60, 843–848. [Google Scholar] [CrossRef]
- Ranjbar, A. Comparative study on the effect of water stress and rootstock on photosynthetic function in pistachio (Pistacia vera L). Trees 2017, 8, 151–159. [Google Scholar]
- Sun, J.; Zhang, Q.; Tabassum, M.A.; Ye, M.; Peng, S.; Li, Y. The inhibition of photosynthesis under water deficit conditions is more severe in flecked than uniform irradiance in rice (Oryza sativa) plants. Funct. Plant Biol. 2017, 44, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Andolfi, A.; Maddau, L.; Franceschini, A.; Marras, F. Biscopyran, a phytotoxic hexasubstituted pyranopyran produced by Biscogniauxia mediterranea, a fungus pathogen of cork oak. J. Nat. Prod. 2005, 68, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Farouk, S.; Abdul Qados, A.M.S. Osmotic adjustment and yield of cowpea in response to drought stress and chitosan. Indian J. Appl. Res. 2013, 3, 1–6. [Google Scholar] [CrossRef]
- Fernandez-Martinez, J.; Zacchini, M.; Elena, G.; Fernandez-Marin, B.; Fleck, I. Effect of environmental stress factors on ecophysiological traits and susceptibility to pathogens of five Populus clones throughout the growing season. Tree Physiol. 2013, 33, 618–627. [Google Scholar] [CrossRef]
- González, L.; González-Vilar, M. Determination of relative water content. In Handbook of Plant Eco-Physiology Techniques; Reigosa, M.J., Ed.; Springer: Dordrecht, The Netherlands, 2001; pp. 207–212. [Google Scholar]
- Hutchinson, T.C. Lime Chlorosis as a Factor in Seedling Establishment on Calcareous Soils. New Phytol. 1970, 69, 143–157. [Google Scholar] [CrossRef]
- Fassnacht, F.E.; Stenzel, S.; Gitelson, A.A. Non-destructive estimation of foliar carotenoid content of tree species using merged vegetation indices. J. Plant Physiol. 2015, 176, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Simon, U.K.; Polanschütz, L.M.; Koffler, B.E.; Zechmann, B. High Resolution Imaging of Temporal and Spatial Changes of Subcellular Ascorbate, Glutathione and H2O2 Distribution during Botrytis cinerea Infection in Arabidopsis. PLoS ONE 2013, 8, e65811. [Google Scholar] [CrossRef] [PubMed]
- Heuser, T.; Zimmer, W. Quantitative analysis of phytopathogenic ascomycota on leaves of pedunculate oaks (Quercus robur L.) by real-time PCR. FEMS Microbiol. Let. 2002, 209, 295–299. [Google Scholar] [CrossRef][Green Version]
- Chalker-Scott, L. Environmental Significance of Anthocyanins in Plant Stress Responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Karageorgou, P.; Manetas, Y. The importance of being red when young: Anthocyanins and the protection of young leaves of Quercus coccifera from insect herbivory and excess light. Tree Physiol. 2006, 26, 613–621. [Google Scholar] [CrossRef]
- Sivankalyani, V.; Feygenberg, O.; Diskin, S.; Wright, B.; Alkan, N. Increased anthocyanin and flavonoids in mango fruit peel are associated with cold and pathogen resistance. Postharvest Biol. Technol. 2016, 111, 132–139. [Google Scholar] [CrossRef]
- Hatier, J.H.B.; Gould, K.S. Anthocyanin Function in Vegetative Organs. In Anthocyanins; Winefield, C., Davies, K., Gould, K., Eds.; Springer: New York, NY, USA, 2008; pp. 1–19. [Google Scholar]
- Iriti, M.; Rossoni, M.; Borgo, M.; Faoro, F. Benzothiadiazole Enhances Resveratrol and Anthocyanin Biosynthesis in Grapevine, Meanwhile Improving Resistance to Botrytis cinerea. J. Agric. Food Chem. 2004, 52, 4406–4413. [Google Scholar] [CrossRef] [PubMed]
