Impact of Soil-Applied Microbial Inoculant and Fertilizer on Fungal and Bacterial Communities in the Rhizosphere of Robinia sp. and Populus sp. Plantations
Abstract
1. Introduction
2. Materials and Methods
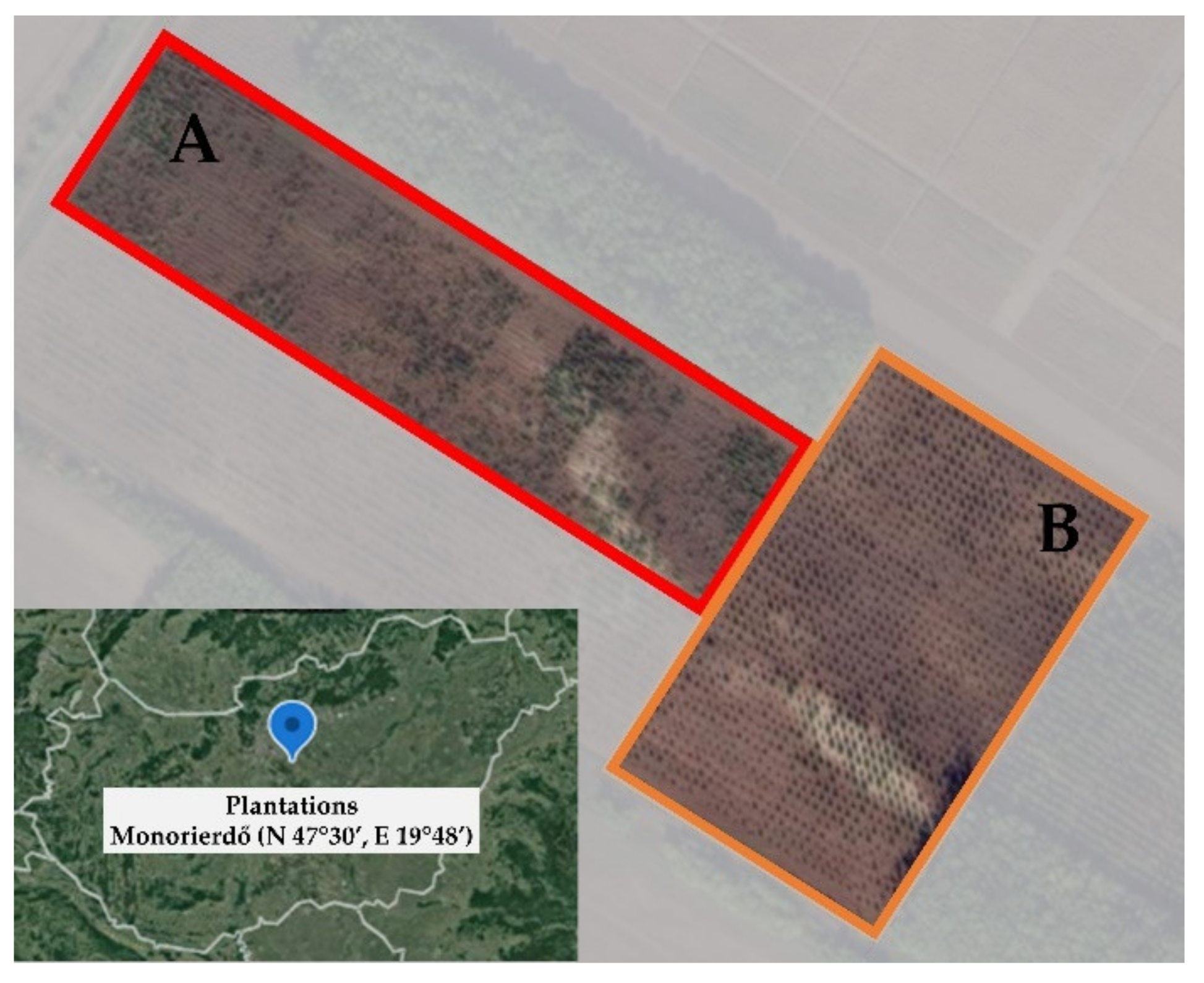
2.1. Study Site
2.2. Intensively Cultivated Plantations Experiment Setup
