Effects of Fertilization on Wood Formation in Naturally Regenerated Juvenile Silver Birch in a Norway Spruce Stand in South Sweden
Abstract
:1. Introduction
- (1)
- increase growth ring width;
- (2)
- decrease cell wall thickness;
- (3)
- decrease wood density.
2. Materials and Methods
3. Results
3.1. Differences in Anatomy between Treatments
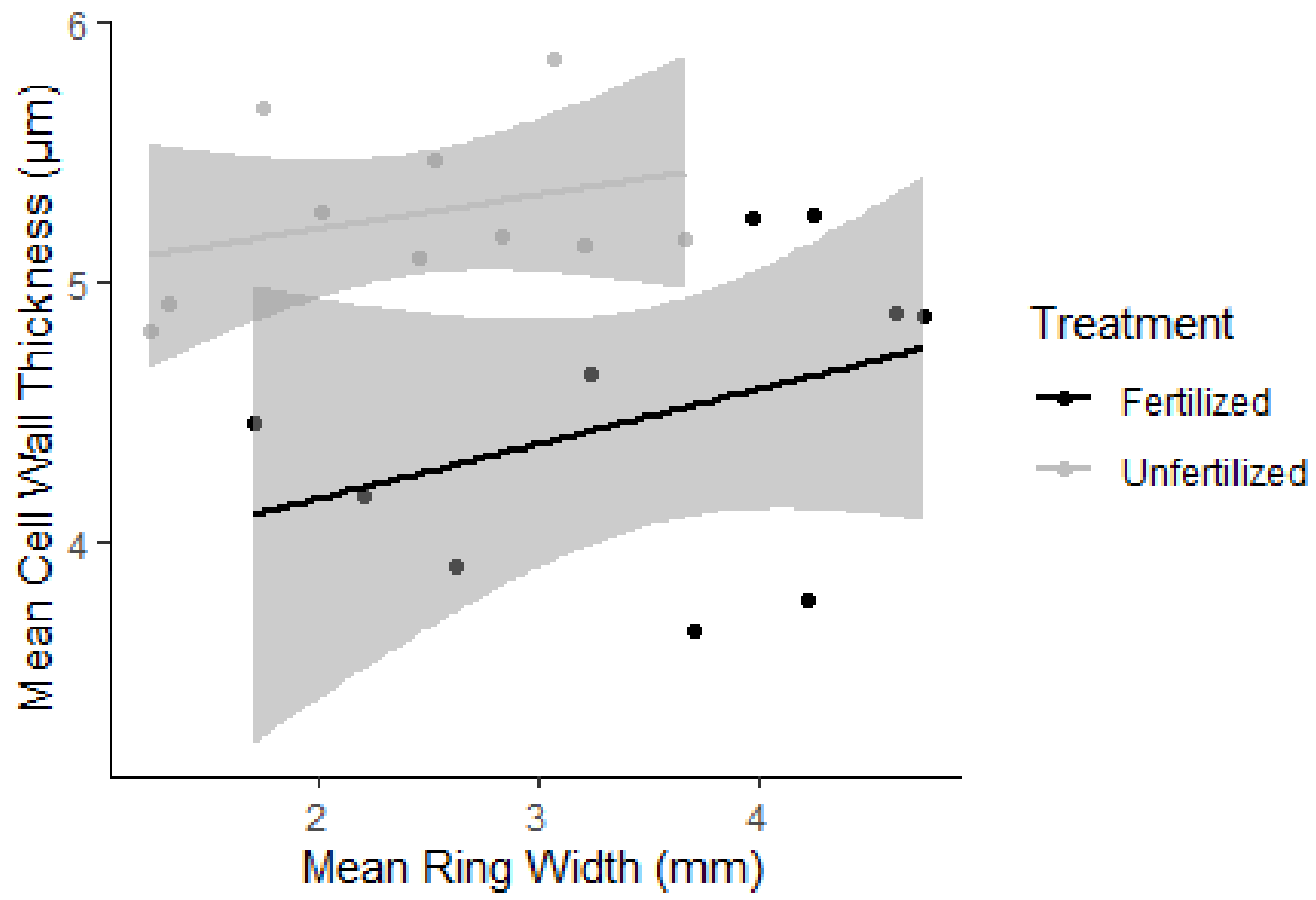
3.2. Growth Rate and Wood Anatomy
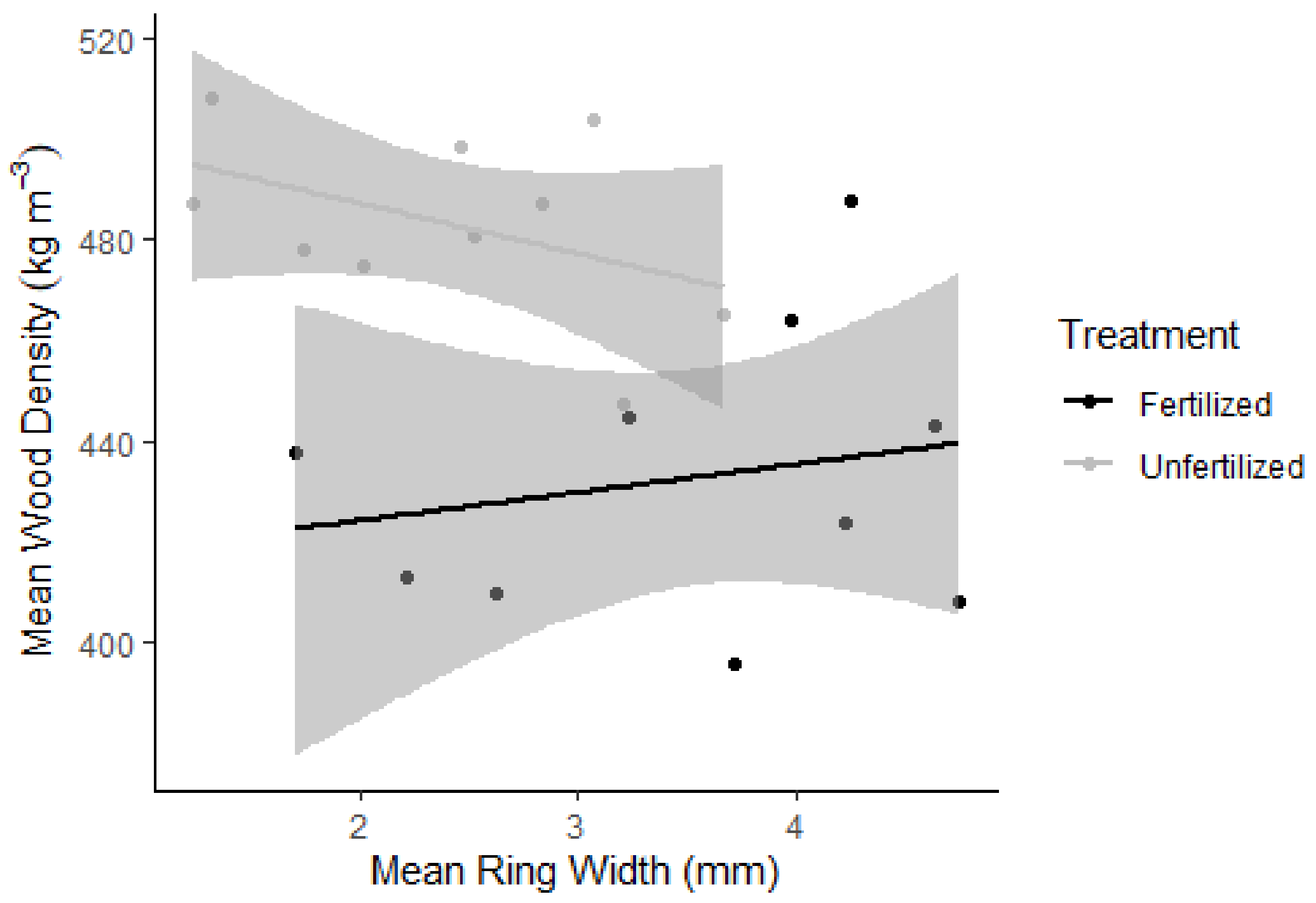
3.3. Growth Rate and Wood Density
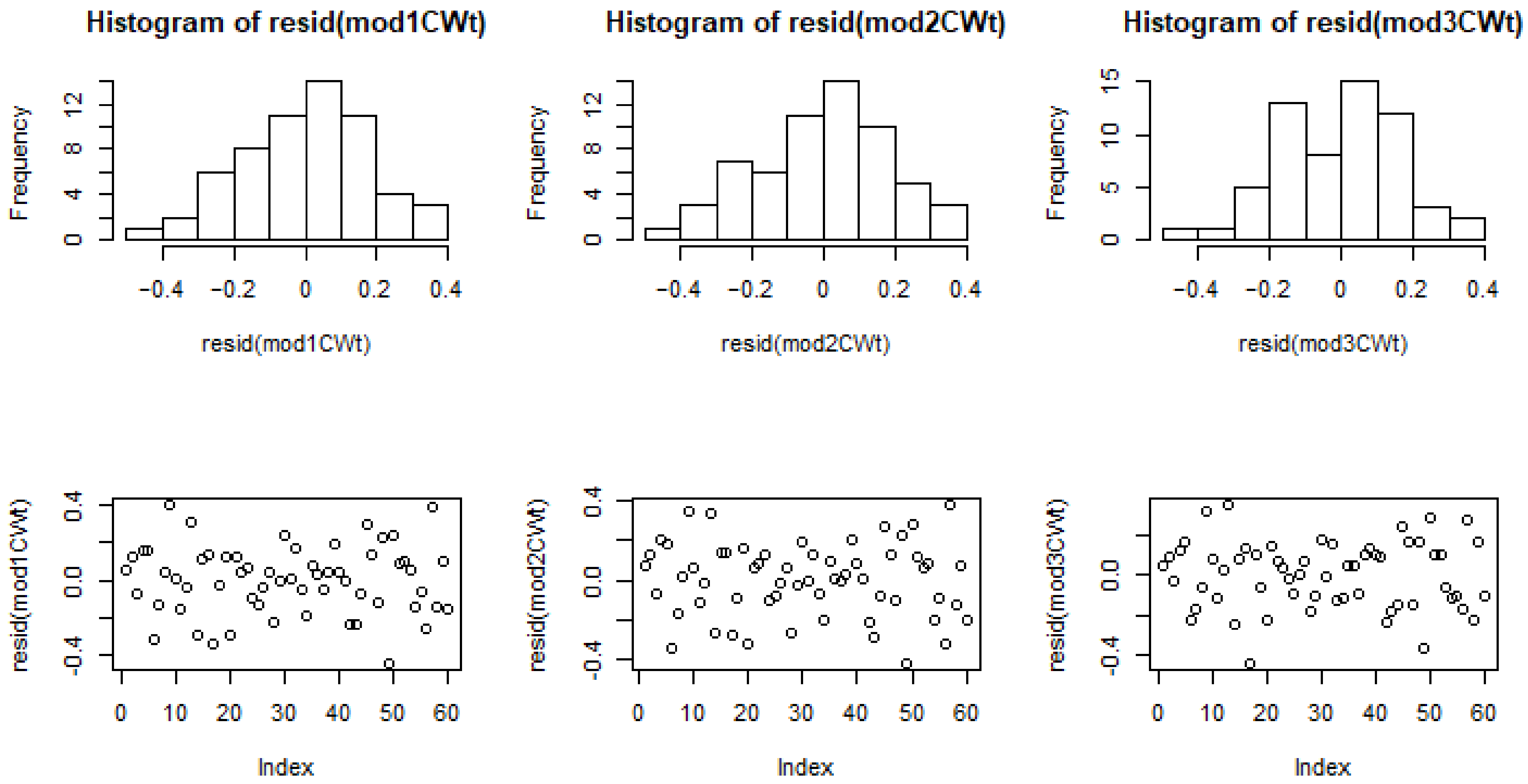
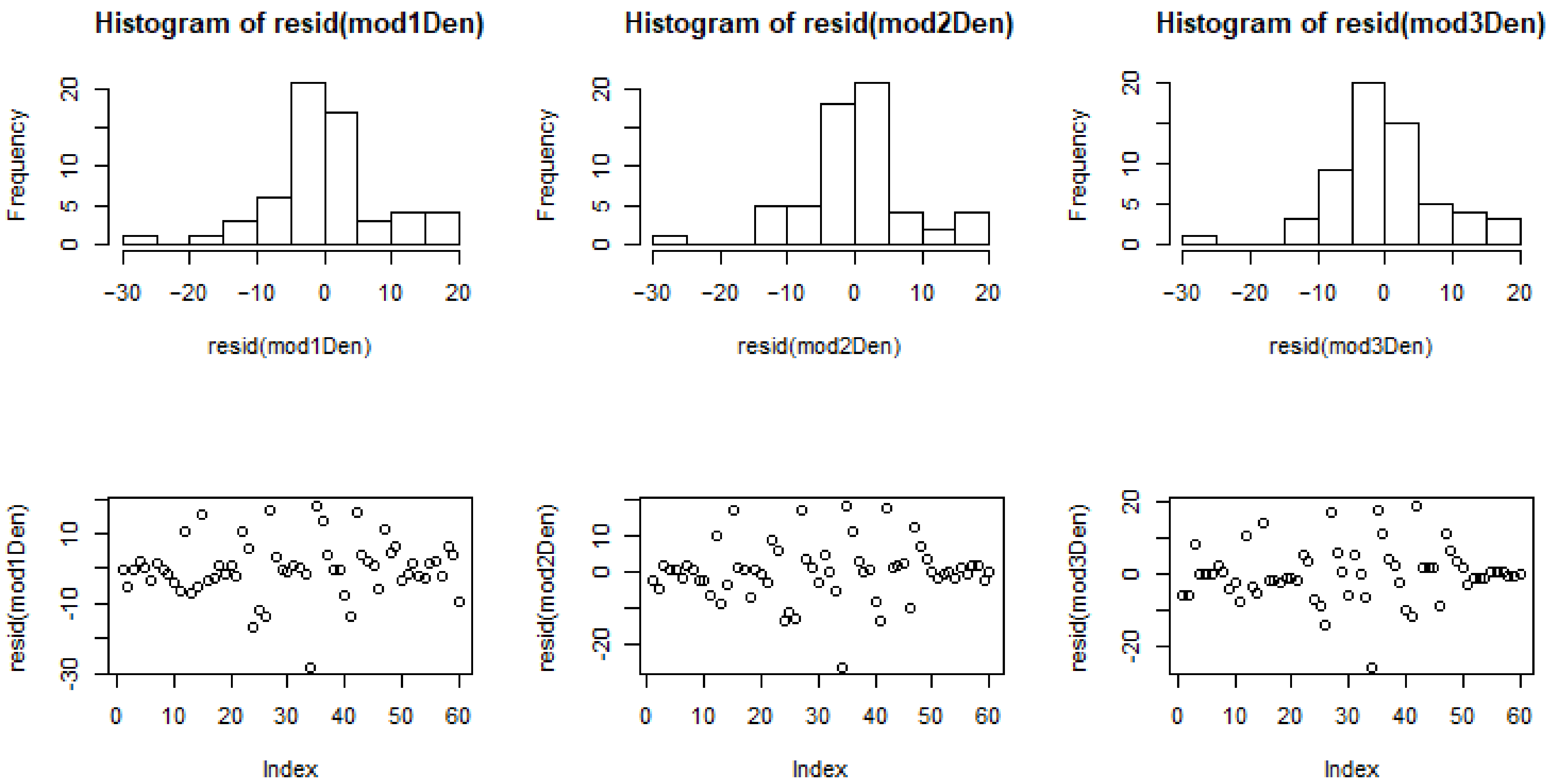
3.4. Model Comparisons
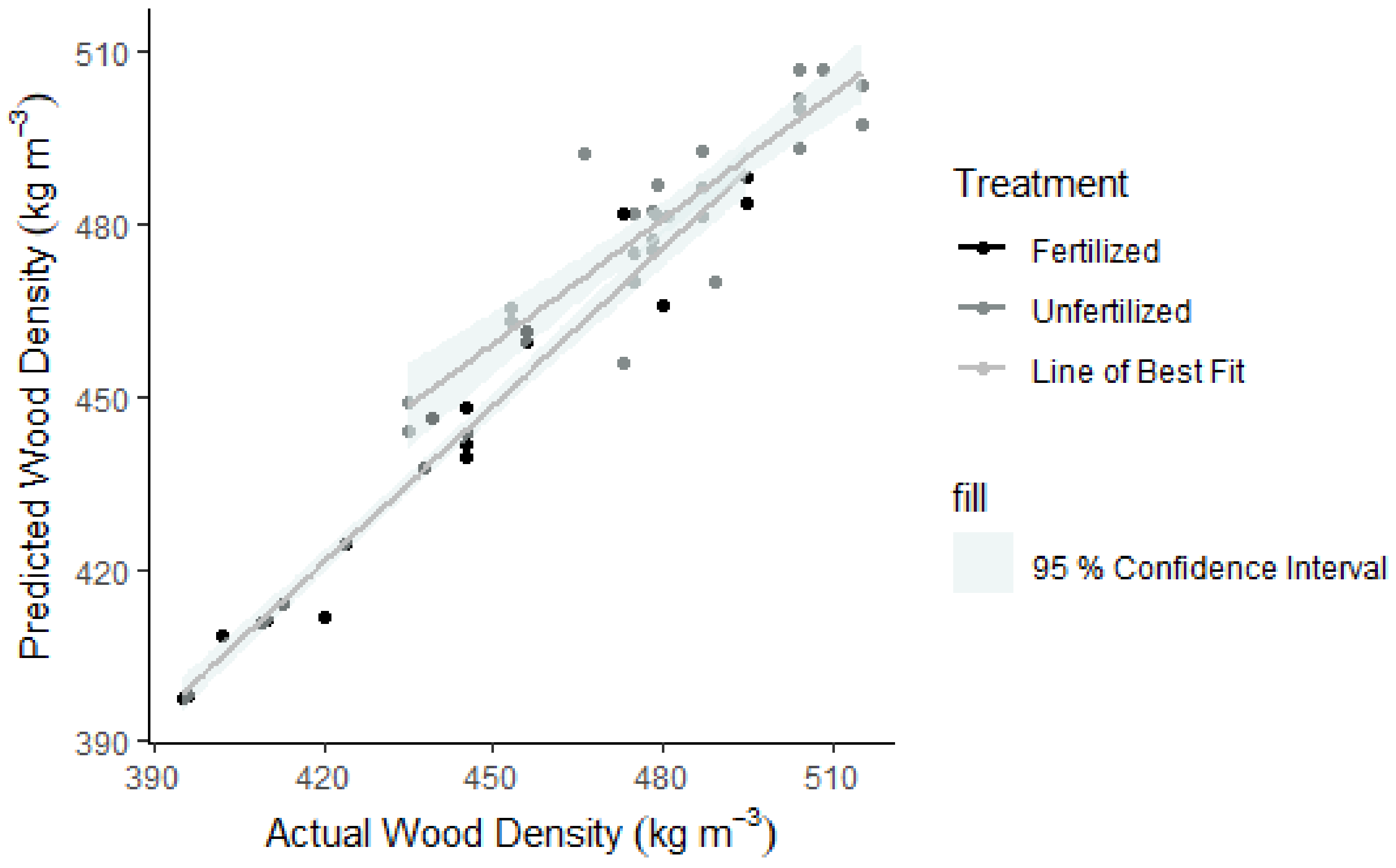
3.5. Modelling Cell Wall Thickness and Average Density
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Poudel, B.C.; Sathre, R.; Bergh, J.; Gustavsson, L.; Lundström, A.; Hyvönen, R. Potential effects of intensive forestry on biomass production and total carbon balance in north-central Sweden. Environ. Sci. Policy 2012, 15, 106–124. [Google Scholar] [CrossRef]
- Heräjärvi, H.; Jouhiaho, A.; Tammiruusu, V.; Verkasalo, E. Small-diameter Scots Pine and Birch timber as raw materials for engineered wood products. Int. J. For. Eng. 2004, 15, 23–34. [Google Scholar] [CrossRef]
- Nohrstedt, H.-Ö. Response of coniferous forest ecosystems on mineral soils to nutrient additions: A review of Swedish experiences. Scand. J. For. Res. 2001, 16, 555–573. [Google Scholar] [CrossRef]
- Jacobson, S.; Pettersson, F. Growth responses following nitrogen and NPK Mg additions to previously N-fertilized Scots pine and Norway spruce stands on mineral soils in Sweden. Can. J. For. Res. 2001, 31, 899–909. [Google Scholar]
- Bergh, J.; Nilsson, U.; Grip, H.; Hedwall, P.-O.; Lundmark, T. Effects of frequency of fertilisation on production, foliar chemistry and nutrient leaching in young Norway spruce stands in Sweden. Silva Fenn. 2008, 42, 721–733. [Google Scholar] [CrossRef] [Green Version]
- Vadeboncoeur, M.A. Meta-analysis of fertilization experiments indicates multiple limiting nutrients in northeastern deciduous forests. Can. J. For. Res. 2010, 40, 1766–1780. [Google Scholar] [CrossRef] [Green Version]
- Muukkonen, P.; Lehtonen, A. Needle and branch biomass turnover rates of Norway spruce (Picea abies). Can. J. For. Res. 2004, 34, 2517–2527. [Google Scholar] [CrossRef]
