Phytophthora mediterranea sp. nov., a New Species Closely Related to Phytophthora cinnamomi from Nursery Plants of Myrtus communis in Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surveys and Sampling Procedure
2.2. Isolation of Phytophthora Species
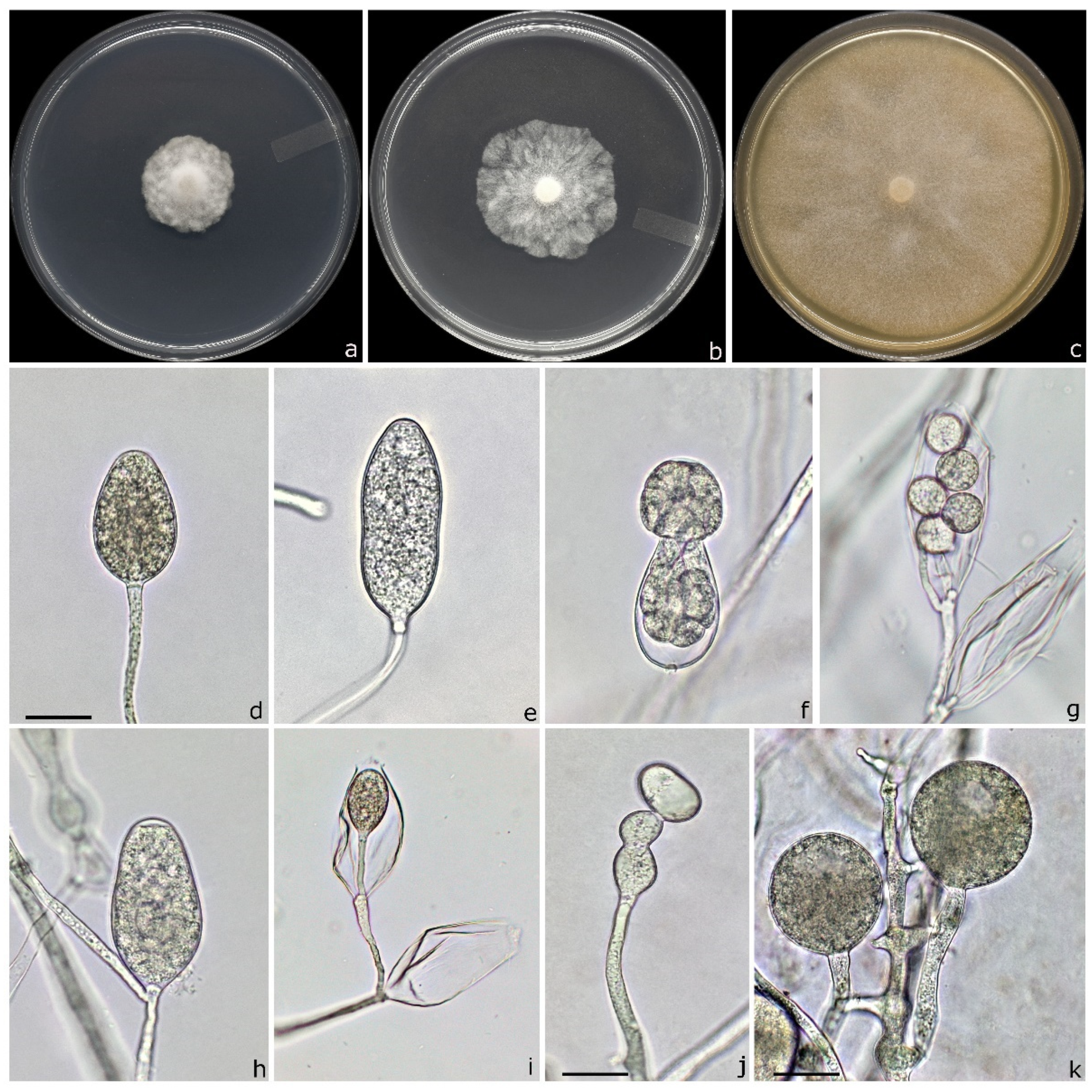
2.3. Morphological Identification and Characterization of Isolates
2.4. DNA Extraction, Polymerase Chain Reaction (PCR) Amplification and Sequencing
2.5. Phylogenetic Analysis
2.6. Pathogenicity Test
2.7. Data Analysis
3. Results
3.1. Symptomatology
3.2. Phytophthora Species Associated with Nursery Plants
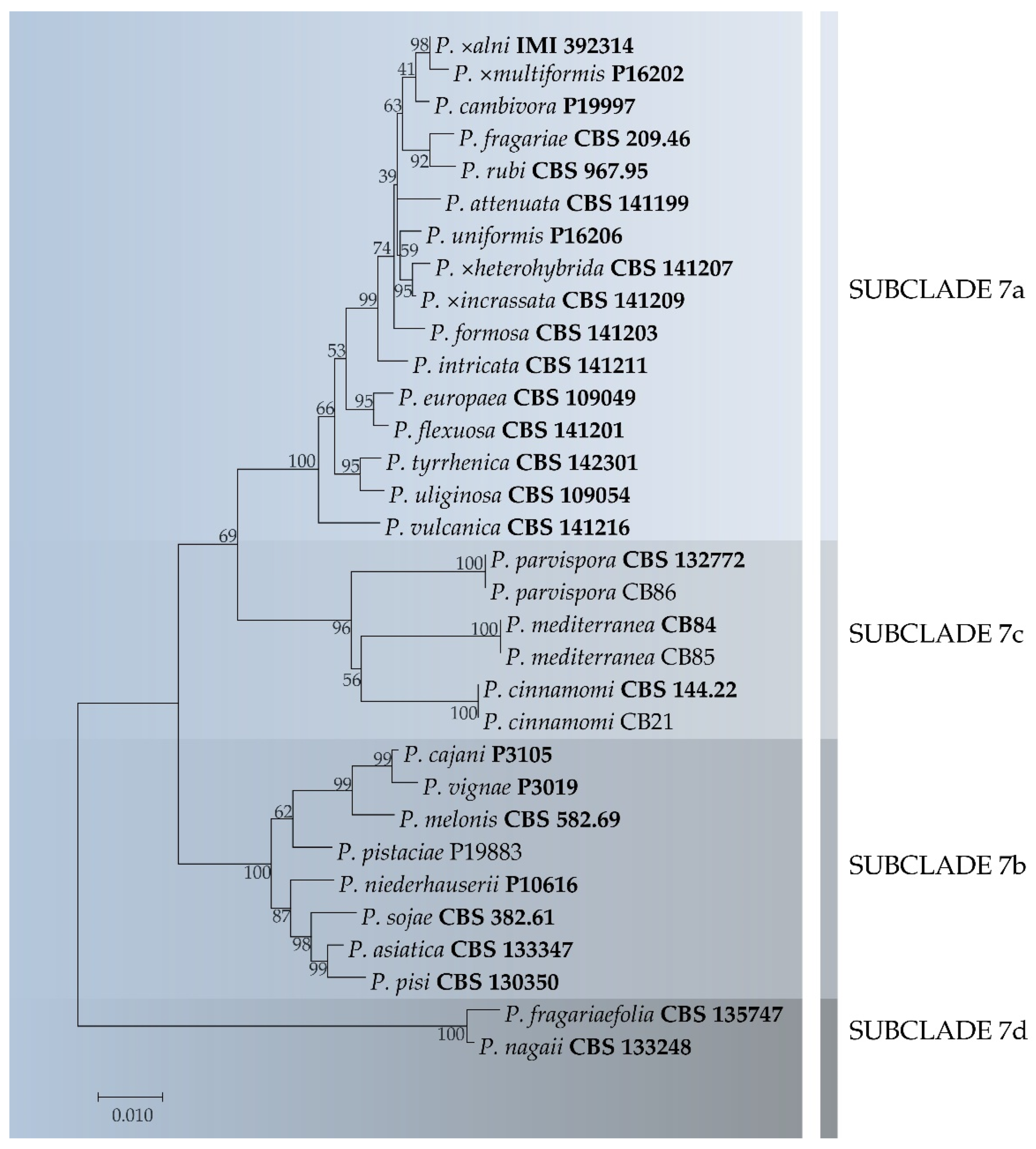
3.3. Phylogenetic Analysis
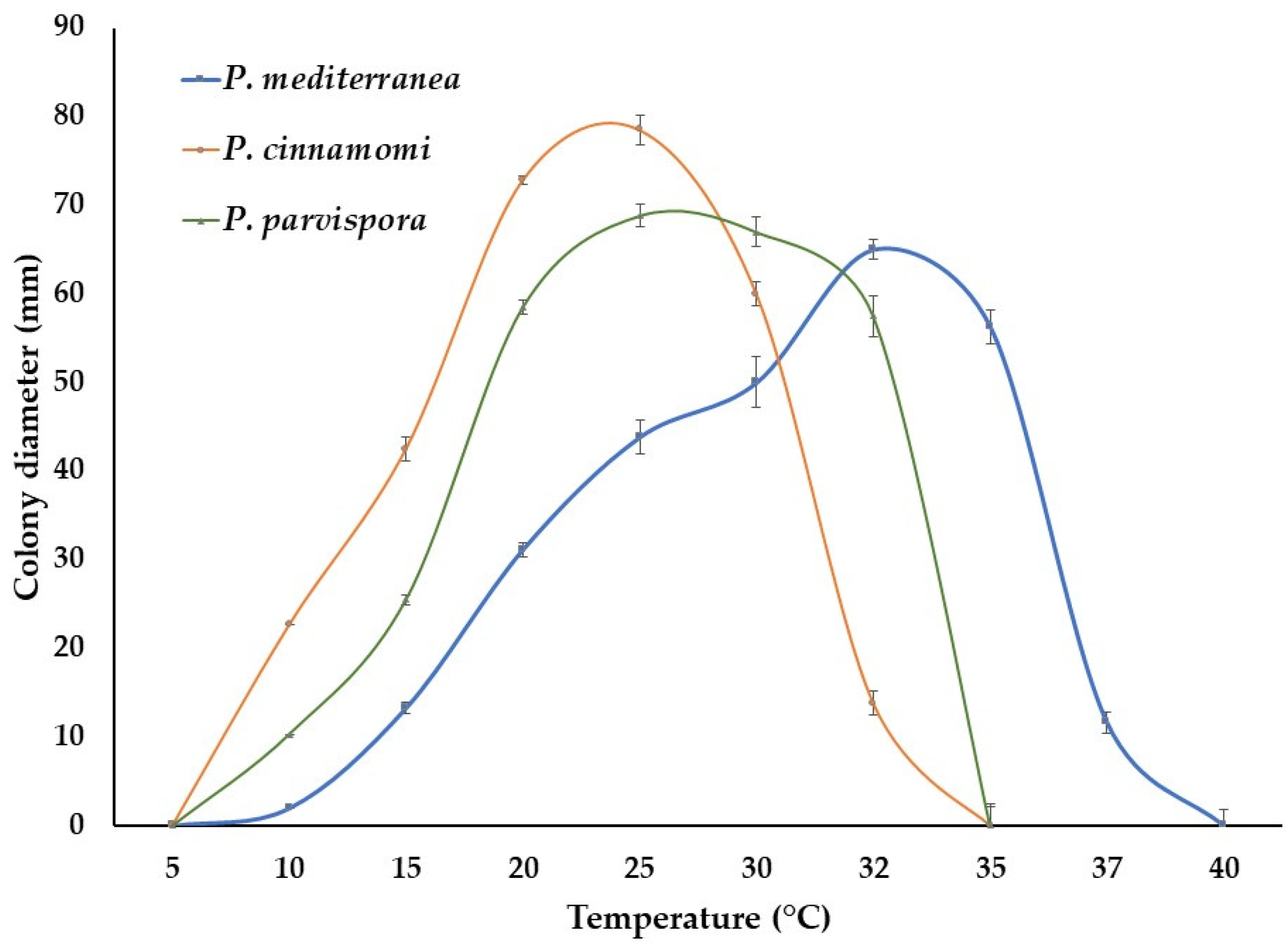
3.4. Taxonomy
3.5. Pathogenicity Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hulme, P.E. Trade, transport and trouble: Managing invasive species pathways in an era of globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar] [CrossRef]
- Bradley, B.A.; Blumenthal, D.M.; Regan, E.; Grosholz, E.D.; Lawler, J.J.; Miller, L.P.; Sorte, C.J.B.; D’Antonio, C.M.; Diez, J.M.; Dukes, J.S.; et al. Global Change, Global Trade, and the Next Wave of Plant Invasions. Front. Ecol. Environ. 2012, 10, 20–28. [Google Scholar] [CrossRef] [Green Version]
- ISTAT. Stima Preliminare dei Conti Economici Dell’agricoltura; Archivio, id252819; ISTAT: Rome, Italy, 2021. [Google Scholar]
- Brasier, C. The biosecurity threat to the UK and global environment from international trade in plants. Plant Pathol. 2008, 57, 792–808. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Brockehoff, E.G.; Garret, L.J.; Parke, J.L.; Britton, K.O. Live plant imports: The major pathway for forest insect and pathogen invasions of the US. Front. Ecol. Environ. 2012, 10, 135–143. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Brockerhoff, E.G.; Kalisz, S.; Nuñez, M.A.; Wardle, D.A.; Wingfield, M.J. Biological invasions in forest ecosystems. Biol. Invasions 2017, 19, 3437–3458. [Google Scholar] [CrossRef]
- Ghelardini, L.; Luchi, N.; Pecori, F.; Pepori, A.L.; Danti, R.; Rocca, G.D.; Capretti, P.; Tsopelas, P.; Santini, A. Ecology of invasive forest pathogens. Biol. Invasions 2017, 19, 3183–3200. [Google Scholar] [CrossRef]
- Yang, X.; Tyler, B.M.; Hong, C. An expanded phylogeny for the genus Phytophthora. IMA Fungus 2017, 8, 355–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, T.; Horta Jung, M.; Cacciola, S.O.; Cech, T.; Bakonyi, J.; Seress, D.; Mosca, S.; Schena, L.; Seddaiu, S.; Pane, A.; et al. Multiple new cryptic pathogenic Phytophthora species from Fagaceae forests in Austria, Italy, and Portugal. IMA Fungus 2017, 8, 219–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, P.; Bader, M.K.F.; Burgess, T.I.; Hardy, G.; Williams, N. Global biogeography and invasion risk of the plant pathogen genus Phytophthora. Environ. Sci. Policy 2019, 101, 175–182. [Google Scholar] [CrossRef]
