Functional Trait Responses to Strip Clearcutting in a Moso Bamboo Forest
Abstract
1. Introduction
2. Material and Methods
2.1. Site Description
2.2. Leaves and Branch Sampling and Analysis
2.3. Fine Root Sampling and Analysis
2.4. Soil Sampling and Chemical Analysis
2.5. Data Analysis
3. Results
3.1. Soil Characteristics
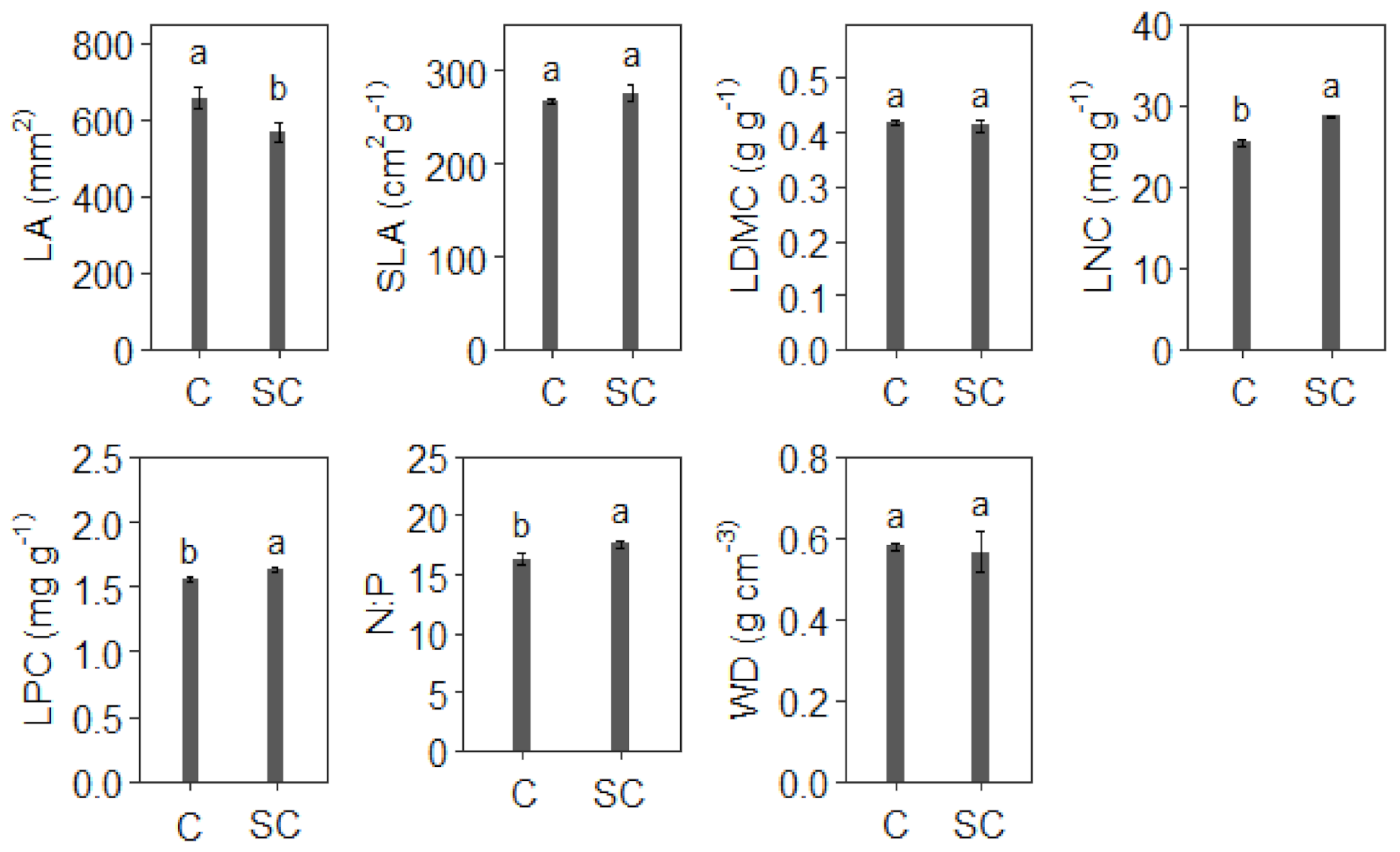
3.2. Leaf Trait and Wood Density
3.3. Relationships between Leaf Traits, Wood Density, and Soil Nutrients
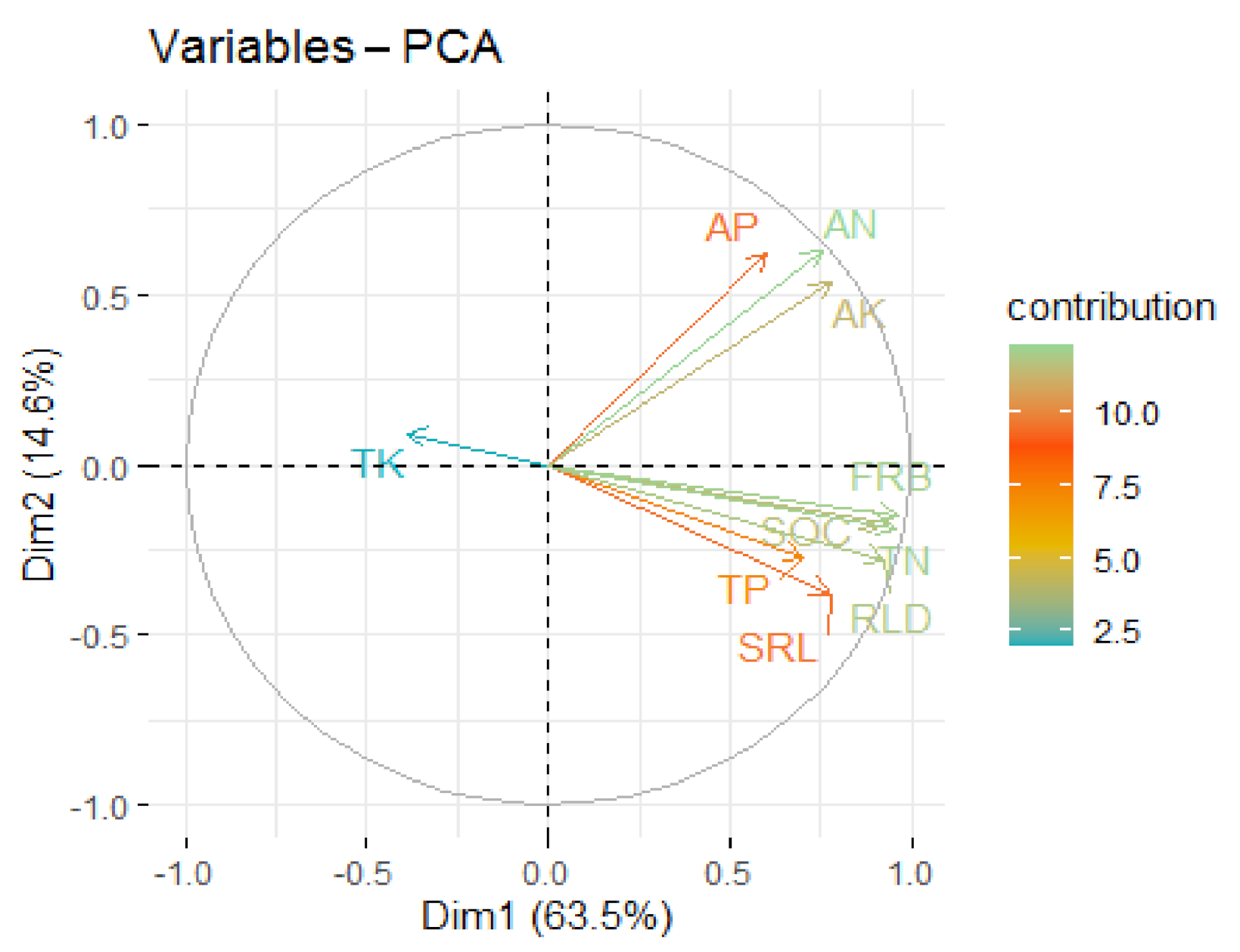
3.4. Associations between Leaf Traits and Stand
3.5. Fine Root Traits
3.6. Associations between Fine Root Trait and Stand and Soil Nutrients
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jiang, Z.H. Bamboo and Rattan in the World; China Forest Publishing House: Beijng, China, 2007. [Google Scholar]
- National Forestry and Grassland Administration. China Forest Resources Report; China Forest Publishing House: Beijng, China, 2019. [Google Scholar]
- Fan, S.; Zhao, J.; Su, W.; Yu, L.; Yan, Y. Comprehensive Evaluation of Soil Quality in Phyllostachys edulis Stands of Different Stocking Stocking Densities. Sci. Silvae Sin. 2015, 51, 1–9. [Google Scholar] [CrossRef]
- Wang, L.; Fan, C.; Liang, K.; Fan, Y.; Yang, G.; Zhang, L.; Shi, J. An Approach to a Labor-saving Cutting Model for Bamboo Forest Management—A Study Based on Clonal Integration. Acta Agric. Univ. Jiangxiensis 2016, 38, 1110–1118. [Google Scholar] [CrossRef]
- Li, X.; Lai, J.; Yu, Z.; Li, S.; Chen, L.; He, T.; Rong, J. Effect of Different Clear Cutting Band on Physiological Indexes of Phyllostachys pubescens. Mod. Agric. Sci. Technol. 2018, 18, 122–125. [Google Scholar]
- Zeng, X.; Su, W.; Fan, S.; Jin, Y. Qualitative Recovery Characteristics of Moso Bamboo Forests under Strip Clearcutting. Acta Bot. Boreali Occident. Sin. 2019, 39, 917–924. [Google Scholar] [CrossRef]
- Lilli, K.; Janne, V.; Mikael, M.; Sofie, H.; Mikko, K.; Anna, S.; Marjo, P.; Helmisaari, H.-S. Stump harvesting in Picea abies stands: Soil surface disturbance and biomass distribution of the harvested stumps and roots. For. Ecol. Manag. 2018, 425, 27–34. [Google Scholar] [CrossRef]