- Kouki, J.; Tatu, H. Long-Term Needle Litterfall of a Scots Pine Pinus sylvestris Stand: Relation to Temperature Factors. Oecologia 1992, 89, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Bergh, J.; Linder, S.; Lundmark, T.; Elfving, B. The effect of water and nutrient availability on the productivity of Norway spruce in northern and southern Sweden. For. Ecol. Manag. 1999, 119, 51–62. [Google Scholar] [CrossRef]
- Landsberg, J.; Sands, P. Physiological Ecology of Forest Production: Principles, Processes and Models; Elsevier Academic Press: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Lundmark, T.; Bergh, J.; Hofer, P.; Lundstrom, A.; Nordin, A.; Poudel, B.C.; Sathre, R.; Taverna, R.; Werner, F. Potential Roles of Swedish Forestry in the Context of Climate Change Mitigation. Forests 2014, 5, 557–578. [Google Scholar] [CrossRef]
- Bergh, J. Climatic and Nutritional Constraints to Productivity in Norway Spruce; Swedish University of Agricultural Sciences: Uppsala, Sweden, 1997. [Google Scholar]
- Paavilainen, E. Effect of Refertilization of Pine and Birch Stands on a Drained Fertile Mire; Silva Fennica: Helsinki, Finland, 1990; Volume 24. [Google Scholar] [CrossRef] [Green Version]
- Hoyle, M.C.; Bjorkbom, J.C. Birch nutrition. In Proceedings of the Birch Symposium, Durham, NH, USA, 19–21 August 1969; Doolittle, W.T., Bruns, P.E., Eds.; US Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Upper Darby, PA, USA, 1969; pp. 102–105. [Google Scholar]
- Viro, P.J. Fertilization of Birch. Commun. Inst. For. Fenn. 1974, 81, 1–38. [Google Scholar]
- Moilanen, M. Lannoituksen ja Harvennuksen Vaikutus Hieskoivun Kasvuun Ohutturpeisilla Ojitetuilla rämeillä. Commun. Inst. For. Fenn. 1985, 629, 1–29, (In Finnish with English Summary). [Google Scholar]
- Kaunisto, S. Lannoituksen ja Muokkauksen Vaikutus Männyn ja Rauduskoivun Istutustaimien Kasvuun Suonpohjilla. Commun. Inst. For. Fenn. 1987, 681, 1–23, (In Finnish with English Summary). [Google Scholar]
- Sellin, A.; Rohejärv, A.; Rahi, M. Distribution of vessel size, vessel density and xylem conducting efficiency within a crown of silver birch (Betula pendula). Trees 2008, 22, 205–216. [Google Scholar] [CrossRef]