- Erwin, C.D.; Ribeiro, O.K. Phytophthora Diseases Worldwide; APS Press: Saint Paul, MN, USA, 1996. [Google Scholar]
- Benson, D.M.; Jones, J.K. Diseases of Woody Ornamentals and Trees in Nurseries; APS Press: St. Paul, MN, USA, 2001. [Google Scholar]
- Parke, J.L.; Redekar, N.R.; Eberhart, J.L.; Funahashi, F. Hazard analysis for Phytophthora species in container nurseries: Three case studies. HortTechnology 2019, 29, 745–755. [Google Scholar] [CrossRef] [Green Version]
- Webber, J.F.; Mullett, M.; Brasier, C.M. Dieback and mortality of plantation Japanese larch (Larix kaempferi) associated with infection by Phytophthora ramorum. New Dis. Rep. 2010, 22, 19. [Google Scholar] [CrossRef] [Green Version]
- Prospero, S.; Vercauteren, A.; Heungens, K.; Belbahri, L.; Rigling, D. Phytophthora diversity and population structure of Phytophthora ramorum in Swiss ornamental nurseries. Plant Pathol. 2013, 62, 1063–1071. [Google Scholar] [CrossRef]
- Simamora, A.V.; Paap, T.; Howard, K.; Stukely, M.J.; Hardy, G.E.S.J.; Burgess, T.I. Phytophthora contamination in a nursery and its potential dispersal into the natural environment. Plant Dis. 2017, 102, 132–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zentmyer, G.A. Origin and Distribution of Phytophthora cinnamomi; California Avocado Society: San Juan Capistrano, CA, USA, 1985; Volume 69, pp. 89–96. Available online: http://www.avocadosource.com/cas_yearbooks/cas_69_1985/cas_1985_pg_89-96.pdf (accessed on 4 May 2021).
- Hansen, E.M.; Streito, J.C.; Delatour, C. First confirmation of Phytophthora lateralis in Europe. Plant Dis. 1999, 83, 587. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, D.M.; Garbelotto, M.; Davidson, J.M.; Slaughter, G.W.; Koike, S.T. Phytophthora ramorum as the cause of extensive mortality of Quercus spp and Lithocarpus densiflorus in California. Plant Dis. 2002, 86, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Cobb, R.C.; Haas, S.E.; Kruskamp, N.; Dillon, W.W.; Swiecki, T.J.; Rizzo, D.M.; Frankel, S.J.; Meentemeyer, R.K. The magnitude of regional-scale tree mortality caused by the invasive pathogen Phytophthora ramorum. Earth’s Future 2020, 8, e2020EF001500. [Google Scholar] [CrossRef]
- Man in’t Veld, W.A.; De Cock, A.W.A.M.; Summerbell, R.C. Natural hybrids of residents and introduced Phytophthora species proliferating on multiple new hosts. J. Plant Pathol. 2007, 117, 25–33. [Google Scholar] [CrossRef]
- McDonald, J.D.; Alishtayeh, M.S.; Kabashima, J.; Stites, J. Occurrence of Phytophthora species in recirculated nursery irrigation effluents. Plant Dis. 1994, 78, 607–611. [Google Scholar] [CrossRef]
