Diurnal Tree Stem CH4 and N2O Flux Dynamics from a Riparian Alder Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Site
2.2. Stem and Soil Gas Flux Measurements
2.3. Measurements of Environmental Parameters
2.4. Data Quality Check and Statistical Analysis
3. Results
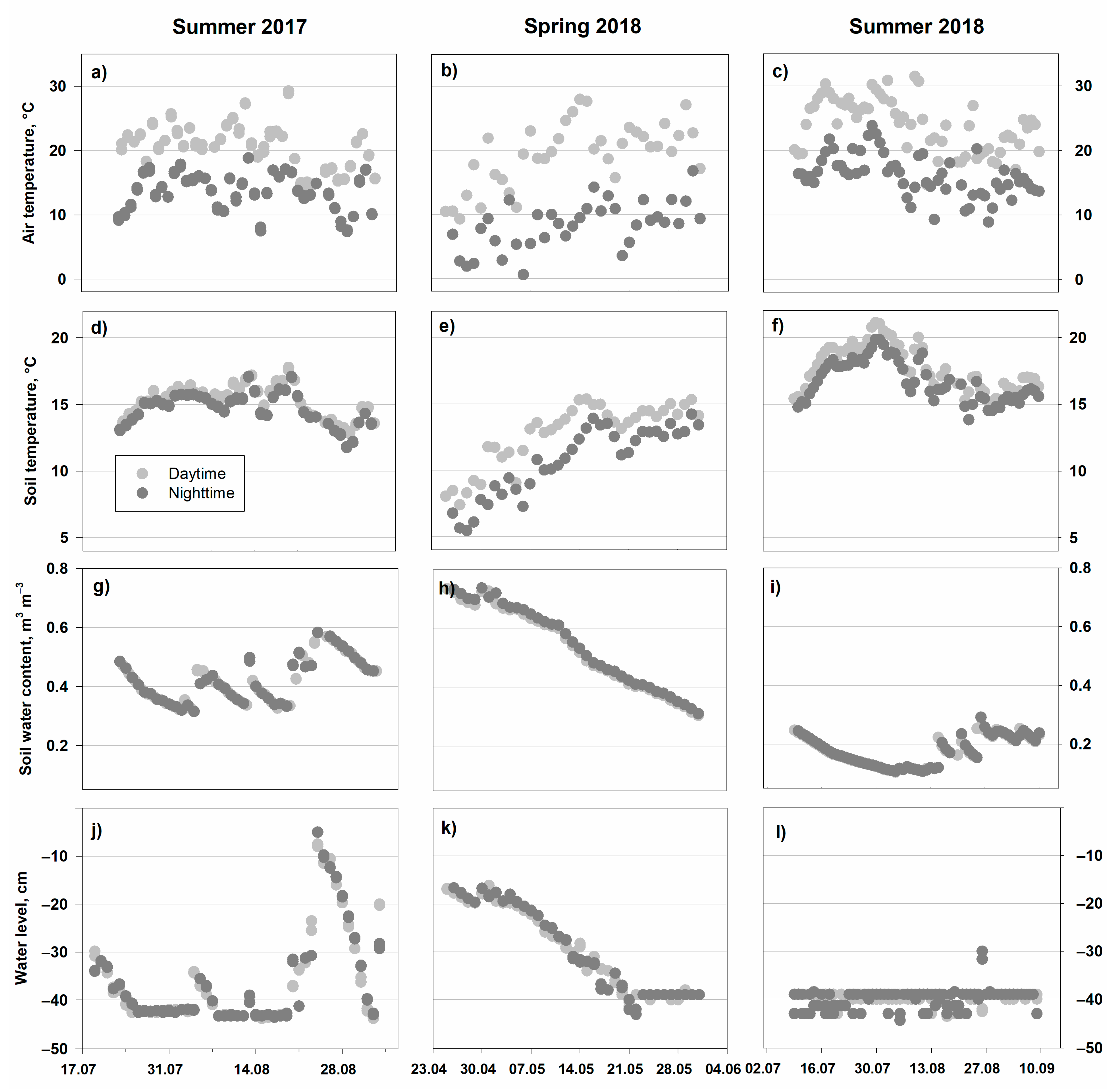
3.1. Environmental Parameters
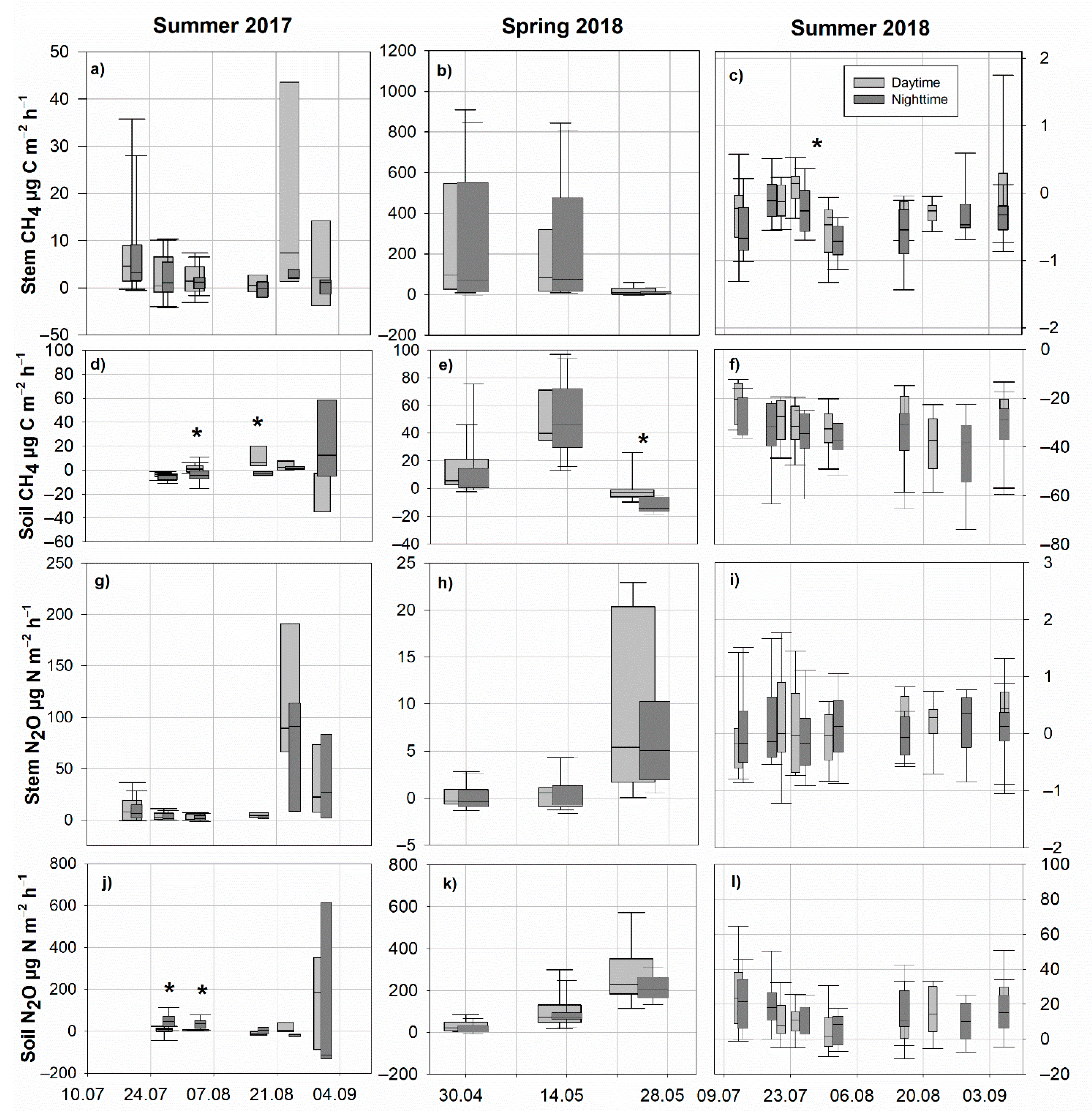
3.2. Soil and Stem CH4 Fluxes
3.3. Soil and Stem N2O Fluxes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maier, M.; Machacova, K.; Lang, F.; Svobodova, K.; Urban, O. Combining soil and tree-stem flux measurements and soil gas profiles to understand CH4 pathways in Fagus sylvatica forests. J. Plant Nutr. Soil Sci. 2018, 181, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Barba, J.; Bradford, M.A.; Brewer, P.E.; Bruhn, D.; Covey, K.; Van Haren, J.; Megonigal, J.P.; Mikkelsen, T.N.; Pangala, S.R.; Pihlatie, M.; et al. Methane emissions from tree stems: A new frontier in the global carbon cycle. New Phytol. 2019, 222, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Machacova, K.; Borak, L.; Agyei, T.; Schindler, T.; Soosaar, K.; Mander, Ü.; Ah-Peng, C. Trees as net sinks for methane (CH4) and nitrous oxide (N2O) in the lowland tropical rain forest on volcanic Réunion Island. New Phytol. 2021, 229, 1983–1994. [Google Scholar] [CrossRef]
- Uri, V.; Lõhmus, K.; Kiviste, A.; Aosaar, J. The dynamics of biomass production in relation to foliar and root traits in a grey alder (Alnus incana (L.) Moench) plantation on abandoned agricultural land. Forestry 2008, 82, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Rytter, L.; Rytter, R.-M. Growth and carbon capture of grey alder (Alnus incana (L.) Moench.) under north European conditions—Estimates based on reported research. For. Ecol. Manag. 2016, 373, 56–65. [Google Scholar] [CrossRef]
- Uri, V.; Tullus, H.; Lõhmus, K. Biomass production and nutrient accumulation in short-rotation grey alder (Alnus incana (L.) Moench) plantation on abandoned agricultural land. For. Ecol. Manag. 2002, 161, 169–179. [Google Scholar] [CrossRef]
- Uri, V.; Kukumägi, M.; Aosaar, J.; Varik, M.; Becker, H.; Soosaar, K.; Morozov, G.; Ligi, K.; Padari, A.; Ostonen, I.; et al. Carbon budgets in fertile grey alder (Alnus incana (L.) Moench.) stands of different ages. For. Ecol. Manag. 2017, 396, 55–67. [Google Scholar] [CrossRef]