- Zobel, B.J.; van Buijtenen, J.P. Wood Variation: Its Causes and Control; Springer: Berlin/Heidelberg, Germany, 1989. [Google Scholar]
- Dinwoodie, J.M. Timber, Its Nature and Behaviour; E & FN Spon: London, UK, 2000. [Google Scholar]
- Moore, J.R.; Cown, D.J. Corewood (Juvenile Wood) and Its Impact on Wood Utilisation. Curr. For. Rep. 2017, 3, 107–118. [Google Scholar] [CrossRef]
- Biondi, F.; Qeadan, F. A Theory-Driven Approach to Tree-Ring Standardization: Defining the Biological Trend from Expected Basal Area Increment. Tree Ring Res. 2008, 64, 81–96. [Google Scholar] [CrossRef] [Green Version]
- Kollmann, F.F.P.; Côté, W.A. Principles of Wood Science and Technology; Springer: Berlin/Heidelberg, Germany, 1968. [Google Scholar]
- Funada, R.; Yamagishi, Y.; Begum, S.; Kudo, K.; Nabeshima, E.; Nugroho, W.D.; Hasnat, R.; Oribe, Y.; Nakaba, S. Xylogenesis in trees: From cambial cell division to cell death. In Secondary Xylem Biology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 25–43. [Google Scholar]
- Panshin, A.J.; De Zeeuw, C. Textbook of Wood Technology: Structure, Identification, Properties and Uses of the Commercial Woods of the United States and Canada; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Saranpää, P. Wood density and growth. In Wood Quality and Its Biological Basis; Barnett, J.R., Jeronimidis, G., Eds.; Blackwell Publishing: Oxford, UK, 2003; Volume 87. [Google Scholar]
- Fukazawa, K. Juvenile wood of hardwoods judged by density variation. IAWA J. 1984, 5, 65–73. [Google Scholar] [CrossRef]
- Pompa-García, M.; Venegas-González, A. Temporal Variation of Wood Density and Carbon in Two Elevational Sites of Pinus cooperi in Relation to Climate Response in Northern Mexico. PLoS ONE 2016, 11, e0156782. [Google Scholar] [CrossRef]
- Sjökvist, T.; Niklewski, J.; Blom, Å. Effect of wood density and cracks on the moisture content of coated Norway spruce (Picea abies (L.) Karst.). Wood Fiber Sci. 2019, 51, 160–172. [Google Scholar] [CrossRef]
