Using a Bottom-Up Approach to Scale Leaf Photosynthetic Traits of Oil Palm, Rubber, and Two Coexisting Tropical Woody Species
Abstract
1. Introduction
2. Methods
2.1. Experimental Sites
2.2. Sampling Procedure and Gas Exchange Measurements
2.3. Response Curve Analyses
2.4. Leaf Nutrient Status and Specific Leaf Area
2.5. Theory for Within-Canopy Gradients in Photosynthetic Capacity
2.6. Measured Data-Sets
2.7. Scaling Up Photosynthetic Capacity and Data Availability
3. Results
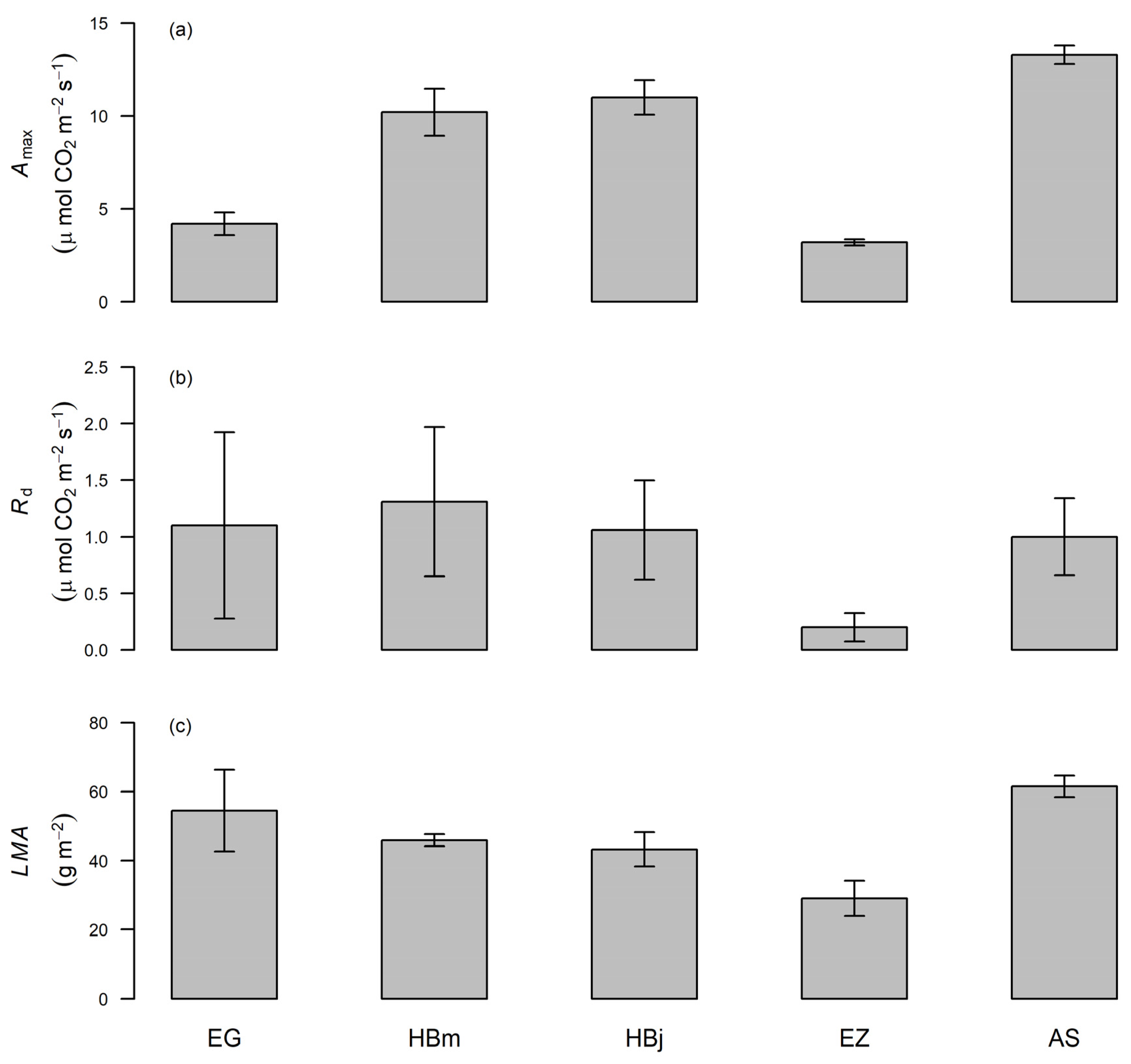
3.1. Variation of Photosynthetic Capacity at the Lower Part of the Canopy
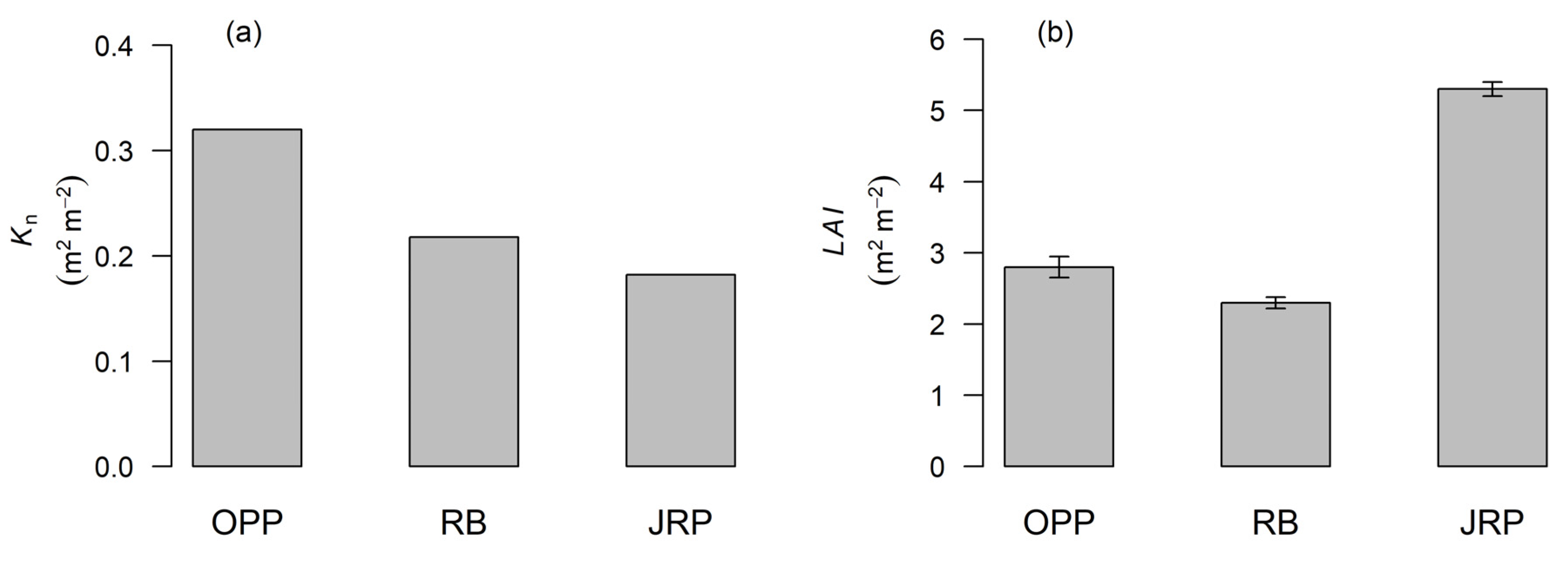
3.2. Extinction of Light in the Canopy Profile
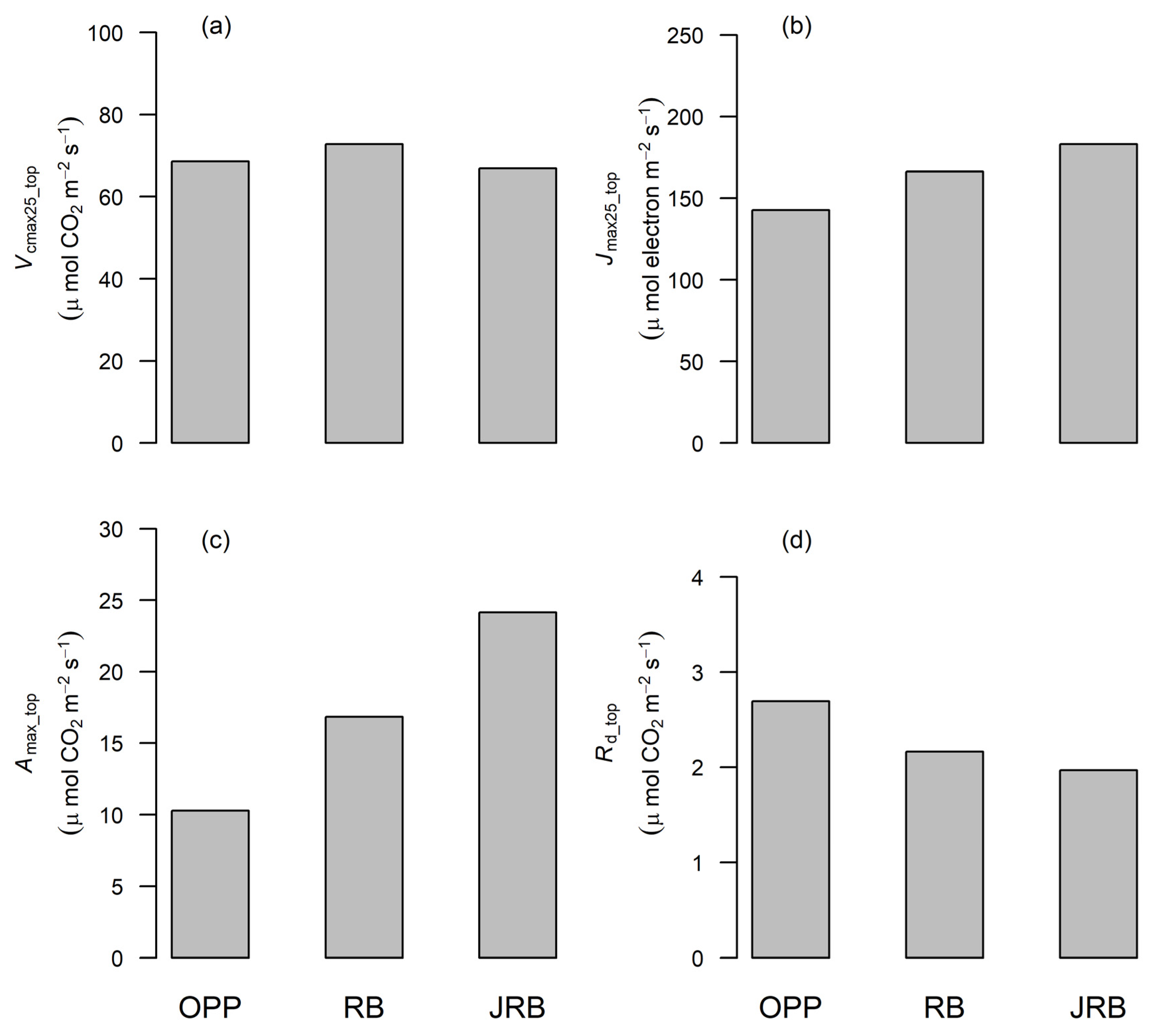
3.3. Variation of Photosynthetic Capacity at the Top of the Canopy
3.4. Area-Based Leaf Nitrogen Content and Leaf Mass Per Area
3.5. Within-Canopy Gradients for Forest Ecosystems
4. Discussion
4.1. Interspecific Variability in Photosynthetic Traits at the Bottom of the Canopy
4.2. Light Extinction in the Canopy Profile
4.3. Photosynthetic Trait Variability at the Top of the Canopy
4.4. Limitations and Implications of Scaling Method Used in This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Estimating Kn Values of Plantations
Appendix B. Leaf Area Index Measurements
Appendix C. Calculation of the Photosynthetic Capacity Using the Scaling Method
References
- Ziegler, A.D.; Phelps, J.; Yuen, J.Q.; Webb, E.L.; Lawrence, D.; Fox, J.M.; Bruun, T.B.; Leisz, S.J.; Ryan, C.M.; Dressler, W.; et al. Carbon Outcomes of Major Land-Cover Transitions in SE Asia: Great Uncertainties and REDD+ Policy Implications. Glob. Chang. Biol. 2012, 18, 3087–3099. [Google Scholar] [CrossRef]
- Houghton, R.A.; House, J.I.; Pongratz, J.; van der Werf, G.R.; DeFries, R.S.; Hansen, M.C.; Quéré, C.L.; Ramankutty, N. Carbon Emissions from Land Use and Land-Cover Change. Biogeosciences 2012, 9, 5125–5142. [Google Scholar] [CrossRef]
- Margono, B.A.; Turubanova, S.; Zhuravleva, I.; Potapov, P.; Tyukavina, A.; Baccini, A.; Goetz, S.; Hansen, M.C. Mapping and Monitoring Deforestation and Forest Degradation in Sumatra (Indonesia) Using Landsat Time Series Data Sets from 1990 to 2010. Environ. Res. Lett. 2012, 7, 034010. [Google Scholar] [CrossRef]
- Clough, Y.; Krishna, V.V.; Corre, M.D.; Darras, K.; Denmead, L.H.; Meijide, A.; Moser, S.; Musshoff, O.; Steinebach, S.; Veldkamp, E.; et al. Land-Use Choices Follow Profitability at the Expense of Ecological Functions in Indonesian Smallholder Landscapes. Nat. Commun. 2016, 7, 13137. [Google Scholar] [CrossRef] [PubMed]
- Luskin, M.S.; Christina, E.D.; Kelley, L.C.; Potts, M.D. Modern Hunting Practices and Wild Meat Trade in the Oil Palm Plantation-Dominated Landscapes of Sumatra, Indonesia. Hum. Ecol. 2014, 42, 35–45. [Google Scholar] [CrossRef]
- Rist, L.; Feintrenie, L.; Levang, P. The Livelihood Impacts of Oil Palm: Smallholders in Indonesia. Biodivers. Conserv. 2010, 19, 1009–1024. [Google Scholar] [CrossRef]