- Uri, V.; Lõhmus, K.; Mander, Ü.; Ostonen, I.; Aosaar, J.; Maddison, M.; Helmisaari, H.-S.; Augustin, J. Long-term effects on the nitrogen budget of a short-rotation grey alder (Alnus incana (L.) Moench) forest on abandoned agricultural land. Ecol. Eng. 2011, 37, 920–930. [Google Scholar] [CrossRef]
- Aosaar, J.; Varik, M.; Lõhmus, K.; Ostonen, I.; Becker, H.; Uri, V. Long-term study of above- and below-ground biomass production in relation to nitrogen and carbon accumulation dynamics in a grey alder (Alnus incana (L.) Moench) plantation on former agricultural land. Eur. J. For. Res. 2013, 132, 737–749. [Google Scholar] [CrossRef]
- Sorz, J.; Hietz, P. Gas diffusion through wood: Implications for oxygen supply. Trees Struct. Funct. 2006, 20, 34–41. [Google Scholar] [CrossRef]
- Rusch, H.; Rennenberg, H. Black alder (Alnus Glutinosa (L.) Gaertn.) trees mediate methane and nitrous oxide emission from the soil to the atmosphere. Plant Soil 1998, 201, 1–7. [Google Scholar] [CrossRef]
- Schröder, P. Aeration of the root system in Alnus glutinosa L. Gaertn. Ann. Sci. For. 1989, 46, 310s–314s. [Google Scholar] [CrossRef]
- Huth, V.; Hoffmann, M.; Bereswill, S.; Popova, Y.; Zak, D.; Augustin, J. The climate warming effect of a fen peat meadow with fluctuating water table is reduced by young alder trees. Mires Peat 2018, 21, 1–18. [Google Scholar] [CrossRef]
- Schindler, T.; Mander, Ü.; Machacova, K.; Espenberg, M.; Krasnov, D.; Escuer-Gatius, J.; Veber, G.; Pärn, J.; Soosaar, K. Short-term flooding increases CH4 and N2O emissions from trees in a riparian forest soil-stem continuum. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Butterbach-Bahl, K.; Baggs, L.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.A.; Ball, T.; Conen, F.; Dobbie, K.E.; Massheder, J.; Rey, A. Exchange of greenhouse gases between soil and atmosphere: Interactions of soil physical factors and biological processes. Eur. J. Soil Sci. 2018, 69, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Machacova, K.; Vainio, E.; Urban, O.; Pihlatie, M. Seasonal dynamics of stem N2O exchange follow the physiological activity of boreal trees. Nat. Commun. 2019, 10, 4989. [Google Scholar] [CrossRef]
- Machacova, K.; Maier, M.; Svobodova, K.; Lang, F.; Urban, O. Cryptogamic stem covers may contribute to nitrous oxide consumption by mature beech trees. Sci. Rep. 2017, 7, 13243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smart, D.R.; Bloom, A.J. Wheat leaves emit nitrous oxide during nitrate assimilation. Proc. Natl. Acad. Sci. USA 2001, 98, 7875–7878. [Google Scholar] [CrossRef] [Green Version]