- Violle, C.; Enquist, B.J.; Mcgill, B.J.; Jiang, L.; Albert, C.H.; Hulshof, C.; Jung, V.; Messier, J. The return of the variance: Intraspecific variability in community ecology. Trends Ecol. Evol. 2012, 27, 244–252. [Google Scholar] [CrossRef]
- Funk, J.L.; Larson, J.E.; Ames, G.M.; Butterfield, B.J.; Cavender-Bares, J.; Firn, J.; Laughlin, D.C.; Sutton-Grier, A.; Williams, L.; Wright, J. Revisiting the Holy Grail: Using plant functional traits to understand ecological processes. Biol. Rev. 2017, 92, 1156–1173. [Google Scholar] [CrossRef]
- Freschet, G.T.; Bellingham, P.J.; Lyver, P.O.B.; Bonner, K.I.; Wardle, D.A. Plasticity in above-and belowground resource acquisition traits in response to single and multiple environmental factors in three tree species. Ecol. Evol. 2013, 3, 1065–1078. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Bhaskar, R.; Dawson, T.E.; Balvanera, P. Community assembly and functional diversity along succession post-management. Funct. Ecol. 2014, 28, 1256–1265. [Google Scholar] [CrossRef]
- Ding, Y.; Zang, R. Effects of thinning on the demography and functional community structure of a secondary tropical lowland rain forest. J. Environ. Manag. 2021, 279, 111805. [Google Scholar] [CrossRef]
- Cheng, J.; Chu, P.; Chen, D.; Bai, Y. Functional correlations between specific leaf area and specific root length along a regional environmental gradient in Inner Mongolia grasslands. Funct. Ecol. 2016, 30, 985–997. [Google Scholar] [CrossRef]
- Jianmin, S.; Xuehua, Y.; Fusheng, C.; Qingpei, Y.; Zuyao, L.; Kai, F. Adaptation of bamboo to heterogeneous habitat: Phenotypic plasticity. Acta Ecol. Sin. 2014, 34, 5687–5695. [Google Scholar] [CrossRef]
- Hui, C.M.; Du, F.; Yang, Y.M. Cultivation and Utilization of Bamboo; China Forestry Press: Beijing, China, 1996. [Google Scholar]
- Wang, Y.; Bai, S.; Binkley, D.; Zhou, G.; Fang, F. The independence of clonal shoot’s growth from light availability supports moso bamboo invasion of closed-canopy forest. For. Ecol. Manag. 2016, 368, 105–110. [Google Scholar] [CrossRef]
- Liu, X.; Feng, H.; Cai, C.; Fan, S.; Liu, G. Response of leaf functional traits of Moso bamboo during the invading process into the broad-leaved forest. J. Beijing For. Univ. 2015, 37, 8–17. [Google Scholar] [CrossRef]