- Chiwetalu, U.J.; Mbajiorgu, C.C.; Ogbuagu, N.J. Remedial ability of maize (Zea mays) on lead contamination under potted condition and non-potted field soil condition. J. Bioresour. Bioprod. 2020, 5, 51–59. [Google Scholar] [CrossRef]
- Hughes, N.M.; Neufeld, H.S.; Burkey, K.O. Functional role of anthocyanins in high-light winter leaves of the evergreen herb Galax urceolata. New Phytol. 2005, 168, 575–587. [Google Scholar] [CrossRef]
- Coram, T.E.; Wang, M.; Chen, X. Transcriptome analysis of the wheat–Puccinia striiformis f. sp. tritici interaction. Mol. Plant Pathol. 2008, 9, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Del Río, L.A. ROS and RNS in plant physiology: An overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Jones, J.D.; Dangl, J.L. Reactive Oxygen Species Signaling in Response to Pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Asada, K. Production and Scavenging of Reactive Oxygen Species in Chloroplasts and Their Functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Y.; Wang, M.; Wang, C.; Ling, N.; Mur, L.A.; Shen, Q.; Guo, S. Redox imbalance contributed differently to membrane damage of cucumber leaves under water stress and Fusarium infection. Plant Sci. 2018, 274, 171–180. [Google Scholar] [CrossRef]
- Henmi, T.; Miyao, M.; Yamamoto, Y. Release and Reactive-Oxygen-Mediated Damage of the Oxygen-Evolving Complex Subunits of PSII during Photoinhibition. Plant Cell Physiol. 2004, 45, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Allakhverdiev, S.I.; Murata, N. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim. Biophys. Acta BBA Bioenerg. 2006, 1757, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Su, L.-J.; Chen, J.-W.; Zeng, X.-Q.; Sun, B.-Y.; Peng, C.-L. The antioxidative role of anthocyanins in Arabidopsis under high-irradiance. Biol. Plant. 2012, 56, 97–104. [Google Scholar] [CrossRef]
- Geat, N.; Singh, D.; Khirbat, S. Effect of Non-conventional Chemicals and Synthetic Fungicide on Biochemical Characteristics of Chilli against Fruit Rot Pathogen Colletotrichum capsici. J. Plant Pathol. Microbiol. 2016, 7, 605–613. [Google Scholar] [CrossRef]
- Ali, M.B.; Hahn, E.-J.; Paek, K.-Y. Effects of temperature on oxidative stress defense systems, lipid peroxidation and lipoxygenase activity in Phalaenopsis. Plant Physiol. Biochem. 2005, 43, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, R.; Nasibi, F.; Farahbakhsh, H. Effect of exogenous salicylic acid on some physiological parameters and alleviation of drought stress in Nigella sativa plants under hydroponic hulture. Plant Protec. Sci. 2014, 50, 43–51. [Google Scholar] [CrossRef]
- Costa, M.; Civell, P.M.; Chaves, A.R.; Martinez, G.A. Effects of ethephon and 6-benzylaminopurine on chlorophyll degrading enzymes and peroxidase-linked chlorophyll bleaching during post-harvest senescence of broccoli (Brassica oleracea L.) at 20 °C. Postharvest Biol. Technol. 2005, 35, 191–199. [Google Scholar] [CrossRef]
- Chakhchar, A.; Wahbi, S.; Lamaoui, M.; Ferradous, A.; El Mousadik, A.; Ibnsouda-Koraichi, S.; Filali-Maltouf, A.; El Modafar, C. Physiological and biochemical traits of drought tolerance in Argania spinosa. J. Plant Interact. 2015, 10, 252–261. [Google Scholar] [CrossRef]
- Liang, C.; Liu, T.; Zhao, Y.; Feng, Y.; Wan, T.; Cai, Y. Defense Responses of Cherry Rootstock ‘Gisela 6’ Elicited by Agrobacterium tumefaciens Infection. J. Plant Growth Regul. 2019, 38, 1082–1093. [Google Scholar] [CrossRef]
- Montillet, J.-L.; Agnel, J.-P.; Ponchet, M.; Vailleau, F.; Roby, M.; Triantaphylidès, C. Lipoxygenase-mediated production of fatty acid hydroperoxides is a specific signature of the hypersensitive reaction in plants. Plant Physiol. Biochem. 2002, 40, 633–639. [Google Scholar] [CrossRef]