- Keppler, F.; Hamilton, J.T.G.; Brass, M.; Röckmann, T. Methane emissions from terrestrial plants under aerobic conditions. Nature 2006, 439, 187–191. [Google Scholar] [CrossRef]
- Flanagan, L.B.; Nikkel, D.J.; Scherloski, L.M.; Tkach, R.E.; Smits, K.M.; Selinger, L.B.; Rood, S.B. Multiple processes contribute to methane emission in a riparian cottonwood forest ecosystem. New Phytol. 2021, 229, 1970–1982. [Google Scholar] [CrossRef]
- Lenhart, K.; Weber, B.; Elbert, W.; Steinkamp, J.; Clough, T.; Crutzen, P.; Pöschl, U.; Keppler, F. Nitrous oxide and methane emissions from cryptogamic covers. Glob. Chang. Biol. 2015, 21, 3889–3900. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, L.C.; Maher, D.T.; Chiri, E.; Leung, P.M.; Nauer, P.A.; Arndt, S.K.; Tait, D.R.; Greening, C.; Johnston, S.G. Bark-dwelling methanotrophic bacteria decrease methane emissions from trees. Nat. Commun. 2021, 12, 1–8. [Google Scholar] [CrossRef]
- Putkinen, A.; Siljanen, H.M.P.; Laihonen, A.; Paasisalo, I.; Porkka, K.; Tiirola, M.; Haikarainen, I.; Tenhovirta, S.; Pihlatie, M. New insight to the role of microbes in the methane exchange in trees: Evidence from metagenomic sequencing. New Phytol. 2021, 231, 524–536. [Google Scholar] [CrossRef]
- Terazawa, K.; Yamada, K.; Ohno, Y.; Sakata, T.; Ishizuka, S. Spatial and temporal variability in methane emissions from tree stems of Fraxinus mandshurica in a cool-temperate floodplain forest. Biogeochemistry 2015, 123, 349–362. [Google Scholar] [CrossRef]
- Pitz, S.L.; Megonigal, J.P.; Chang, C.-H.; Szlavecz, K. Methane fluxes from tree stems and soils along a habitat gradient. Biogeochemistry 2018, 137, 307–320. [Google Scholar] [CrossRef]
- Pangala, S.R.; Gowing, D.J.; Hornibrook, E.; Gauci, V. Controls on methane emissions from Alnus glutinosa saplings. New Phytol. 2014, 201, 887–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barba, J.; Poyatos, R.; Vargas, R. Automated measurements of greenhouse gases fluxes from tree stems and soils: Magnitudes, patterns and drivers. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Jeffrey, L.C.; Maher, D.T.; Tait, D.R.; Euler, S.; Johnston, S.G. Tree stem methane emissions from subtropical lowland forest (Melaleuca quinquenervia) regulated by local and seasonal hydrology. Biogeochemistry 2020, 151, 273–290. [Google Scholar] [CrossRef]