- Lundgren, C.J.S.F. Cell wall thickness and tangential and radial cell diameter of fertilized and irrigated Norway spruce. Silva Fenn. 2004, 38, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Cuny, H.E.; Rathgeber, C.B.; Frank, D.; Fonti, P.; Fournier, M. Kinetics of tracheid development explain conifer tree-ring structure. New Phytol. 2014, 203, 1231–1241. [Google Scholar] [CrossRef]
- Hoadley, R.B. Identifying Wood: Accurate Results with Simple Tools; Taunton: Newtown, CT, USA, 1990. [Google Scholar]
- Pretzsch, H.; Biber, P.; Schütze, G.; Kemmerer, J.; Uhl, E. Wood density reduced while wood volume growth accelerated in Central European forests since 1870. For. Ecol. Manag. 2018, 429, 589–616. [Google Scholar] [CrossRef]
- Peters, R.L.; von Arx, G.; Nievergelt, D.; Ibrom, A.; Stillhard, J.; Trotsiuk, V.; Mazurkiewicz, A.; Babst, F. Axial changes in wood functional traits have limited net effects on stem biomass increment in European beech (Fagus sylvatica). Tree Physiol. 2020, 40, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Ollinmaa, P.J. Eräistä ojitetuilla soilla kasvavan puun fysikaalisista ominaisuuksista. Acta For. Fenn. 1960, 72, 1–24, (In Finnish with English summary). [Google Scholar] [CrossRef] [Green Version]
- Loustarinen, K.; Verkasalo, E. Birch as sawn timber and in mechanical further processing in Finland. A literature Study. Silva Fenn. 2000, 1, 40. [Google Scholar]
- Velling, P. Wood density in two Betula pendula Roth. Progeny trials. Folia For. 1979, 416, 1–24. [Google Scholar]
- Bhat, K.M. Variation in structure and selected properties of finnish birch wood: I. Interrelationships of some structural features, basic density and shrinkage. Silva Fenn. 1980, 14, 384–396. [Google Scholar] [CrossRef]
- Liepiòð, K.; Rieksts-Riekstiòð, J. Stemwood Density of Juvenile Silver Birch Trees (Betula pendula Roth.) From Plantations on Former Farmlands. Balt. For. 2013, 19, 179–186. [Google Scholar]
- Heräjärvi, H. Properties of Birch (Betula Pendula, B. Pubescens) for Sawmilling and Further Processing in Finland. Ph.D. Thesis, Finnish Forest Research Institute, Joensuu Research Centre, Joensuu, Finland, 2002. [Google Scholar]
- Hakkila, P. Investigations on the Basic Density of Finnish Pine, Spruce and Birch Wood. Commun. Inst. For. Fenn. 1966, 61, 1–98. [Google Scholar]
- Liepiņš, R. Die Technischen Eigenschaften der Birke Lettlands; Finska forstsamfundet, Commentationes Forestalis: Helsinki, Finland, 1933; pp. 1–15. [Google Scholar]