- Grass, I.; Kubitza, C.; Krishna, V.V.; Corre, M.D.; Mußhoff, O.; Pütz, P.; Drescher, J.; Rembold, K.; Ariyanti, E.S.; Barnes, A.D.; et al. Trade-Offs between Multifunctionality and Profit in Tropical Smallholder Landscapes. Nat. Commun. 2020, 11, 1186. [Google Scholar] [CrossRef] [PubMed]
- van Straaten, O.; Corre, M.D.; Wolf, K.; Tchienkoua, M.; Cuellar, E.; Matthews, R.B.; Veldkamp, E. Conversion of Lowland Tropical Forests to Tree Cash Crop Plantations Loses up to One-Half of Stored Soil Organic Carbon. Proc. Natl. Acad. Sci. USA 2015, 112, 9956–9960. [Google Scholar] [CrossRef]
- Kotowska, M.M.; Leuschner, C.; Triadiati, T.; Meriem, S.; Hertel, D. Quantifying Above- and Belowground Biomass Carbon Loss with Forest Conversion in Tropical Lowlands of Sumatra (Indonesia). Glob. Chang. Biol. 2015, 21, 3620–3634. [Google Scholar] [CrossRef]
- Guillaume, T.; Kotowska, M.M.; Hertel, D.; Knohl, A.; Krashevska, V.; Murtilaksono, K.; Scheu, S.; Kuzyakov, Y. Carbon Costs and Benefits of Indonesian Rainforest Conversion to Plantations. Nat. Commun. 2018, 9, 2388. [Google Scholar] [CrossRef]
- Allen, K.; Corre, M.D.; Tjoa, A.; Veldkamp, E. Soil Nitrogen-Cycling Responses to Conversion of Lowland Forests to Oil Palm and Rubber Plantations in Sumatra, Indonesia. PLoS ONE 2015, 10, e0133325. [Google Scholar] [CrossRef] [PubMed]
- Hassler, E.; Corre, M.D.; Tjoa, A.; Damris, M.; Utami, S.R.; Veldkamp, E. Soil Fertility Controls Soil–Atmosphere Carbon Dioxide and Methane Fluxes in a Tropical Landscape Converted from Lowland Forest to Rubber and Oil Palm Plantations. Biogeosciences 2015, 12, 5831–5852. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A Biochemical Model of Photosynthetic CO2 Assimilation in Leaves of C3 Species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.T.; Woodrow, I.E.; Berry, J.A. A Model Predicting Stomatal Conductance and its Contribution to the Control of Photosynthesis under Different Environmental Conditions. In Progress in Photosynthesis Research: Volume 4, Proceedings of the VIIth International Congress on Photosynthesis Providence, Rhode Island, USA, 10–15 August 1986; Biggins, J., Ed.; Springer: Dordrecht, The Netherlands, 1987; pp. 221–224. ISBN 978-94-017-0519-6. [Google Scholar]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting Photosynthetic Carbon Dioxide Response Curves for C3 Leaves. Plant Cell Environ. 2007, 30, 1035–1040. [Google Scholar] [CrossRef]
- Kumarathunge, D.P.; Medlyn, B.E.; Drake, J.E.; Rogers, A.; Tjoelker, M.G. No Evidence for Triose Phosphate Limitation of Light-Saturated Leaf Photosynthesis under Current Atmospheric CO2 Concentration. Plant Cell Environ. 2019. [Google Scholar] [CrossRef]
- Stefanski, A.; Bermudez, R.; Sendall, K.M.; Montgomery, R.A.; Reich, P.B. Surprising Lack of Sensitivity of Biochemical Limitation of Photosynthesis of Nine Tree Species to Open-Air Experimental Warming and Reduced Rainfall in a Southern Boreal Forest. Glob. Chang. Biol. 2019. [Google Scholar] [CrossRef]
- Rogers, A.; Medlyn, B.E.; Dukes, J.S.; Bonan, G.; von Caemmerer, S.; Dietze, M.C.; Kattge, J.; Leakey, A.D.B.; Mercado, L.M.; Niinemets, Ü.; et al. A Roadmap for Improving the Representation of Photosynthesis in Earth System Models. New Phytol. 2017, 213, 22–42. [Google Scholar] [CrossRef] [PubMed]