- Pitz, S.; Megonigal, J.P. Temperate forest methane sink diminished by tree emissions. New Phytol. 2017, 214, 1432–1439. [Google Scholar] [CrossRef] [Green Version]
- Kupper, P.; Sõber, J.; Sellin, A.; Lõhmus, K.; Tullus, A.; Räim, O.; Lubenets, K.; Tulva, I.; Uri, V.; Zobel, M.; et al. An experimental facility for free air humidity manipulation (FAHM) can alter water flux through deciduous tree canopy. Environ. Exp. Bot. 2011, 72, 432–438. [Google Scholar] [CrossRef]
- Soosaar, K.; Mander, Ü.; Maddison, M.; Kanal, A.; Kull, A.; Lõhmus, K.; Truu, J.; Augustin, J. Dynamics of gaseous nitrogen and carbon fluxes in riparian alder forests. Ecol. Eng. 2011, 37, 40–53. [Google Scholar] [CrossRef]
- Livingston, G.P.; Hutchinson, G.L. Enclosure-based measurement of trace gas exchange: Applications and sources of error. In Biogenic Trace Gases: Measuring Emissions from Soil and Water; Matson, P.A., Harris, R.C., Eds.; Blackwell Science Ltd.: Oxford, UK, 1995; pp. 14–51. [Google Scholar]
- Pangala, S.R.; Hornibrook, E.; Gowing, D.J.G.; Gauci, V. The contribution of trees to ecosystem methane emissions in a temperate forested wetland. Glob. Chang. Biol. 2015, 21, 2642–2654. [Google Scholar] [CrossRef] [PubMed]
- Mikkelä, C.; Sundh, I.; Svensson, B.H.; Nilsson, M. Diurnal variation in methane emission in relation to the water table, soil temperature, climate and vegetation cover in a Swedish acid mire. Biogeochemistry 1995, 28, 93–114. [Google Scholar] [CrossRef]
- Le Mer, J.; Roger, P. of methane by soils: A review. Eur. J. Soil Biol. 2001, 37, 25–50. [Google Scholar] [CrossRef]
- Koch, O.; Tscherko, D.; Kandeler, E. Seasonal and Diurnal Net Methane Emissions from Organic Soils of the Eastern Alps, Austria: Effects of Soil Temperature, Water Balance, and Plant Biomass. Arctic Antarct. Alp. Res. 2007, 39, 438–448. [Google Scholar] [CrossRef] [Green Version]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Chem. Erde 2016, 76, 327–352. [Google Scholar] [CrossRef] [Green Version]
- Whiting, G.J.; Chanton, J.P. Plant-dependant CH4 emission in a subartic Canadia fen. Glob. Biogeochem. Cycles 1992, 6, 225–231. [Google Scholar] [CrossRef]
- Joabsson, A.; Christensen, T.R.; Wallén, B. Vascular plant controls on methane emissions from northern peatforming wetlands. Trends Ecol. Evol. 1999, 14, 385–388. [Google Scholar] [CrossRef]