- Fontaine, F.; Pinto, C.; Vallet, J.; Clément, C.; Gomes, A.C.; Spagnolo, A. The effects of grapevine trunk diseases (GTDs) on vine physiology. Eur. J. Plant Pathol. 2015, 144, 707–721. [Google Scholar] [CrossRef]
- Ge, Y.; Bi, Y.; Guest, D.I. Defense responses in leaves of resistant and susceptible melon (Cucumis melo L.) cultivars infected with Colletotrichum lagenarium. Physiol. Mol. Plant Pathol. 2013, 81, 13–21. [Google Scholar] [CrossRef]
- Koç, E.; Üstün, A.S.; Islek, C.; Arıcı, Y.K.; Arici, Y.K. Defence responses in leaves of resistant and susceptible pepper (Capsicum annuum L.) cultivars infected with different inoculum concentrations of Phytophthora capsici Leon. Sci. Hortic. 2011, 128, 434–442. [Google Scholar] [CrossRef]
- Liu, B.; Li, M.; Cheng, L.; Liang, N.; Zou, Y.; Ma, F. Influence of rootstock on antioxidant system in leaves and roots of young apple trees in response to drought stress. Plant Growth Regul. 2012, 67, 247–256. [Google Scholar] [CrossRef]
- Mohammadi, H.; Moradi, F. Effects of growth regulators on enzymatic and non-enzymatic antioxidants in leaves of two contrasting wheat cultivars under water stress. Braz. J. Bot. 2016, 39, 495–505. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Prochazkova, D.; Sairam, R.; Srivastava, G.; Singh, D. Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci. 2001, 161, 765–771. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Guha, A.; Sengupta, D.; Rasineni, G.K.; Reddy, A.R. Non-enzymatic antioxidative defense in drought-stressed mulberry (Morus indica L.) genotypes. Trees 2012, 26, 903–918. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Intracellular redox compartmentation and ROS-related communication in regulation and signaling. Plant Physiol. 2016, 171, 1581–1592. [Google Scholar] [CrossRef]
- Mullineaux, P.M.; Rausch, T. Glutathione, photosynthesis and the redox regulation of stress-responsive gene expression. Photosynth. Res. 2005, 86, 459–474. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Glutathione-induced drought stress tolerance in mung bean: Coordinated roles of the antioxidant defense and methylglyoxal detoxification systems. Aob Plants 2015, 7, plv069. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Pandey, P.; Senthil-Kumar, M. Tailored Responses to Simultaneous Drought Stress and Pathogen Infection in Plants. In Drought Stress Tolerance in Plants; Hossain, M., Wani, S., Bhattacharjee, S., Burritt, D., Tran, L.S., Eds.; Springer: Cham, Germany, 2016; Volume 1, pp. 427–438. [Google Scholar]
- Gong, B.; Sun, S.; Yan, Y.; Jing, X.; Shi, Q. Glutathione Metabolism and Its Function in Higher Plants Adapting to Stress. In Antioxidant Enzymes in Higher Plants; Gupta, D., Palma, J., Corpas, F., Eds.; Springer: Cham, Germany, 2018; pp. 181–205. [Google Scholar]
- Großkinsky, D.K.; Koffler, B.E.; Roitsch, T.; Maier, R.; Zechmann, B. Compartment-Specific Antioxidative Defense in Arabidopsis Against Virulent and Avirulent Pseudomonas syringae. Phytopathology 2012, 102, 662–673. [Google Scholar] [CrossRef]
- Modarres, R.; Sarhadi, A.; Burn, D.H. Changes of extreme drought and flood events in Iran. Glob. Planet. Chang. 2016, 144, 67–81. [Google Scholar] [CrossRef]
- Bader, M.-F.; Leuzinger, S. Hydraulic Coupling of a Leafless Kauri Tree Remnant to Conspecific Hosts. iScience 2019, 19, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).