- Hynynen, J.; Niemistö, P.; Viherä-Aarnio, A.; Brunner, A.; Hein, S.; Velling, P. Silviculture of birch (Betula pendula Roth. and Betula pubescens Ehrh.) in northern Europe. Forestry 2010, 83, 103–119. [Google Scholar] [CrossRef]
- Dubois, H.; Verkasalo, E.; Claessens, H. Potential of Birch (Betula pendula Roth. and B. pubescens Ehrh.) for Forestry and Forest-Based Industry Sector within the Changing Climatic and Socio-Economic Context of Western Europe. Forests 2020, 11, 336. [Google Scholar] [CrossRef] [Green Version]
- SMHI. Swedish Meteorological and Hydrological Institute. Available online: http://www.smhi.se/klimatdata/meteorologi/nederbord (accessed on 5 July 2018).
- Hägglund, B.; Lundmark, J.-E. Site Index Estimation by Means of Site Properties; Studia Forestalia Suecica: Stockholm, Sweden, 1977; pp. 1–34. [Google Scholar]
- Linder, S. Foliar Analysis for Detecting and Correcting Nutrient Imbalances in Norway Spruce. Ecol. Bull. 1995, 44, 178–190. [Google Scholar]
- Ericsson, T.; Kähr, M. Growth and nutrition of birch seedlings in relation to potassium supply rate. Trees 1993, 7, 78–85. [Google Scholar] [CrossRef]
- Linder, S. Nutritional control of forest yield. In Proceedings of the Marcus Wallenberg Foundation Symposia, Saltsjöbaden, Sweden, 9–13 August 1977. [Google Scholar]
- Brække, F.H. Diagnostiske Grenseverdier for Naeringselementer i Gran-Og Furunaaler; Aktuelt fra Skogforsk Nr.: Ås, Norway, 1994; pp. 15–94. (In Norwegian) [Google Scholar]
- Bonham, V.; Barnett, J. Fibre length and microfibril angle in silver birch (Betula pendula Roth.). Holzforschung 2001, 55, 159–162. [Google Scholar] [CrossRef]
- Dobrowolska, E.; Wroniszewska, P.; Jankowska, A. Density Distribution in Wood of European Birch (Betula pendula Roth.). Forests 2020, 11, 445. [Google Scholar] [CrossRef] [Green Version]
- Zobel, B.J.; Jett, J.B. Genetics of Wood Production; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Siau, J.F. Wood: Influence of Moisture on Physical Properties; Deptartment of Wood Science and Forest Products, Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 1995. [Google Scholar]