- Machacova, K.; Papen, H.; Kreuzwieser, J.; Rennenberg, H. Inundation strongly stimulates nitrous oxide emissions from stems of the upland tree Fagus sylvatica and the riparian tree Alnus glutinosa. Plant Soil 2013, 364, 287–301. [Google Scholar] [CrossRef]
- Klemedtsson, L.; Svensson, B.; Rosswall, T. Relationships between soil moisture content and nitrous oxide production during nitrification and denitrification. Biol. Fertil. Soils 1988, 6, 106–111. [Google Scholar] [CrossRef]
- Bateman, E.J.; Baggs, E.M. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Christiansen, J.R.; Vesterdal, L.; Gundersen, P. Nitrous oxide and methane exchange in two small temperate forest catchments—effects of hydrological gradients and implications for global warming potentials of forest soils. Biogeochemistry 2010, 107, 437–454. [Google Scholar] [CrossRef]
- Barrat, H.A.; Evans, J.; Chadwick, D.R.; Clark, I.M.; Le Cocq, K.; Cardenas, L.M. The impact of drought and rewetting on N2O emissions from soil in temperate and Mediterranean climates. Eur. J. Soil Sci. 2020, 1–13. [Google Scholar] [CrossRef]
- Schützenmeister, K.; Meurer, K.H.E.; Gronwald, M.; Hartmann, A.B.D.; Gansert, D.; Jungkunst, H.F. N2O emissions from plants are reduced under photosynthetic activity. Plant Environ. Interact. 2020, 1, 48–56. [Google Scholar] [CrossRef]
- Megonigal, J.P.; Brewer, P.E.; Knee, K.L. Radon as a natural tracer of gas transport through trees. New Phytol. 2020, 225, 1470–1475. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schindler, T.; Machacova, K.; Mander, Ü.; Escuer-Gatius, J.; Soosaar, K. Diurnal Tree Stem CH4 and N2O Flux Dynamics from a Riparian Alder Forest. Forests 2021, 12, 863. https://doi.org/10.3390/f12070863
Schindler T, Machacova K, Mander Ü, Escuer-Gatius J, Soosaar K. Diurnal Tree Stem CH4 and N2O Flux Dynamics from a Riparian Alder Forest. Forests. 2021; 12(7):863. https://doi.org/10.3390/f12070863
Chicago/Turabian StyleSchindler, Thomas, Katerina Machacova, Ülo Mander, Jordi Escuer-Gatius, and Kaido Soosaar. 2021. "Diurnal Tree Stem CH4 and N2O Flux Dynamics from a Riparian Alder Forest" Forests 12, no. 7: 863. https://doi.org/10.3390/f12070863
APA StyleSchindler, T., Machacova, K., Mander, Ü., Escuer-Gatius, J., & Soosaar, K. (2021). Diurnal Tree Stem CH4 and N2O Flux Dynamics from a Riparian Alder Forest. Forests, 12(7), 863. https://doi.org/10.3390/f12070863