2.3. Metagenomic Sequencing
2.4. Statistical Analysis
3. Results
3.1. Biomass Production and Plant Growth Rate
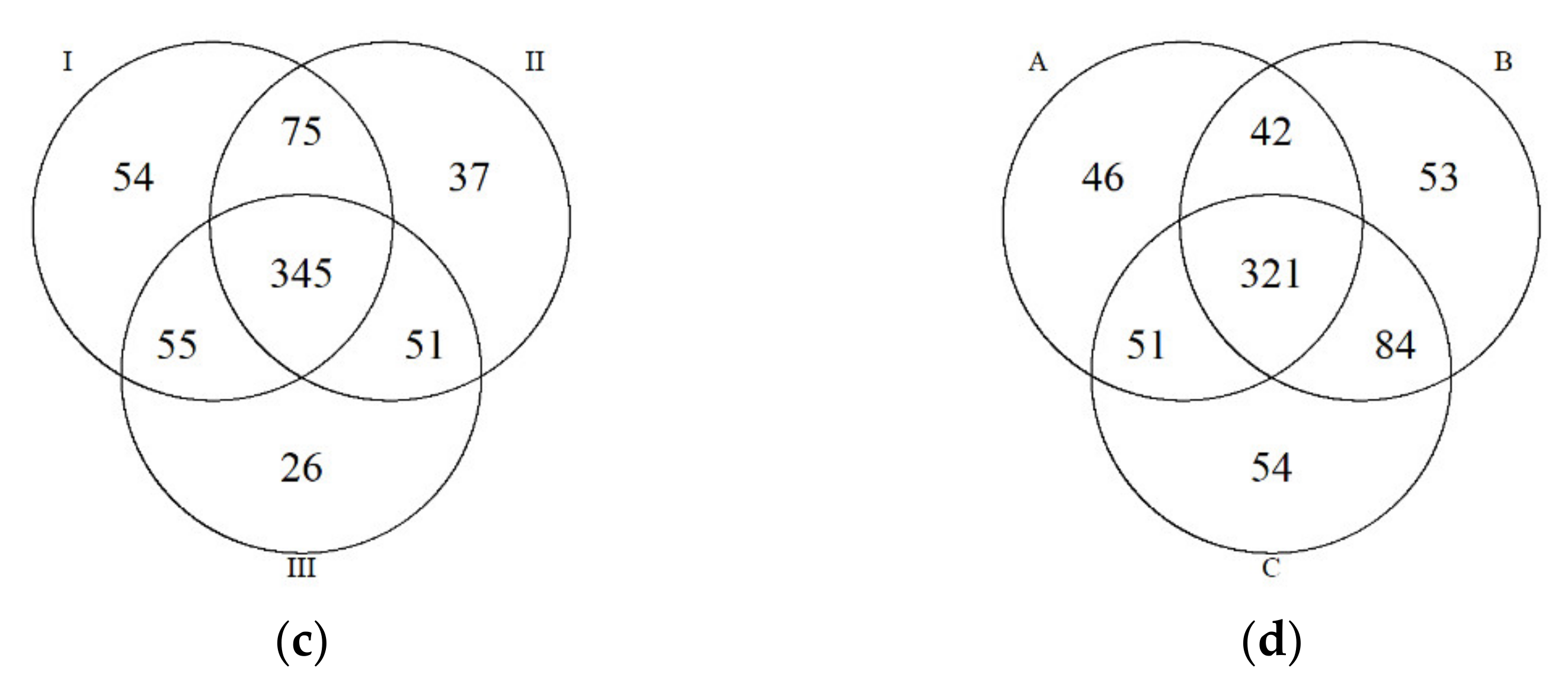
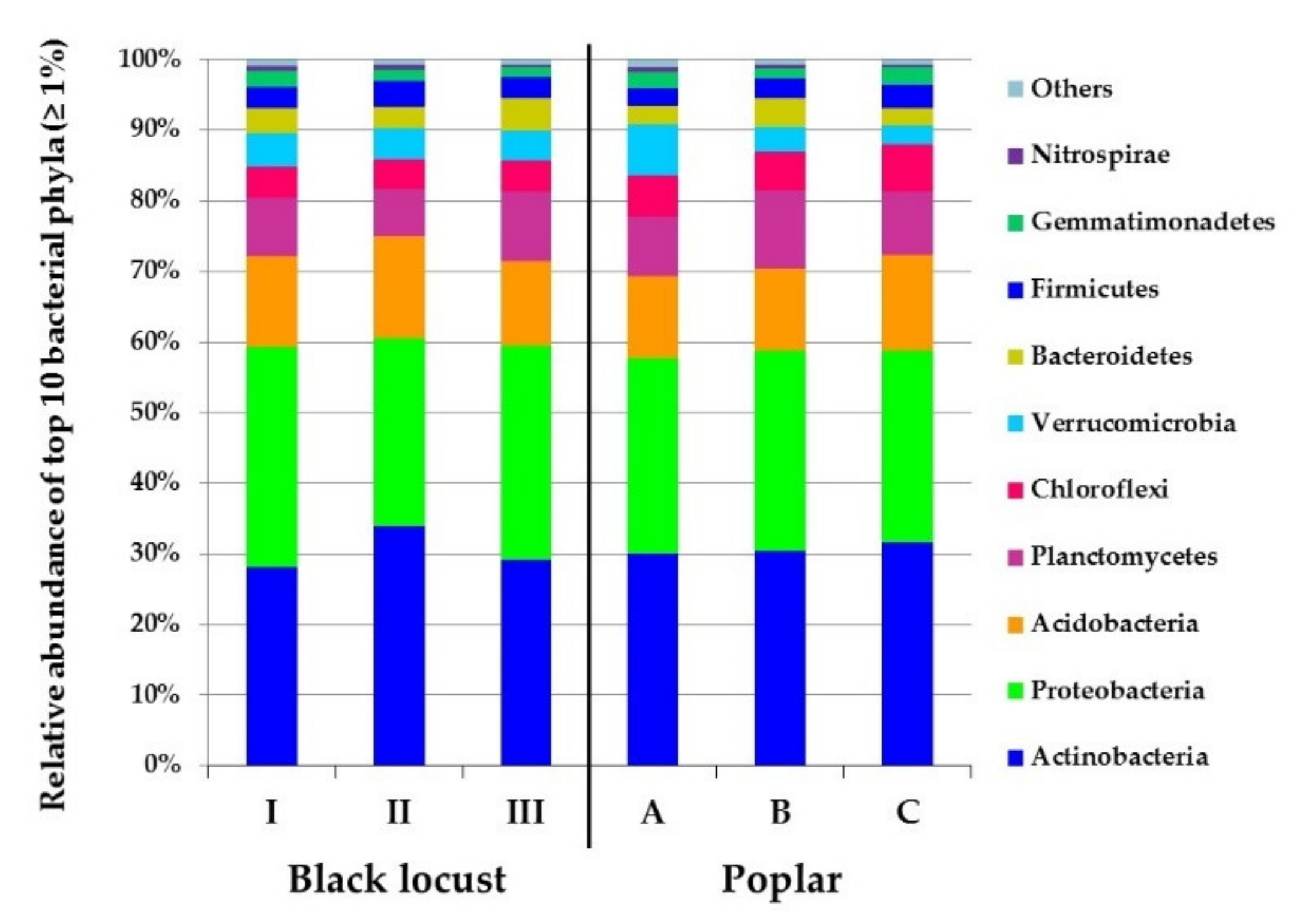
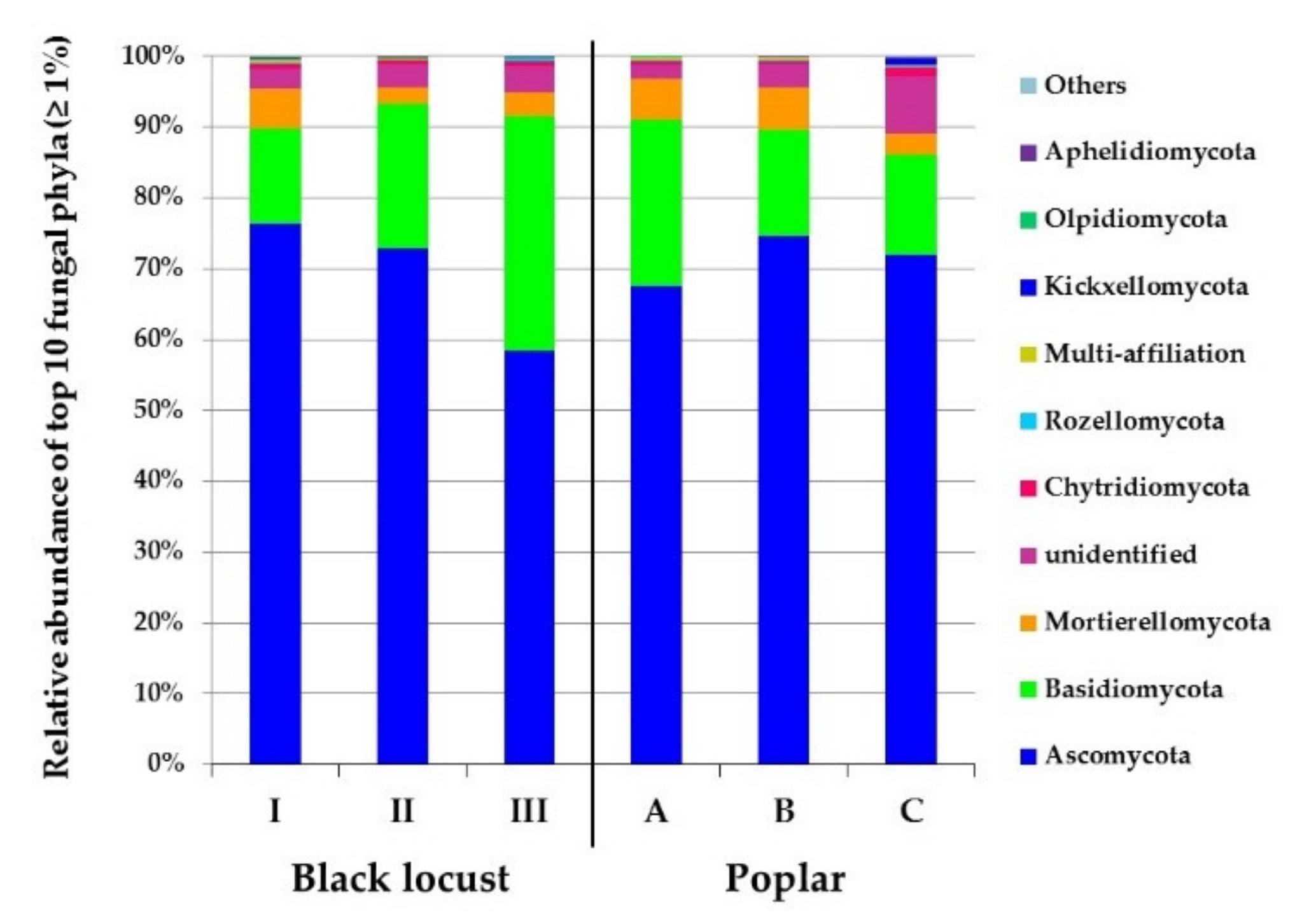
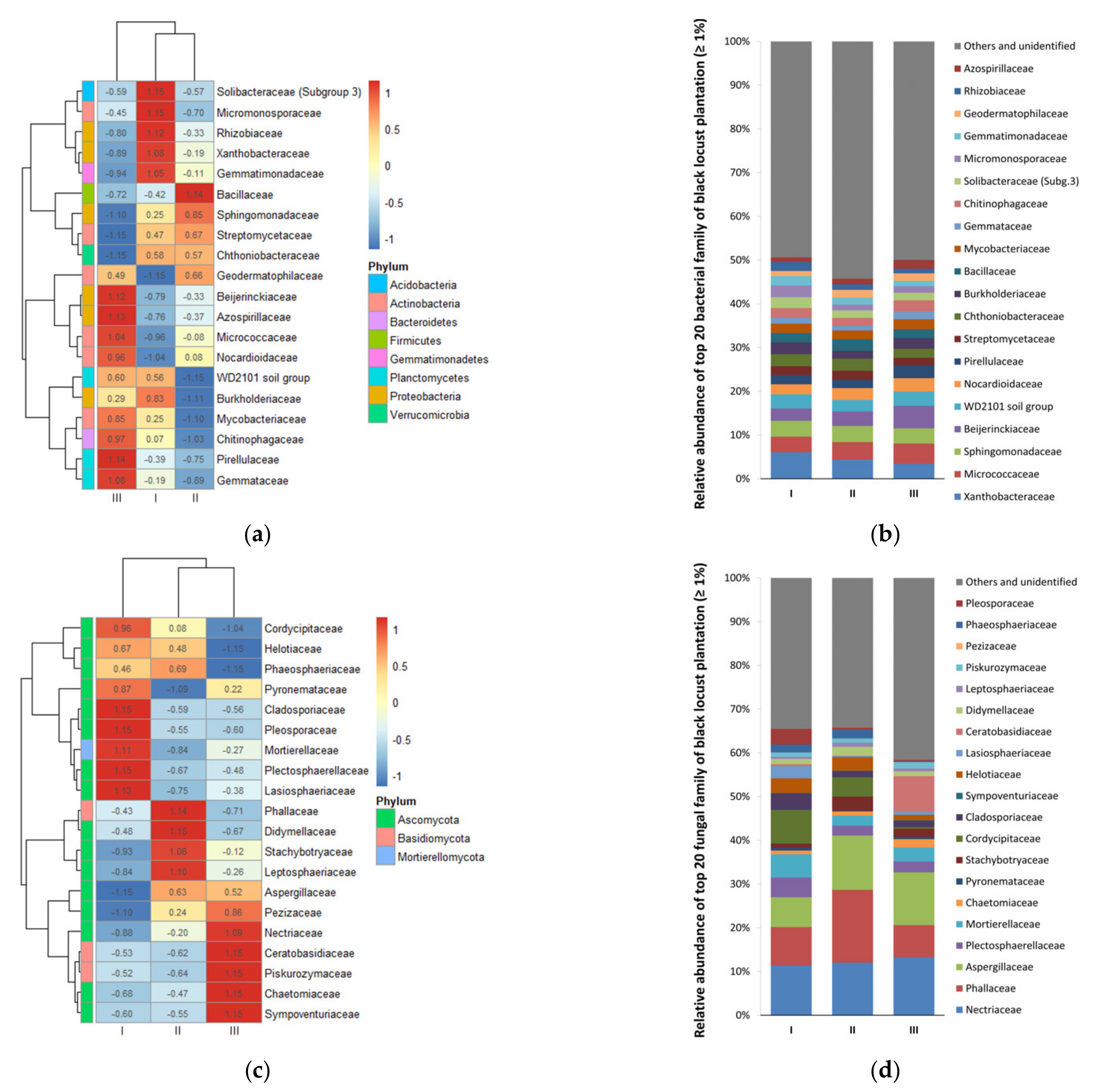
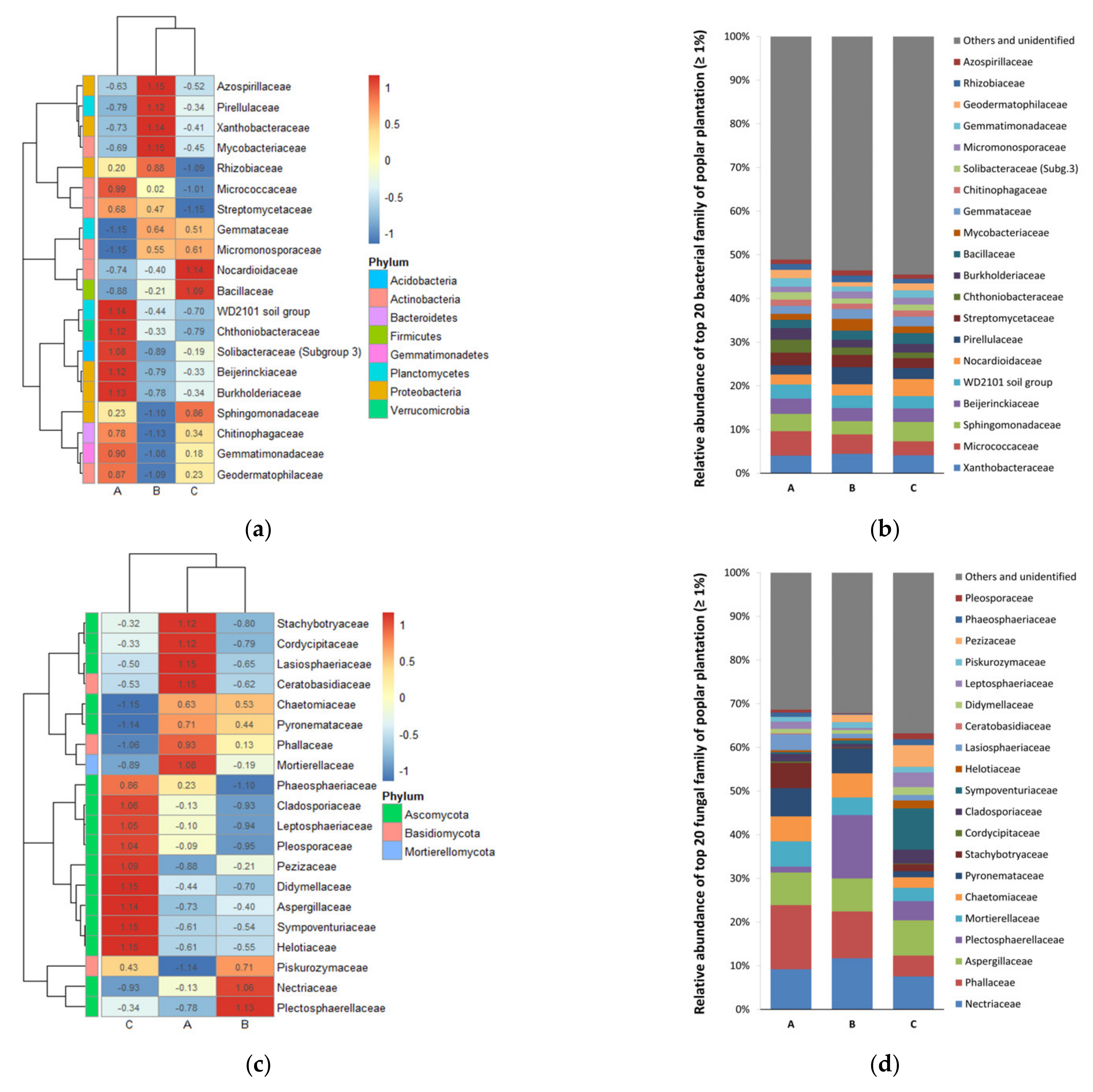