- Von Arx, G.; Crivellaro, A.; Prendin, A.L.; Čufar, K.; Carrer, M. Quantitative Wood Anatomy-Practical Guidelines. Front. Plant Sci. 2016, 7, 781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gärtner, H.; Nievergelt, D. The core-microtome: A new tool for surface preparation on cores and time series analysis of varying cell parameters. Dendrochronologia 2010, 28, 85–92. [Google Scholar] [CrossRef]
- Fujii, T. Application of the “NT-cutter” knife blade to microtome sectioning of wood. IAWA J. 2003, 24, 241–245. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.A.; Morén, T.; Hagman, O.; Cloutier, A.; Fang, C.-H.; Elustondo, D. Anatomical properties and process parameters affecting blister/blow formation in densified European aspen and downy birch sapwood boards by thermo-hygro-mechanical compression. J. Mater. Sci. 2013, 48, 8571–8579. [Google Scholar] [CrossRef]
- Tng, D.Y.P.; Apgaua, D.M.G.; Ishida, Y.F.; Mencuccini, M.; Lloyd, J.; Laurance, W.F.; Laurance, S.G.W. Rainforest trees respond to drought by modifying their hydraulic architecture. Ecol. Evol. 2018, 8, 12479–12491. [Google Scholar] [CrossRef] [Green Version]
- Luostarinen, K.; Möttönen, V. Radial variation in the anatomy of Betula pendula wood from different growing sites. Balt. For. 2010, 16, 209–316. [Google Scholar]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning; Springer: Berlin/Heidelberg, Germany, 2013; Volume 112. [Google Scholar]
- Mutikainen, P.; Walls, M.; Ovaska, J.; Keinanen, M.; Julkunen-Tiitto, R.; Vapaavuori, E. Herbivore Resistance in Betula pendula: Effect of Fertilization, Defoliation, and Plant Genotype. Ecology 2000, 81, 49–65. [Google Scholar] [CrossRef]
- Lumme, I. Early effects of peat ash on growth and mineral nutrition of the silver birch (Betula pendula) on a mined peatland. Silva Fenn. 1988, 22, 5344. [Google Scholar] [CrossRef] [Green Version]
- Han, C.-S. Study on the Radial Variation of Structural Element in the Diffuse-Porous Woods. J. Korean Wood Sci. Technol. 1987, 15, 26–52. [Google Scholar]
- Hacke, U.G.; Sperry, J.S.; Pockman, W.T.; Davis, S.D.; McCulloh, K.A.J.O. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 2001, 126, 457–461. [Google Scholar] [CrossRef]