- Rui, S.; Shangbin, B.; Guomo, Z.; Yixiang, W.; Nan, W.; Guosheng, W.; Juan, C. The response of root morphological plasticity to the expansion of a population of Phyllostachys edulis into a mixed needle-and broad-leaved forest. Acta Ecol. Sin. 2016, 36, 326–334. [Google Scholar] [CrossRef]
- Zeng, X.; Su, W.; Fan, S.; Jing, X.; Chu, H. Soil quality assessment in Moso bamboo forests under different strip clearcutting. Chin. J. Ecol. 2019, 38, 3015–3023. [Google Scholar] [CrossRef]
- Zhan, M.; Guan, F.; Yan, Y.; Zhang, M.; Zheng, Y. Effects of strip harvesting on species diversity of undergrowth in bamboo (Phyllostachys Edulis) forest. Acta Ecol. Sin. 2020, 40, 4169–4179. [Google Scholar] [CrossRef]
- Li, L.; Li, N.; Lu, D.; Chen, Y. Mapping Moso bamboo forest and its on-year and off-year distribution in a subtropical region using time-series Sentinel-2 and Landsat 8 data. Remote Sens. Environ. 2019, 231, 111265. [Google Scholar] [CrossRef]
- JT, T.; DJ, R. Use of nitrogen to phosphorus ratios in plant tissue as an indicator of nutrient limitation and nitrogen saturation. J. Appl. Ecol. 2003, 40, 523–534. [Google Scholar] [CrossRef]
- State Bureau of Technical Supervision. Testing Methods for Physical and Mechanical Properties of Bamboo; Standard Press of China Beijing: Beijing, China, 1995. [Google Scholar]
- Shaohui, F.; Fuming, X.; Silong, W.; Wenhui, S.; Xiaojun, Y.; Zhengqi, S. Fine Root Biomass and Turnover in Moso Bamboo Plantation in Huitong Forest Station, Hunan Province. Sci. Silvae Sin. 2009, 45, 1–6. [Google Scholar] [CrossRef]
- Liu, G.; Fan, S.; Cai, C.; Liu, X.; Li, Y.; Luo, T. Fine Root Biomass Distribution of Moso Bamboo at Different Ages. J. Trop. Subtrop. Bot. 2017, 25, 472–479. [Google Scholar]
- Lawren, S.; Christine, S.; John, G.P.; Hendrik, P.; Mason, C.M.; Rodrigo, M.A.; Donovan, L.A. How do leaf veins influence the worldwide leaf economic spectrum? Review and synthesis. J. Exp. Bot. 2013, 64, 4053–4080. [Google Scholar] [CrossRef]
- Wen, G.; Jian, Z.; Lianghua, Q.; Ze’an, S.; Rui, W.; Chang, Y. Leaf functional traits and influencing factors of Phyllostachys edulis and its varieties. J. For. Environ. 2020, 40, 260–268. [Google Scholar] [CrossRef]