- Lombardozzi, D.L.; Smith, N.G.; Cheng, S.J.; Dukes, J.S.; Sharkey, T.D.; Rogers, A.; Fisher, R.; Bonan, G.B. Triose Phosphate Limitation in Photosynthesis Models Reduces Leaf Photosynthesis and Global Terrestrial Carbon Storage. Environ. Res. Lett. 2018, 13, 074025. [Google Scholar] [CrossRef]
- Ellsworth, D.S.; Crous, K.Y.; Lambers, H.; Cooke, J. Phosphorus Recycling in Photorespiration Maintains High Photosynthetic Capacity in Woody Species. Plant Cell Environ. 2015, 38, 1142–1156. [Google Scholar] [CrossRef]
- Wullschleger, S.D. Biochemical Limitations to Carbon Assimilation in C3 Plants—A Retrospective Analysis of the A/Ci Curves from 109 Species. J. Exp. Bot. 1993, 44, 907–920. [Google Scholar] [CrossRef]
- Medlyn, B.E.; Badeck, F.-W.; De Pury, D.G.G.; Barton, C.V.M.; Broadmeadow, M.; Ceulemans, R.; Angelis, P.D.; Forstreuter, M.; Jach, M.E.; Kellomäki, S.; et al. Effects of Elevated [CO2] on Photosynthesis in European Forest Species: A Meta-Analysis of Model Parameters. Plant Cell Environ. 1999, 22, 1475–1495. [Google Scholar] [CrossRef]
- Bonan, G.B.; Levis, S.; Sitch, S.; Vertenstein, M.; Oleson, K.W. A Dynamic Global Vegetation Model for Use with Climate Models: Concepts and Description of Simulated Vegetation Dynamics. Glob. Chang. Biol. 2003, 9, 1543–1566. [Google Scholar] [CrossRef]
- Kattge, J.; Knorr, W.; Raddatz, T.; Wirth, C. Quantifying Photosynthetic Capacity and Its Relationship to Leaf Nitrogen Content for Global-Scale Terrestrial Biosphere Models. Glob. Chang. Biol. 2009, 15, 976–991. [Google Scholar] [CrossRef]
- Bonan, G.B.; Lawrence, P.J.; Oleson, K.W.; Levis, S.; Jung, M.; Reichstein, M.; Lawrence, D.M.; Swenson, S.C. Improving Canopy Processes in the Community Land Model Version 4 (CLM4) Using Global Flux Fields Empirically Inferred from FLUXNET Data. J. Geophys. Res. Biogeosciences 2011, 116. [Google Scholar] [CrossRef]
- Rogers, A. The Use and Misuse of V(c,Max) in Earth System Models. Photosyn. Res. 2014, 119, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.C.; Winner, W.E. Photosynthetic Differences between Saplings and Adult Trees: An Integration of Field Results by Meta-Analysis. Tree Physiol. 2002, 22, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Coste, S.; Roggy, J.-C.; Imbert, P.; Born, C.; Bonal, D.; Dreyer, E. Leaf Photosynthetic Traits of 14 Tropical Rain Forest Species in Relation to Leaf Nitrogen Concentration and Shade Tolerance. Tree Physiol. 2005, 25, 1127–1137. [Google Scholar] [CrossRef]
- Vincent, G. Leaf Life Span Plasticity in Tropical Seedlings Grown under Contrasting Light Regimes. Ann. Bot. 2006, 97, 245–255. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J.; Wright, I.J. Leaf Phosphorus Influences the Photosynthesis-Nitrogen Relation: A Cross-Biome Analysis of 314 Species. Oecologia 2009, 160, 207–212. [Google Scholar] [CrossRef]