- Schniewind, A.P. Horizontal specific gravity variation in tree stems in relation to their support function. For. Sci. 1962, 8, 111–118. [Google Scholar]
- Woodcock, D.; Shier, A. Wood specific gravity and its radial variations: The many ways to make a tree. Trees 2002, 16, 437–443. [Google Scholar] [CrossRef]
- Dunham, R.; Cameron, A.; Petty, J. The effect of growth rate on the strength properties of sawn beams of silver birch (Betula pendula Roth.). Scand. J. For. Res. 1999, 14, 18–26. [Google Scholar] [CrossRef]
- Bouriaud, O.; Breda, N.; Le Moguedec, G.; Nepveu, G. Modelling variability of wood density in beech as affected by ring age, radial growth and climate. Trees 2004, 18, 264–276. [Google Scholar] [CrossRef]
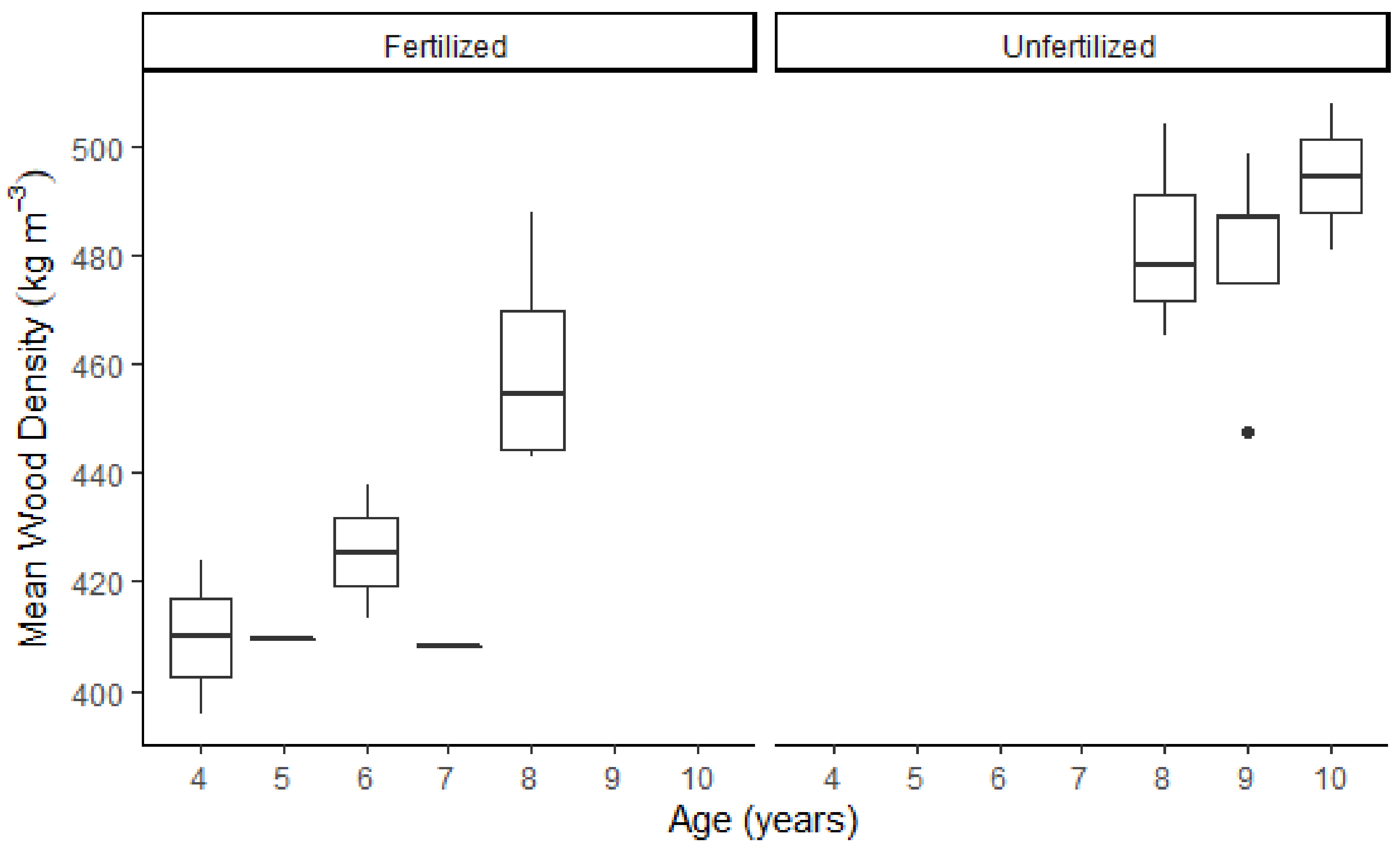

| Fertilized | Unfertilized | |||||
|---|---|---|---|---|---|---|
| Mean | Min | Max | Mean | Min | Max | |
| Diameter 1 (mm) | 53 | 31 | 79 | 60 | 33 | 83 |
| Height (m) | 5.8 | 3.3 | 8.2 | 6.8 | 4.7 | 8.3 |
| Number of growth rings | 6.4 | 4.0 | 8.0 | 8.9 | 8.0 | 10.0 |
| Whole tree mean ring width 2 (mm) | 3.8 | 2.6 | 6.8 | 2.8 | 1.5 | 4.0 |
| Whole tree mean density (kg m−3) | 420 | 390 | 470 | 460 | 430 | 490 |
| Fertilized | Unfertlized | ||||
|---|---|---|---|---|---|
| Year | Mean | Std. Dev | Mean | Std. Dev | |
| Ring width (mm) | 2015 | 3.5 | 1.4 | 2.5 | 0.7 |
| 2016 | 3.3 | 1.0 | 2.2 | 0.9 | |
| 2017 | 3.8 | 1.9 | 2.5 | 1.1 | |
| Basal Area Increment (mm2) | 2015 | 433 | 355 | 392 | 212 |
| 2016 | 438 | 230 | 401 | 243 | |
| 2017 | 614 | 408 | 492 | 297 | |
| Cell wall thickness (µm) | 2015 | 4.5 | 0.9 | 5.1 | 0.9 |
| 2016 | 4.3 | 1.0 | 5.3 | 0.9 | |
| 2017 | 4.7 | 0.9 | 5.4 | 0.9 | |
| Vessels (No mm−2) | 2015 | 140 | 54 | 120 | 20 |
| 2016 | 143 | 42 | 121 | 31 | |
| 2017 | 102 | 29 | 99 | 28 | |
| Fertilized | Unfertilized | |||
|---|---|---|---|---|
| Mean | Std. Dev | Mean | Std. Dev | |
| Growth before treatment (mm year−1) | 1.7 | 0.7 | 2.4 | 0.7 |
| Relative growth year—2014 (%) | 4.0 | 1.5 | 1.4 | 0.4 |
| Relative growth year—2016 (%) | 2.1 | 0.8 | 1.0 | 0.3 |
| Increase in basal area since treatment (%) | 7.3 | 3.5 | 2.1 | 0.6 |
| CWt (µm) | Dens (kg m−3) | |||||
|---|---|---|---|---|---|---|
| Model | Variable | Estimates | p-Value | Variable | Estimates | p-Value |