- Themann, K.; Werres, S.; Lüttmann, R.; Diener, H.A. Observations of Phytophthora spp. in water recirculation systems in commercial hardy ornamental nursery stock. Eur. J. Plant Pathol. 2002, 108, 337–343. [Google Scholar] [CrossRef]
- Hong, C.X.; Moorman, G.W. Plant pathogens in irrigation water: Challenges and opportunities. Crit. Rev. Plant Sci. 2005, 24, 189–208. [Google Scholar] [CrossRef]
- Redekar, N.R.; Eberhart, J.L.; Parke, J.L. Diversity of Phytophthora, Pythium and Phytopythium species in recycled irrigation water in a container nursery. Phytobiomes 2019, 3, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Ivors, K.L.; Moorman, G.W. Oomycete plant pathogens in irrigation water. In Biology, Detection, and Management of Plant pathogens in Irrigation Water; Hong, C., Moorman, G.W., Wohanka, W., Büttner, C., Eds.; APS Press: St. Paul, MN, USA, 2017; pp. 57–64. [Google Scholar]
- Shishkoff, N. Persistence of Phytophthora ramorum in soil mix and roots of nursery ornamentals. Plant Dis. 2007, 91, 1245–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crone, M.; McComb, J.A.; O’Brien, P.A.; Hardy, G.E.S.J. Survival of Phytophthora cinnamomi as oospores, stromata, and thick-walled chlamydospores in roots of symptomatic and asymptomatic annual and herbaceous perennial plant species. Fungal Biol. 2013, 117, 112–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bienapfl, J.C.; Balci, Y. Movement of Phytophthora spp. in Maryland’s nursery trade. Plant Dis. 2014, 98, 134–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruin, G.C.A.; Edgington, L.V. Adaptive resistance in Peronosporales to metalaxyl. Can. J. Plant Pathol. 1981, 3, 201–206. [Google Scholar] [CrossRef]
- Dobrowolski, M.; Shearer, B.; Colquhoun, I.; O’brien, P.; Hardy, G.E.S.J. Selection for decreased sensitivity to phosphite in Phytophthora cinnamomi with prolonged use of fungicide. Plant Pathol. 2008, 57, 928–936. [Google Scholar] [CrossRef]
- González-Tobón, J.; Childers, R.A.; Regnier, M.; Rodriguez, A.; Olave, C.; Fry, W.E.; Restrepo, S.; Danies, G. Is the phenomenon of mefenoxam-acquired resistance in Phytophthora infestans universal? Plant Dis. 2019, 104, 211–221. [Google Scholar] [CrossRef]
- Hardy, G.E.S.J.; Sivasithamparam, K. Phytophthora spp. associated with container-grown plants in nurseries in Western Australia. Plant Dis. 1988, 72, 435–437. [Google Scholar] [CrossRef] [Green Version]
- Davison, E.M.; Drenth, A.; Kumar, S.; Mack, S.; Mackie, A.E.; McKirdy, S. Pathogens associated with nursery plants imported into Western Australia. Australas. Plant Pathol. 2006, 35, 473–475. [Google Scholar]