- Ely, K.S.; Rogers, A.; Agarwal, D.A.; Ainsworth, E.A.; Albert, L.P.; Ali, A.; Anderson, J.; Aspinwall, M.J.; Bellasio, C.; Bernacchi, C.; et al. A Reporting Format for Leaf-Level Gas Exchange Data and Metadata. Ecol. Inform. 2021, 61, 101232. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From Tropics to Tundra: Global Convergence in Plant Functioning. Proc. Natl. Acad. Sci. USA 1997, 94, 13730. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Xu, C.; Rogers, A.; McDowell, N.G.; Medlyn, B.E.; Fisher, R.A.; Wullschleger, S.D.; Reich, P.B.; Vrugt, J.A.; Bauerle, W.L.; et al. Global-Scale Environmental Control of Plant Photosynthetic Capacity. Ecol. Appl. 2015, 25, 2349–2365. [Google Scholar] [CrossRef] [PubMed]
- Gouyon, A.; de Foresta, H.; Levang, P. Does ‘Jungle Rubber’ Deserve Its Name? An Analysis of Rubber Agroforestry Systems in Southeast Sumatra. Agrofor. Syst. 1993, 22, 181–206. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Chen, C. Below-Ground Interspecific Competition for Water in a Rubber Agroforestry System May Enhance Water Utilization in Plants. Sci. Rep. 2016, 6, 19502. [Google Scholar] [CrossRef]
- Baltzer, J.L.; Thomas, S.C.; Nilus, R.; Burslem, D.F.R.P. Edaphic Specialization in Tropical Trees: Physiological Correlates and Responses to Reciprocal Transplantation. Ecology 2005, 86, 3063–3077. [Google Scholar] [CrossRef]
- Kurokawa, H.; Yoshida, T.; Nakamura, T.; Lai, J.; Nakashizuka, T. The Age of Tropical Rain-Forest Canopy Species, Borneo Ironwood (Eusideroxylon Zwageri), Determined by 14C Dating. J. Trop. Ecol. 2003, 19, 1–7. [Google Scholar] [CrossRef]
- Irawan, B. Growth Performance of One Year Old Seedlings of Ironwood (Eusideroxylon Zwageri Teijsm. & Binn.) Varieties. J. Manaj. Hutan Trop. 2012, 18, 184–190. [Google Scholar]
- de Pury, D.G.G.; Farquhar, G.D. Simple Scaling of Photosynthesis from Leaves to Canopies without the Errors of Big-Leaf Models. Plant Cell Environ. 1997, 20, 537–557. [Google Scholar] [CrossRef]
- Leuning, R.; Kelliher, F.M.; de Pury, D.G.G.; Schulze, E.-D. Leaf Nitrogen, Photosynthesis, Conductance and Transpiration: Scaling from Leaves to Canopies. Plant Cell Environ. 1995, 18, 1183–1200. [Google Scholar] [CrossRef]
- Drescher, J.; Rembold, K.; Allen, K.; Beckschäfer, P.; Buchori, D.; Clough, Y.; Faust, H.; Fauzi, A.M.; Gunawan, D.; Hertel, D.; et al. Ecological and Socio-Economic Functions across Tropical Land Use Systems after Rainforest Conversion. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371. [Google Scholar] [CrossRef] [PubMed]
- Kotowska, M.M.; Leuschner, C.; Triadiati, T.; Hertel, D. Conversion of Tropical Lowland Forest Reduces Nutrient Return through Litterfall, and Alters Nutrient Use Efficiency and Seasonality of Net Primary Production. Oecologia 2016, 180, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Meijide, A.; Badu, C.S.; Moyano, F.; Tiralla, N.; Gunawan, D.; Knohl, A. Impact of Forest Conversion to Oil Palm and Rubber Plantations on Microclimate and the Role of the 2015 ENSO Event. Agric. For. Meteorol. 2018, 252, 208–219. [Google Scholar] [CrossRef]