- Jager, M.M.; Richardson, S.J.; Bellingham, P.J.; Clearwater, M.J.; Laughlin, D.C. Soil fertility induces coordinated responses of multiple independent functional traits. J. Ecol. 2015, 103, 374–385. [Google Scholar] [CrossRef]
- Ordoñez, J.C.; Bodegom, P.M.V.; Witte, J.P.M.; Wright, I.J.; Reich, P.B.; Aerts, R. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Glob. Ecol. Biogeogr. 2010, 18, 137–149. [Google Scholar] [CrossRef]
- Inoue; Tateishi; Sakuta; Yamamoto; Mizoue; Kitahara. Relationships of light environment to stand attributes in a stand of bamboo, Phyllostachys pubescens. Ecol. Eng. 2012, 38, 135–139. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Bai, Y.-F.; Han, X.-G. Application of N:P Stoichiometry to Ecology Studies. Acta Bot. Sin. 2003, 45, 1009–1018. [Google Scholar]
- Yin, Q.; Tian, T.; Han, X.; Xu, J.; Chai, Y.; Mo, J.; Lei, M.; Wang, L.; Yue, M. The relationships between biomass allocation and plant functional trait. Ecol. Indic. 2019, 102, 302–308. [Google Scholar] [CrossRef]
- Schreeg, L.A.; Santiago, L.S.; Wright, S.J.; Turner, B.L. Stem, root, and older leaf N:P ratios are more responsive indicators of soil nutrient availability than new foliage. Ecology 2014, 95, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Koerselman, W.; Meuleman, A. The Vegetation N:P Ratio: A New Tool to Detect the Nature of Nutrient Limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Hume, A.M.; Chen, H.Y.H.; Taylor, A.R. Intensive forest harvesting increases susceptibility of northern forest soils to carbon, nitrogen and phosphorus loss. J. Appl. Ecol. 2018, 55, 246–255. [Google Scholar] [CrossRef]
- White, P. Long-distance Transport in the Xylem and Phloem. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier: Adelaide, Australia, 2012; pp. 49–70. [Google Scholar]
- Liu, G. Study on the Mechanism of Maintaining Long-term Productivity of Bamboo Forest; Chinese Academy of Forestry: Beijing, China, 2019. [Google Scholar]
- Freschet, G.T.; Valverde-Barrantes, O.J.; Tucker, C.M.; Craine, J.M.; Mccormack, M.L.; Violle, C.; Fort, F.; Blackwood, C.B.; Urban-Mead, K.R.; Iversen, C.M. Climate, soil and plant functional types as drivers of global fine-root trait variation. J. Ecol. 2017, 105, 1182–1196. [Google Scholar] [CrossRef]