- Moralejo, E.; Pérez-Sierra, A.M.; Alvarez, L.A.; Belbahri, L.; Lefort, F.; Descals, E. Multiple alien Phytophthora taxa discovered on diseased ornamental plants in Spain. Plant Pathol. 2009, 58, 100–110. [Google Scholar] [CrossRef]
- Yakabe, L.E.; Blomquist, C.L.; Thomas, S.L.; MacDonald, J.D. Identification and frequency of Phytophthora species associated with foliar diseases in California ornamental nurseries. Plant Dis. 2009, 93, 883–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Sierra, A.; Jung, T. Phytophthora in woody ornamental nurseries. In Phytophthora: A Global Perspective; Lamour, K., Ed.; CABI: Wallingford, UK, 2013; pp. 166–177. [Google Scholar]
- Parke, J.L.; Knaus, B.J.; Fieland, V.J.; Lewis, C.; Grünwald, N.J. Phytophthora community structure analyses in Oregon nurseries inform systems approaches to disease management. Phytopathology 2014, 104, 1052–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molnar, C.; Nikolaeva, E.; Kim, S.; Olson, T.; Bily, D.; Kim, J.-E.; Kang, S. Phytophthora diversity in Pennsylvania nurseries and greenhouses inferred from clinical samples collected over four decades. Microorganisms 2020, 8, 1056. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.; Taylor, P.; Gardner, J.; Puértolas, A.; Panda, P.; Addison, S.; Hood, I.; Burgess, T.; Horner, I.; Williams, N.; et al. Phytophthora aleatoria sp. nov., associated with root and collar damage on Pinus radiata from nurseries and plantations. Australas. Plant Pathol. 2019, 48, 313–321. [Google Scholar] [CrossRef]
- Hong, C.X.; Gallegly, M.E.; Richardson, P.A.; Kong, P.; Moorman, G.W.; Lea-Cox, J.D.; Ross, D.S. Phytophthora hydropathica, a new pathogen identified from irrigation water, Rhododendron catawbiense and Kalmia latifolia. Plant Pathol. 2010, 59, 913–921. [Google Scholar] [CrossRef]
- Yang, X.; Copes, W.E.; Hong, C.X. Phytophthora mississippiae sp. nov., a new species recovered from irrigation reservoirs at a plant nursery in Mississippi. J. Plant Pathol. Microb. 2013, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bertier, L.; Brouwer, H.; de Cock, A.W.A.M.; Cooke, D.E.L.; Olsson, C.H.B.; Höfte, M. The expansion of Phytophthora clade 8b: Three new species associated with winter grown vegetable crops. Persoonia 2013, 31, 63–76. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Gallegly, M.E.; Hong, C. A high-temperature tolerant species in Clade 9 of the genus Phytophthora: P. hydrogena sp. nov. Mycologia 2014, 106, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Brasier, C.M.; Beales, P.A.; Kirk, S.A.; Denman, S.; Rose, J. Phytophthora kernoviae sp. nov., an invasive pathogen causing bleeding stem lesions on forest trees and foliar necrosis of ornamentals in the UK. Mycol. Res. 2005, 109, 853–859. [Google Scholar] [CrossRef] [Green Version]