- Allen, K.; Corre, M.D.; Kurniawan, S.; Utami, S.R.; Veldkamp, E. Spatial Variability Surpasses Land-Use Change Effects on Soil Biochemical Properties of Converted Lowland Landscapes in Sumatra, Indonesia. Geoderma 2016, 284, 42–50. [Google Scholar] [CrossRef]
- Hassler, E.; Corre, M.D.; Kurniawan, S.; Veldkamp, E. Soil Nitrogen Oxide Fluxes from Lowland Forests Converted to Smallholder Rubber and Oil Palm Plantations in Sumatra, Indonesia. Biogeosciences 2017, 14, 2781–2798. [Google Scholar] [CrossRef]
- Chandrashekar, T.R.; Nazeer, M.A.; Marattukalam, J.G.; Prakash, G.P.; Annamalainathan, K.; Thomas, J. An Analysis of Growth and Drought Tolerance in Rubber during the Immature Phase in a Dry Subhumid Climate. Exp. Agric. 1998, 34, 287–300. [Google Scholar] [CrossRef]
- Saputra, J.; Stevanus, C.T.; Cahyo, A.N. The Effect of El-Nino 2015 on the Rubber Plant (Hevea Brasiliensis) Growth in the Experimental Field Sembawa Research Centre. Widyariset 2016, 2, 37–46. [Google Scholar] [CrossRef][Green Version]
- Purwaningrum, Y.; Asbur, Y. Junaidi Latex Quality and Yield Parameters of Hevea Brasiliensis (Willd. Ex A. Juss.) Mull.Arg.Clone PB260 for Different Tapping and Stimulant Application Frequencies. Chil. J. Agric. Res. 2019, 79, 347–355. [Google Scholar] [CrossRef]
- Cahyo, A.N.; Stevanus, C.T.; Aji, M. Production of PB260 Rubber Clone in Relation with Field Water Balance. In Proceedings of the International Rubber Conference, Jakarta, Indonesia, 15–18 November 2017; pp. 763–773. [Google Scholar]
- Albert, L.P.; Wu, J.; Prohaska, N.; de Camargo, P.B.; Huxman, T.E.; Tribuzy, E.S.; Ivanov, V.Y.; Oliveira, R.S.; Garcia, S.; Smith, M.N.; et al. Age-Dependent Leaf Physiology and Consequences for Crown-Scale Carbon Uptake during the Dry Season in an Amazon Evergreen Forest. New Phytol. 2018, 219, 870–884. [Google Scholar] [CrossRef]
- Koch, G.W.; Amthor, J.S.; Goulden, M.L. Diurnal Patterns of Leaf Photosynthesis, Conductance and Water Potential at the Top of a Lowland Rain Forest Canopy in Cameroon: Measurements from the Radeau Des Cimes. Tree Physiol. 1994, 14, 347–360. [Google Scholar] [CrossRef]
- von Caemmerer, S.; Farquhar, G.D. Some Relationships between the Biochemistry of Photosynthesis and the Gas Exchange of Leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef]
- Duursma, R.A. Plantecophys—An R Package for Analysing and Modelling Leaf Gas Exchange Data. PLoS ONE 2015, 10, e0143346. [Google Scholar] [CrossRef] [PubMed]
- Medlyn, B.E.; Dreyer, E.; Ellsworth, D.; Forstreuter, M.; Harley, P.C.; Kirschbaum, M.U.F.; Le Roux, X.; Montpied, P.; Strassemeyer, J.; Walcroft, A.; et al. Temperature Response of Parameters of a Biochemically Based Model of Photosynthesis. II. A Review of Experimental Data. Plant Cell Environ. 2002, 25, 1167–1179. [Google Scholar] [CrossRef]
- Farquhar, G.; Wong, S. An Empirical Model of Stomatal Conductance. Funct. Plant Biol. 1984, 11, 191–210. [Google Scholar] [CrossRef]