- Wurzburger, N.; Wright, S.J. Fine-root responses to fertilization reveal multiple nutrient limitation in a lowland tropical forest. Ecol. A Publ. Ecol. Soc. Am. 2015, 96, 2137–2146. [Google Scholar] [CrossRef]
- Weng, F.; Wang, K.; He, Q.; Wu, R. Absorbing ability of root and rhizome system of Phyllostachys pubescens shoot forest at different ages. J. Zhejiang A F Univ. 2001, 18, 28–30. [Google Scholar] [CrossRef]
- Wang, D.; Olatunji, O.A.; Xiao, J. Thinning increased fine root production, biomass, turnover rate and understory vegetation yield in a Chinese fir plantation. For. Ecol. Manag. 2019, 440, 92–100. [Google Scholar] [CrossRef]
- Tang, X.; Qi, L.; Fan, S.; Guan, F.; Zhang, H. Soil respiration and net ecosystem production in relation to intensive management in Moso bamboo forests. Catena 2016, 137, 219–228. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, G.; Zhang, R.; Wang, D.; Zhang, J. Climate change response using a simulation study of photosynthetic physiology on Phyllostachys pubescens. J. Zhejiang A F Univ. 2011, 28, 555–561. [Google Scholar] [CrossRef]
- Umemura, M.; Takenaka, C. Retranslocation and localization of nutrient elements in various organs of moso bamboo (Phyllostachys pubescens). Sci. Total Environ. 2014, 493, 845–853. [Google Scholar] [CrossRef]
- Tong, L.; Li, P.; Zhou, G.; Zhou, Y.; Li, C. A review of research about rhizome-root system in bamboo forest. J. Zhejiang A F Univ. 2019, 36, 183–192. [Google Scholar] [CrossRef]
- Chapin, F.S.; Matson, P.A.; Mooney, H.A. Principles of Terrestrial Ecosystem Ecology; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Zhong, Y.; Jiang, X.; Lou, C. Research progress in growth rhythm of bamboos. World Bamboo Ratt. 2014, 12, 35–44. [Google Scholar] [CrossRef]
- Wu, M.; Han, D.; Wand, J. Studies on the roots of young artificial seedling stands of Phyllostachys pubescens. Sci. Silv. Sin. 1984, 20, 201–204. [Google Scholar]
- Jiang, G.; Yu, L.; Li, Z.; Niu, H.; Shi, L. Hierarchical system and its quantitative attribute of moso bamboo rhizome. Chin. J. Ecol. 2017, 36, 3479–3484. [Google Scholar] [CrossRef]