| Mod 1 | Intercept | 4.28 | <0.0001 | Intercept | 432.83 | <0.0001 |
| Vessel Number | −0.01 | 0.0269 | Ring width | −7.44 | 0.0164 | |
| DBH | 0.11 | 0.0175 | BAyear | 0.05 | 0.0019 | |
| Treatment | 0.65 | 0.0005 | Treatment | 45.52 | 0.0002 | |
| Mod 2 | Intercept | 5.04 | <0.0001 | Intercept | 424.40 | <0.0001 |
| Vessel Number | −0.02 | 0.0013 | BAyear | 0.02 | 0.0654 | |
| Treatment | 0.70 | 0.0007 | Treatment | 51.46 | 0.0001 | |
| Mod 3 | Intercept | 4.46 | <0.0001 | Intercept | 432.50 | <0.0001 |
| Treatment | 0.78 | 0.0012 | Treatment | 50.34 | 0.0002 | |
| CWt (µm) | Dens (kg m−3) | |||||
|---|---|---|---|---|---|---|
| ANOVA Value | Mod 1 | Mod 2 | Mod 3 | Mod 1 | Mod 2 | Mod 3 |
| AIC | 46.96 | 51.70 | 59.36 | 510.71 | 514.36 | 515.72 |
| BIC | 61.62 | 64.27 | 69.84 | 525.37 | 526.93 | 526.19 |
| LL | −16.48 | −19.85 | −24.68 | −248.35 | −251.18 | −252.86 |
| CWt Mod 1 | Dens Mod 3 | |||
|---|---|---|---|---|
| Random Effect | Variance | Standard Deviation | Variance | Standard Deviation |
| Age:Year | 0.01 | 0.11 | 21.38 | 4.62 |
| Tree | 0.09 | 0.30 | 523.54 | 22.88 |
| Residual | 0.05 | 0.30 | 95.90 | 9.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nilsson, J.A.; Jones, G.; Håkansson, C.; Blom, Å.; Bergh, J. Effects of Fertilization on Wood Formation in Naturally Regenerated Juvenile Silver Birch in a Norway Spruce Stand in South Sweden. Forests 2021, 12, 415. https://doi.org/10.3390/f12040415
Nilsson JA, Jones G, Håkansson C, Blom Å, Bergh J. Effects of Fertilization on Wood Formation in Naturally Regenerated Juvenile Silver Birch in a Norway Spruce Stand in South Sweden. Forests. 2021; 12(4):415. https://doi.org/10.3390/f12040415
Chicago/Turabian StyleNilsson, Josefin A., Grace Jones, Charlotta Håkansson, Åsa Blom, and Johan Bergh. 2021. "Effects of Fertilization on Wood Formation in Naturally Regenerated Juvenile Silver Birch in a Norway Spruce Stand in South Sweden" Forests 12, no. 4: 415. https://doi.org/10.3390/f12040415
APA StyleNilsson, J. A., Jones, G., Håkansson, C., Blom, Å., & Bergh, J. (2021). Effects of Fertilization on Wood Formation in Naturally Regenerated Juvenile Silver Birch in a Norway Spruce Stand in South Sweden. Forests, 12(4), 415. https://doi.org/10.3390/f12040415