- Norby, R.J.; Gu, L.; Haworth, I.C.; Jensen, A.M.; Turner, B.L.; Walker, A.P.; Warren, J.M.; Weston, D.J.; Xu, C.; Winter, K. Informing Models through Empirical Relationships between Foliar Phosphorus, Nitrogen and Photosynthesis across Diverse Woody Species in Tropical Forests of Panama. New Phytol. 2017, 215, 1425–1437. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Keenan, T.F.; Hallik, L. A Worldwide Analysis of Within-Canopy Variations in Leaf Structural, Chemical and Physiological Traits across Plant Functional Types. New Phytol. 2015, 205, 973–993. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.; Patiño, S.; Paiva, R.Q.; Nardoto, G.B.; Quesada, C.A.; Santos, A.J.B.; Baker, T.R.; Brand, W.A.; Hilke, I.; Gielmann, H.; et al. Optimisation of Photosynthetic Carbon Gain and Within-Canopy Gradients of Associated Foliar Traits for Amazon Forest Trees. Biogeosciences 2010, 7, 1833–1859. [Google Scholar] [CrossRef]
- Lowman, M.D. Leaf Growth Dynamics and Herbivory in Five Species of Australian Rain- Forest Canopy Trees. J. Ecol. 1992, 80, 433–447. [Google Scholar] [CrossRef]
- Kitajima, K.; Mulkey, S.S.; Wright, S.J. Variation in Crown Light Utilization Characteristics among Tropical Canopy Trees. Ann. Bot. 2005, 95, 535–547. [Google Scholar] [CrossRef]
- Posada, J.M.; Lechowicz, M.J.; Kitajima, K. Optimal Photosynthetic Use of Light by Tropical Tree Crowns Achieved by Adjustment of Individual Leaf Angles and Nitrogen Content. Ann. Bot. 2009, 103, 795–805. [Google Scholar] [CrossRef]
- Rijkers, T.; Pons, T.L.; Bongers, F. The Effect of Tree Height and Light Availability on Photosynthetic Leaf Traits of Four Neotropical Species Differing in Shade Tolerance. Funct. Ecol. 2000, 14, 77–86. [Google Scholar] [CrossRef]
- Thomas, S.C.; Bazzaz, F.A. Asymptotic Height as a Predictor of Photosynthetic Characteristics in Malaysian Rain Forest Trees. Ecology 1999, 80, 1607–1622. [Google Scholar] [CrossRef]
- Domingues, T.F.; Berry, J.A.; Martinelli, L.A.; Ometto, J.P.H.B.; Ehleringer, J.R. Parameterization of Canopy Structure and Leaf-Level Gas Exchange for an Eastern Amazonian Tropical Rain Forest (Tapajós National Forest, Pará, Brazil). Earth Interact. 2005, 9, 1–23. [Google Scholar] [CrossRef]
- Buckley, T.; Miller, J.; Farquhar, G. The Mathematics of Linked Optimisation for Water and Nitrogen Use in a Canopy. Silva Fenn. 2002, 36, 639–669. [Google Scholar] [CrossRef]
- Meir, P.; Kruijt, B.; Broadmeadow, M.; Barbosa, E.; Kull, O.; Carswell, F.; Nobre, A.; Jarvis, P.G. Acclimation of Photosynthetic Capacity to Irradiance in Tree Canopies in Relation to Leaf Nitrogen Concentration and Leaf Mass per Unit Area. Plant Cell Environ. 2002, 25, 343–357. [Google Scholar] [CrossRef]
- Wright, I.J.; Leishman, M.R.; Read, C.; Westoby, M. Gradients of Light Availability and Leaf Traits with Leaf Age and Canopy Position in 28 Australian Shrubs and Trees. Funct. Plant Biol. 2006, 33, 407–419. [Google Scholar] [CrossRef]
- Kosugi, Y.; Takanashi, S.; Yokoyama, N.; Philip, E.; Kamakura, M. Vertical Variation in Leaf Gas Exchange Parameters for a Southeast Asian Tropical Rainforest in Peninsular Malaysia. J. Plant Res. 2012, 125, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü.; Tenhunen, J.D. A Model Separating Leaf Structural and Physiological Effects on Carbon Gain along Light Gradients for the Shade-Tolerant Species Acer Saccharum. Plant Cell Environ. 1997, 20, 845–866. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kull, O.; Tenhunen, J.D. An Analysis of Light Effects on Foliar Morphology, Physiology, and Light Interception in Temperate Deciduous Woody Species of Contrasting Shade Tolerance. Tree Physiol. 1998, 18, 681–696. [Google Scholar] [CrossRef]