- Eissenstat, D.; Yanai, R. The ecology of root lifespan. In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 1997; Volume 27, pp. 1–60. [Google Scholar]
- Schoettle, A.W.; Fahey, T.J.; Shoettle, A.W. Foliage and fine root longevity of pines. Ecol. Bull. 1994, 43, 136–153. [Google Scholar]
- Ostertag; Rebecca. Effects of Nitrogen and Phosphorus Availability on Fine-Root Dynamics in Hawaiian Montane Forests. Ecology 2001, 82, 485–499. [Google Scholar] [CrossRef]
- Zhu, K.; Song, Y.; Qin, C. Forest age improves understanding of the global carbon sink. Proc. Natl. Acad. Sci. USA 2019, 116, 3962–3964. [Google Scholar] [CrossRef]
- Yachi, S.; Loreau, M. Does complementary resource use enhance ecosystem functioning? A model of light competition in plant communities. Ecol. Lett. 2010, 10, 54–62. [Google Scholar] [CrossRef]
- Yirdaw, E.; Luukkanen, O. Photosynthetically active radiation transmittance of forest plantation canopies in the Ethiopian highlands. For. Ecol. Manag. 2004, 188, 17–24. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y. A Review on Within-gap Micro-environmental Heterogeneity and Species’ Response. J. Nanjing For. Univ. 2002, 26, 69–74. [Google Scholar] [CrossRef]
- Chuanbao, Y.; Xiaoping, Z.; Huijing, N.; Xu, G.; Zheke, Z. Soil carbon and associated bacterial community shifts driven by fine root traits along a chronosequence of Moso bamboo (Phyllostachys edulis) plantations in subtropical China. Sci. Total Environ. 2021, 752, 142333. [Google Scholar] [CrossRef]
- Westoby, M.; Wright, I. Land-plant ecology on the basis of functional traits. Trends Ecol. Evol. 2006, 21, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, D. The Effect of Thinning on Fine Root Morphology, Nutrients and Turnover of Chinese Fir Plantations; Nanjing Forestry University: Nanjing, China, 2013. [Google Scholar]


| Site | Soil Layers | SOC | TN | TP | TK | AN | AP | AK |
|---|---|---|---|---|---|---|---|---|
| (cm) | (g/kg) | (g/kg) | (g/kg) | (g/kg) | (mg/kg) | (mg/kg) | (mg/kg) | |
| SC | 0–10 | 27.20 ± 0.54 Aa | 2.15 ± 0.07 Aa | 0.28 ± 0.02 Aa | 9.17 ± 0.57 Aa | 167.95 ± 15.06 Aa | 5.66 ± 0.85 Aa | 50.61 ± 6.02 Aa |