- Abad, Z.G.; Abad, J.A.; Cacciola, S.O.; Pane, A.; Faedda, R.; Moralejo, E.; Pérez-Sierra, A.; Abad-Campos, P.; Alvarez-Bernaola, L.A.; Bakonyi, J.; et al. Phytophthora niederhauserii sp. nov., a polyphagous species associated with ornamentals, fruit trees and native plants in 13 countries. Mycologia 2014, 106, 431–447. [Google Scholar] [CrossRef]
- Migliorini, D.; Ghelardini, L.; Tondini, E.; Luchi, N.; Santini, A. The potential of symptomless potted plants for carrying invasive soilborne plant pathogens. Divers. Distrib. 2015, 21, 1218–1229. [Google Scholar] [CrossRef]
- Prigigallo, M.I.; Mosca, S.; Cacciola, S.O.; Cooke, D.E.L.; Schena, L. Molecular analysis of Phytophthora diversity in nursery-grown ornamental and fruit plants. Plant Pathol. 2015, 64, 1308–1319. [Google Scholar] [CrossRef] [Green Version]
- Scanu, B.; Hunter, G.C.; Linaldeddu, B.T.; Franceschini, A.; Maddau, L.; Jung, T.; Denman, S. A taxonomic re-evaluation reveals that Phytophthora cinnamomi and P. cinnamomi var. parvispora are separate species. For. Pathol. 2014, 44, 1–20. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Mulas, A.A.; Bregant, C.; Piras, G.; Montecchio, L. First Report of Phytophthora pistaciae causing root and collar rot on nursery plants of Pistacia lentiscus in Italy. Plant Dis. 2020, 104, 1564. [Google Scholar] [CrossRef]
- Bregant, C.; Sanna, G.P.; Bottos, A.; Maddau, L.; Montecchio, L.; Linaldeddu, B.T. Diversity and pathogenicity of Phytophthora species associated with declining alder trees in Italy and description of Phytophthora alpina sp. nov. Forests 2020, 11, 848. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols, a Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Martin, F.N.; Tooley, P.W. Phylogenetic relationships among Phytophthora species inferred from sequence analysis of mitochondrially encoded cytochrome oxidase I and II genes. Mycologia 2003, 95, 269–284. [Google Scholar] [CrossRef]
- Kroon, L.P.N.M.; Bakker, F.T.; Van Den Bosch, G.B.M.; Bonants, P.J.M.; Flier, W.G. Phylogenetic analysis of Phytophthora species based on mitochondrial and nuclear DNA sequences. Fungal Genet. Biol. 2004, 41, 766–782. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [Green Version]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic Acids Symposium Series; Oxfrod University Press: Oxfrod, UK, 1999; Volume 41, pp. 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Hardham, A.R.; Blackman, L.M. Phytophthora cinnamomi. Mol. Plant Pathol. 2018, 19, 260–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nechwatal, J.; Jung, T. A study of Phytophthora spp. in declining highbush blueberry in Germany reveals that P. cinnamomi can thrive under Central European outdoor conditions. J. Plant. Dis. Prot. 2021. [Google Scholar] [CrossRef]