- Lawrence, D.M.; Fisher, R.A.; Koven, C.D.; Oleson, K.W.; Swenson, S.C.; Bonan, G.; Collier, N.; Ghimire, B.; van Kampenhout, L.; Kennedy, D.; et al. The Community Land Model Version 5: Description of New Features, Benchmarking, and Impact of Forcing Uncertainty. J. Adv. Modeling Earth Syst. 2019, 11, 4245–4287. [Google Scholar] [CrossRef]
- Fisher, R.A.; Wieder, W.R.; Sanderson, B.M.; Koven, C.D.; Oleson, K.W.; Xu, C.; Fisher, J.B.; Shi, M.; Walker, A.P.; Lawrence, D.M. Parametric Controls on Vegetation Responses to Biogeochemical Forcing in the CLM5. J. Adv. Modeling Earth Syst. 2019, 11, 2879–2895. [Google Scholar] [CrossRef]
- Kumagai, T.; Mudd, R.G.; Miyazawa, Y.; Liu, W.; Giambelluca, T.W.; Kobayashi, N.; Lim, T.K.; Jomura, M.; Matsumoto, K.; Huang, M.; et al. Simulation of Canopy CO2/H2O Fluxes for a Rubber (Hevea Brasiliensis) Plantation in Central Cambodia: The Effect of the Regular Spacing of Planted Trees. Ecol. Model. 2013, 265, 124–135. [Google Scholar] [CrossRef]
- Olchev, A.; Radler, K.; Sogachev, A.; Panferov, O.; Gravenhorst, G. Application of a Three-Dimensional Model for Assessing Effects of Small Clear-Cuttings on Radiation and Soil Temperature. Ecol. Model. 2009, 220, 3046–3056. [Google Scholar] [CrossRef]
- Widlowski, J.-L.; Pinty, B.; Clerici, M.; Dai, Y.; De Kauwe, M.; de Ridder, K.; Kallel, A.; Kobayashi, H.; Lavergne, T.; Ni-Meister, W.; et al. RAMI4PILPS: An Intercomparison of Formulations for the Partitioning of Solar Radiation in Land Surface Models. J. Geophys. Res. Biogeosci. 2011, 116. [Google Scholar] [CrossRef]
- Rival, A. Achieving Sustainable Cultivation of Oil Palm Volume 1: Introduction, Breeding and Cultivation Techniques, 1st ed.; Burleigh Dodds Science Publishing: Hong Kong, China, 2018. [Google Scholar]
- Kenzo, T.; Ichie, T.; Watanabe, Y.; Yoneda, R.; Ninomiya, I.; Koike, T. Changes in Photosynthesis and Leaf Characteristics with Tree Height in Five Dipterocarp Species in a Tropical Rain Forest. Tree Physiol. 2006, 26, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Meir, P.; Levy, P.E.; Grace, J.; Jarvis, P.G. Photosynthetic Parameters from Two Contrasting Woody Vegetation Types in West Africa. Plant Ecol. 2007, 192, 277–287. [Google Scholar] [CrossRef]
- Leuning, R.; Dunin, F.X.; Wang, Y.-P. A Two-Leaf Model for Canopy Conductance, Photosynthesis and Partitioning of Available Energy. II. Comparison with Measurements. Agric. For. Meteorol. 1998, 91, 113–125. [Google Scholar] [CrossRef]
- Kositsup, B.; Kasemsap, P.; Thanisawanyangkura, S.; Chairungsee, N.; Satakhun, D.; Teerawatanasuk, K.; Ameglio, T.; Thaler, P. Effect of Leaf Age and Position on Light-Saturated CO2 Assimilation Rate, Photosynthetic Capacity, and Stomatal Conductance in Rubber Trees. Photosynthetica 2010, 48, 67–78. [Google Scholar] [CrossRef]