| 10–20 | 17.00 ± 1.02 Ba | 1.21 ± 0.09 Ba | 0.23 ± 0.01 Ba | 9.26 ± 0.49 Aa | 99.83 ± 6.68 Ba | 3.40 ± 1.20 Ba | 28.73 ± 3.89 Ba | |
| 20–40 | 9.74 ± 0.67 Ca | 0.87 ± 0.03 Ca | 0.24 ± 0.02 Ba | 9.37 ± 0.45 Ba | 60.52 ± 9.94 Ca | 1.42 ± 1.00 Ba | 23.13 ± 4.32 Ba | |
| C | 0–10 | 30.50 ± 1.09 Ab | 2.30 ± 0.03 Ab | 0.29 ± 0.01 Aa | 8.92 ± 0.14 Aa | 128.87 ± 52.35 Aa | 2.38 ± 1.77 Ab | 40.78 ± 10.27 Aa |
| 10–20 | 21.93 ± 1.17 Bb | 1.72 ± 0.03 Bb | 0.26 ± 0.01 Bb | 9.05 ± 0.07 Aa | 130.81 ± 43.23 Aa | 2.61 ± 1.72 Aa | 40.82 ± 10.29 Aa | |
| 20–40 | 15.88 ± 1.42 Cb | 1.22 ± 0.04 Cb | 0.27 ± 0.02 ABa | 9.51 ± 0.25 Ba | 130.37 ± 41.30 Ab | 2.17 ± 1.84 Aa | 40.66 ± 10.12 Aa |
| Soil Layers | LA | SLA | LDMC | LNC | LPC | N:P | WD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | ||
| SOC | 0–10 | 0.707 | 0.160 | −0.463 | 0.356 | 0.471 | 0.346 | −0.939 | 0.005 ** | −0.792 | 0.060 | −0.924 | 0.009 ** | 0.058 | 0.914 |
| 10–20 | 0.838 | 0.037 * | −0.639 | 0.171 | 0.284 | 0.585 | −0.886 | 0.019 * | −0.826 | 0.043 * | −0.825 | 0.043 * | 0.249 | 0.635 | |
| 20–40 | 0.906 | 0.013 * | −0.509 | 0.303 | 0.199 | 0.706 | −0.918 | 0.009 ** | −0.938 | 0.006 ** | −0.809 | 0.051 | 0.157 | 0.766 | |
| TN | 0–10 | 0.924 | 0.009 ** | −0.499 | 0.314 | 0.709 | 0.114 | −0.869 | 0.025 * | −0.911 | 0.012 * | −0.752 | 0.085 | 0.543 | 0.265 |
| 10–20 | 0.937 | 0.006 ** | −0.694 | 0.126 | 0.506 | 0.305 | −0.962 | 0.002 ** | −0.899 | 0.015 * | −0.895 | 0.016 * | 0.414 | 0.415 | |
| 20–40 | 0.921 | 0.009 ** | −0.671 | 0.144 | 0.470 | 0.346 | −0.997 | 0.00 *** | −0.899 | 0.015 * | −0.946 | 0.004 ** | 0.315 | 0.544 | |
| TP | 0–10 | 0.546 | 0.262 | −0.458 | 0.361 | 0.910 | 0.012 * | −0.552 | 0.256 | −0.447 | 0.374 | −0.552 | 0.256 | 0.643 | 0.168 |
| 10–20 | 0.909 | 0.012 * | −0.779 | 0.068 | 0.328 | 0.526 | −0.904 | 0.013 * | −0.786 | 0.064 | −0.876 | 0.022 * | 0.381 | 0.456 | |
| 20–40 | 0.615 | 0.194 | −0.156 | 0.768 | 0.553 | 0.255 | −0.717 | 0.109 | −0.722 | 0.106 | −0.639 | 0.171 | 0.137 | 0.795 | |
| TK | 0–10 | 0.002 | 0.997 | −0.219 | 0.675 | 0.582 | 0.225 | 0.254 | 0.627 | 0.247 | 0.637 | 0.234 | 0.655 | 0.788 | 0.063 |