- Weste, G.; Marks, G. The biology of Phytophthora cinnamomi in Australasian forests. Annu. Rev. Phytopathol. 2020, 25, 207–229. [Google Scholar] [CrossRef]
- Burgess, T.I.; Scott, J.K.; McDougall, K.L.; Stukely, M.J.; Crane, C.; Dunstan, W.A.; Brigg, F.; Andjic, V.; White, D.; Rudman, T.; et al. Current and projected global distribution of Phytophthora cinnamomi, one of the world’s worst plant pathogens. Glob. Chang. Biol. 2017, 23, 1661–1674. [Google Scholar] [CrossRef] [Green Version]
- Mammella, M.A.; Martin, F.N.; Cacciola, S.O.; Coffey, M.D.; Faedda, R.; Schena, L. Analyses of the population structure in a global collection of Phytophthora nicotianae isolates inferred from mitochondrial and nuclear DNA sequences. Phytopathology 2013, 103, 610–622. [Google Scholar] [CrossRef] [Green Version]
- Rooney-Latham, S.; Blomquist, K.L.; Kosta, K.L.; Gou, Y.Y.; Woods, P.W. Phytophthora species are common on nursery stock grown for restoration and revegetation purposes in California. Plant Dis. 2019, 103, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Kurbetli, İ.; Karaca, G.; Aydoğdu, M. Phytophthora species causing root and collar rot of pomegranate in Turkey. Eur. J. Plant. Pathol. 2020, 157, 485–496. [Google Scholar] [CrossRef]
- Mirabolfathy, M.; Cooke, D.E.L.; Duncan, J.M.; Williams, N.A.; Ershad, D.; Alizadeh, A. Phytophthora pistaciae sp. nov. and P. melonis: The principal causes of pistachio gummosis in Iran. Mycol. Res. 2001, 105, 1166–1175. [Google Scholar] [CrossRef]
- Ginetti, B.; Moricca, S.; Squires, J.N.; Cooke, D.E.L.; Ragazzi, A.; Jung, T. Phytophthora acerina sp. nov., a new species causing bleeding cankers and dieback of Acer pseudoplatanus trees in planted forests in northern Italy. Plant Pathol. 2014, 63, 858–876. [Google Scholar] [CrossRef] [Green Version]
- Linaldeddu, B.T.; Bregant, C.; Montecchio, L.; Favaron, F.; Sella, L. First report of Phytophthora acerina, P. pini and P. plurivora causing root rot and sudden death on olive trees in Italy. Plant Dis. 2020, 104, 996. [Google Scholar] [CrossRef]
- Frankel, S.J.; Conforti, C.; Hillman, J.; Ingolia, M.; Shor, A.; Benner, D.; Alexander, J.M.; Bernhardt, E.; Swiecki, T.J. Phytophthora introductions in restoration areas: Responding to protect California native flora from human-assisted pathogen spread. Forests 2020, 11, 1291. [Google Scholar] [CrossRef]
- Medail, F.; Quezel, P. Biodiversity hotspots in the Mediterranean basin: Setting global conservation priorities. Conserv. Biol. 1999, 13, 1510–1513. [Google Scholar] [CrossRef]
- Melito, S.; Chessa, I.; Erre, P.; Podani, J.; Mulas, M. The genetic diversity of Sardinian myrtle (Myrtus communis L.) populations. Electron. J. Biotechnol. 2013, 16, 7. [Google Scholar] [CrossRef]