- Corley, R.H.V. Photosynthesis and Age of Oil Palm Leaves. Photosynthetica 1983, 17, 97–100. [Google Scholar]
- Apichatmeta, K.; Sudsiri, C.J.; Ritchie, R.J. Photosynthesis of Oil Palm (Elaeis Guineensis). Sci. Hortic. 2017, 214, 34–40. [Google Scholar] [CrossRef]
- Meijide, A.; Röll, A.; Fan, Y.; Herbst, M.; Niu, F.; Tiedemann, F.; June, T.; Rauf, A.; Hölscher, D.; Knohl, A. Controls of Water and Energy Fluxes in Oil Palm Plantations: Environmental Variables and Oil Palm Age. Agric. For. Meteorol. 2017, 239, 71–85. [Google Scholar] [CrossRef]
- Stiegler, C.; Meijide, A.; Fan, Y.; Ashween Ali, A.; June, T.; Knohl, A. El Niño–Southern Oscillation (ENSO) Event Reduces CO2 Uptake of an Indonesian Oil Palm Plantation. Biogeosciences 2019, 16, 2873–2890. [Google Scholar] [CrossRef]
- Fan, Y.; Roupsard, O.; Bernoux, M.; Le Maire, G.; Panferov, O.; Kotowska, M.M.; Knohl, A. A Sub-Canopy Structure for Simulating Oil Palm in the Community Land Model (CLM-Palm): Phenology, Allocation and Yield. Geosci. Model Dev. 2015, 8, 3785–3800. [Google Scholar] [CrossRef]
- Ibrom, A.; Oltchev, A.; June, T.; Kreilein, H.; Rakkibu, G.; Ross, T.; Panferov, O.; Gravenhorst, G. Variation in Photosynthetic Light-Use Efficiency in a Mountainous Tropical Rain Forest in Indonesia. Tree Physiol. 2008, 28, 499–508. [Google Scholar] [CrossRef]
- Pearse, G.D.; Watt, M.S.; Morgenroth, J. Comparison of Optical LAI Measurements under Diffuse and Clear Skies after Correcting for Scattered Radiation. Agric. For. Meteorol. 2016, 221, 61–70. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.A.; Nugroho, B.; Moyano, F.E.; Brambach, F.; Jenkins, M.W.; Pangle, R.; Stiegler, C.; Blei, E.; Cahyo, A.N.; Olchev, A.; et al. Using a Bottom-Up Approach to Scale Leaf Photosynthetic Traits of Oil Palm, Rubber, and Two Coexisting Tropical Woody Species. Forests 2021, 12, 359. https://doi.org/10.3390/f12030359
Ali AA, Nugroho B, Moyano FE, Brambach F, Jenkins MW, Pangle R, Stiegler C, Blei E, Cahyo AN, Olchev A, et al. Using a Bottom-Up Approach to Scale Leaf Photosynthetic Traits of Oil Palm, Rubber, and Two Coexisting Tropical Woody Species. Forests. 2021; 12(3):359. https://doi.org/10.3390/f12030359
Chicago/Turabian StyleAli, Ashehad A., Branindityo Nugroho, Fernando E. Moyano, Fabian Brambach, Michael W. Jenkins, Robert Pangle, Christian Stiegler, Emanuel Blei, Andi Nur Cahyo, Alexander Olchev, and et al. 2021. "Using a Bottom-Up Approach to Scale Leaf Photosynthetic Traits of Oil Palm, Rubber, and Two Coexisting Tropical Woody Species" Forests 12, no. 3: 359. https://doi.org/10.3390/f12030359
APA StyleAli, A. A., Nugroho, B., Moyano, F. E., Brambach, F., Jenkins, M. W., Pangle, R., Stiegler, C., Blei, E., Cahyo, A. N., Olchev, A., Irawan, B., Ariani, R., June, T., Tarigan, S., Corre, M. D., Veldkamp, E., & Knohl, A. (2021). Using a Bottom-Up Approach to Scale Leaf Photosynthetic Traits of Oil Palm, Rubber, and Two Coexisting Tropical Woody Species. Forests, 12(3), 359. https://doi.org/10.3390/f12030359