| 10–20 | −0.516 | 0.294 | 0.008 | 0.988 | −0.769 | 0.074 | 0.323 | 0.532 | 0.558 | 0.249 | 0.153 | 0.772 | −0.613 | 0.196 | |
| 20–40 | 0.419 | 0.408 | −0.871 | 0.024 * | 0.421 | 0.405 | −0.353 | 0.493 | −0.072 | 0.892 | −0.474 | 0.343 | 0.666 | 0.149 | |
| AN | 0–10 | −0.226 | 0.667 | 0.564 | 0.244 | −0.423 | 0.403 | 0.604 | 0.204 | 0.201 | 0.703 | 0.769 | 0.074 | 0.005 | 0.993 |
| 10–20 | 0.743 | 0.091 | −0.199 | 0.706 | 0.000 | 0.999 | −0.427 | 0.399 | −0.729 | 0.100 | −0.209 | 0.691 | 0.347 | 0.501 | |
| 20–40 | 0.918 | 0.009 ** | −0.475 | 0.341 | 0.177 | 0.737 | −0.747 | 0.088 | −0.893 | 0.017 * | −0.584 | 0.224 | 0.364 | 0.478 | |
| AP | 0–10 | −0.675 | 0.142 | 0.771 | 0.073 | −0.603 | 0.205 | 0.898 | 0.015 * | 0.598 | 0.210 | 0.972 | 0.001 ** | −0.305 | 0.556 |
| 10–20 | −0.108 | 0.838 | 0.656 | 0.157 | −0.196 | 0.710 | 0.445 | 0.377 | −0.032 | 0.952 | 0.669 | 0.146 | 0.027 | 0.959 | |
| 20–40 | 0.458 | 0.361 | 0.292 | 0.575 | −0.070 | 0.891 | −0.157 | 0.767 | −0.603 | 0.205 | 0.114 | 0.829 | 0.109 | 0.836 | |
| AK | 0–10 | −0.245 | 0.639 | 0.529 | 0.281 | −0.206 | 0.695 | 0.643 | 0.168 | 0.253 | 0.629 | 0.797 | 0.057 | 0.185 | 0.726 |
| 10–20 | 0.852 | 0.031 * | −0.482 | 0.333 | 0.058 | 0.914 | −0.621 | 0.188 | −0.777 | 0.069 | −0.466 | 0.352 | 0.386 | 0.449 | |
| 20–40 | 0.914 | 0.011 * | −0.554 | 0.254 | 0.148 | 0.779 | −0.751 | 0.085 | −0.853 | 0.031 * | −0.613 | 0.196 | 0.374 | 0.465 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Guan, F.; Fan, S.; Zhou, Y.; Jing, X. Functional Trait Responses to Strip Clearcutting in a Moso Bamboo Forest. Forests 2021, 12, 793. https://doi.org/10.3390/f12060793
Zheng Y, Guan F, Fan S, Zhou Y, Jing X. Functional Trait Responses to Strip Clearcutting in a Moso Bamboo Forest. Forests. 2021; 12(6):793. https://doi.org/10.3390/f12060793
Chicago/Turabian StyleZheng, Yaxiong, Fengying Guan, Shaohui Fan, Yang Zhou, and Xiong Jing. 2021. "Functional Trait Responses to Strip Clearcutting in a Moso Bamboo Forest" Forests 12, no. 6: 793. https://doi.org/10.3390/f12060793
APA StyleZheng, Y., Guan, F., Fan, S., Zhou, Y., & Jing, X. (2021). Functional Trait Responses to Strip Clearcutting in a Moso Bamboo Forest. Forests, 12(6), 793. https://doi.org/10.3390/f12060793