- Mulas, M. Problematiche legate alla coltivazione del mirto. Italus Hortus 2004, 11, 308–312. [Google Scholar]


| Nursery | Elevation (m. a.s.l.) | Geographic Coordinates | Plant Species * | |
|---|---|---|---|---|
| N1 | 860 | 40°25′54′′ N | 8°58′43′′ E | Cs (12), Ia (6), Jr (12), Ln (6), Phl (6) |
| N2 | 16 | 39°57′43′′ N | 8°36′02′′ E | Au (4), Hi (4), Lo (2), Mc (3), Pl (7), Qi (6) |
| N3 | 1077 | 46°06′54′′ N | 12°26′05′′ E | Aa (2), Fs (2) |
| N4 | 191 | 46°11′51′′ N | 13°14′13′′ E | Ai (4) |
| Species | Code | Host | GenBank Accession Number | ||
|---|---|---|---|---|---|
| ITS | Btub | Cox1 | |||
| P. asiatica | CBS 133347 | Pueraria lobata | AB688422 | AB539560 | AB740169 |
| P. attenuata | CBS 141199 | Castanopsis carlesii | KU517154 | KU899277 | LC595899 |
| P. cajani | P3105 | Cajanus cajan | MG783386 | MH493912 | MH136859 |
| P. cinnamomi | CBS 144.22 | Cinnamomum sp. | MG865473 | MH493920 | MH136869 |
| P. cinnamomi | CB21 | Abies alba | MW892397 | MW900442 | MW900446 |
| P. cambivora | P19997 | Castanea sativa | MG783387 | MH493913 | MH136860 |
| P. europaea | CBS 109049 | Quercus robur | MG865488 | MH493935 | MH136884 |
| P. flexuosa | CBS 141201 | Fagus hayatae | KU517152 | KU899302 | LC595910 |
| P. formosa | CBS 141203 | Araucaria sp. | KU517153 | KU899270 | LC595912 |
| P. fragariae | CBS 209.46 | Fragaria ×ananassa | MG865494 | MH493938 | MH136890 |
| P. fragariaefolia | CBS 135747 | Fragaria ×ananassa | MG865495 | MH493939 | MH136891 |
| P. intricata | CBS 141211 | Quercus tarokoensis | KU517155 | KU899284 | LC595921 |
| P. niederhauserii | P10616 | Hedera helix | MG865552 | MH493988 | MH136944 |
| P. melonis | CBS 582.69 | Cucumis sativus | MG865536 | MH493974 | MH136931 |
| P. mediterranea | CB84 | Myrtus communis | MW892398 | MW900443 | MW900447 |
| P. mediterranea | CB85 | M. communis | MW892399 | MW900444 | MW900448 |
| P. nagaii | CBS 133248 | Rosa sp. | MG865547 | MN207274 | MH136940 |
| P. parvispora | CBS 132772 | Arbutus unedo | KC478667 | KC609402 | KC609413 |
| P. parvispora | CB86 | A. unedo | MW892401 | MW900445 | MW900449 |
| P. pisi | CBS 130350 | Pisum sativus | MG865567 | MH494000 | MH477754 |
| P. pistaciae | P19883 | Pistacia vera | KT183043 | KX251749 | LC595934 |
| P. rubi | CBS 967.95 | Rubus idaeus | MG865584 | MH494011 | MH136976 |
| P. sojae | CBS 382.61 | Glycine max | MG865587 | MN207279 | MH136979 |
| P. tyrrhenica | CBS 142301 | Quercus ilex | KU899188 | KU899265 | LC595950 |
| P. uliginosa | CBS 109054 | Q. robur | MG865597 | MH494023 | MH136988 |
| P. uniformis | P16206 | Alnus glutinosa | MK496514 | MH493905 | MH136992 |
| P. vignae | P3019 | Vigna sinensis | MG865598 | MH494024 | MH136989 |
| P. vulcanica | CBS 141216 | Fagus sylvatica | MF036209 | MF036235 | LC595951 |
| P. ×alni | IMI 392314 | A. glutinosa | MK496513 | MH493903 | MH136991 |
| P. ×heterohybrida | CBS 141207 | Water | KU517151 | KU899290 | LC595953 |
| P. ×incrassata | CBS 141209 | Water | KU517156 | KU899286 | LC595954 |
| P. ×multiformis | P16202 | A. glutinosa | MG783372 | MH493904 | MK493472 |
| Species | Accession Number | ITS Clade | Plant Species * | Nursery |
|---|---|---|---|---|
| P. acerina | MW892395 | 2 | Ai (1) | N4 |
| P. bilorbang | MW959911 | 6 | Phl (2) | N1 |
| P. cactorum | MW892396 | 1 | Cs (1) | N1 |
| P. cinnamomi | MW892397 | 7 | Cs (8), Fs (2), Aa (2), Jr (8), Qi (6) | N1-2-3 |
| P. citrophthora | MW959916 | 2 | Ln (1) | N1 |
| P. mediterranea | MW892398 | 7 | Mc (2) | N2 |
| P. megasperma | MW959913 | 6 | Ln (3) | N1 |
| P. nicotianae | MW892400 | 1 | Lo (1), Pl (2), Hi (4), Mc (2) | N2 |
| P. palmivora | MW959917 | 4 | Phl (5) | N1 |
| P. parvispora | MW892401 | 7 | Au (3) | N2 |
| P. pistaciae | MW892402 | 7 | Pl (2) | N2 |
| P. plurivora | MW892403 | 2 | Ai (3) | N4 |
| P. pseudocryptogea | MW959912 | 8 | Ln (4) | N1 |
| P. pseudosyringae | MW959914 | 3 | Ia (3) | N1 |
| P. psychrophila | MW959915 | 3 | Ia (1) | N1 |
| Species | Myrtle * | Lentisk * | Re-Isolation Frequency (%) |
|---|---|---|---|
| Phytophthora mediterranea | 1.01 ± 0.4a | 1.9 ± 0.6b | 100 (M) and 100 (L) |
| Phytophthora cinnamomi | 0.68 ± 0.3b | 2.3 ± 0.8a | 100 (M) and 100 (L) |
| Control | - | 0.5 ± 0.2c | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bregant, C.; Mulas, A.A.; Rossetto, G.; Deidda, A.; Maddau, L.; Piras, G.; Linaldeddu, B.T. Phytophthora mediterranea sp. nov., a New Species Closely Related to Phytophthora cinnamomi from Nursery Plants of Myrtus communis in Italy. Forests 2021, 12, 682. https://doi.org/10.3390/f12060682
Bregant C, Mulas AA, Rossetto G, Deidda A, Maddau L, Piras G, Linaldeddu BT. Phytophthora mediterranea sp. nov., a New Species Closely Related to Phytophthora cinnamomi from Nursery Plants of Myrtus communis in Italy. Forests. 2021; 12(6):682. https://doi.org/10.3390/f12060682
Chicago/Turabian StyleBregant, Carlo, Antonio A. Mulas, Giovanni Rossetto, Antonio Deidda, Lucia Maddau, Giovanni Piras, and Benedetto T. Linaldeddu. 2021. "Phytophthora mediterranea sp. nov., a New Species Closely Related to Phytophthora cinnamomi from Nursery Plants of Myrtus communis in Italy" Forests 12, no. 6: 682. https://doi.org/10.3390/f12060682
APA StyleBregant, C., Mulas, A. A., Rossetto, G., Deidda, A., Maddau, L., Piras, G., & Linaldeddu, B. T. (2021). Phytophthora mediterranea sp. nov., a New Species Closely Related to Phytophthora cinnamomi from Nursery Plants of Myrtus communis in Italy. Forests, 12(6), 682. https://doi.org/10.3390/f12060682