3.2. Diversity and Composition of Microbial Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sitzia, T.; Cierjacks, A.; de Rigo, D.; Caudullo, G. Robinia pseudoacacia in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; Publication Office of the European Union: Luxembourg, 2016; pp. 166–167. [Google Scholar]
- Hungarian Central Statistical Office. Available online: https://www.ksh.hu/docs/hun/xstadat/xstadat_eves/i_ome002b.html (accessed on 23 March 2021).
- Straker, K.C.; Quinn, L.D.; Voigt, T.B.; Lee, D.K.; Kling, G.J. Black locust as a bioenergy feedstock: A review. Bioenergy Res. 2015, 8, 1117–1135. [Google Scholar] [CrossRef]
- Rédei, K.; Keserű, Z.; Antal, B. Improved clonal approaches to growing black locust (Robinia pseudoacacia L.) in Hungary: A case study. Int. J. Hortic. Sci. 2015, 21, 53–56. [Google Scholar] [CrossRef][Green Version]
- Rédei, K.; Osvath-Bujtas, Z.; Veperdi, I. Black locust (Robinia pseudoacacia L.) improvement in Hungary: A review. Acta Silv. Lignaria Hung. 2008, 4, 127–132. [Google Scholar]
- Bolat, I.; Kara, Ö.; Sensoy, H.; Yüksel, K. Influences of black locust (Robinia pseudoacacia L.) afforestation on soil microbial biomass and activity. iForest-Biogeosci. For. 2015, 9, 171. [Google Scholar] [CrossRef]
- Papaioannou, A.; Chatzistahis, T.; Papaioannou, E.; Papadopoulos, G. Robinia pseudoacacia as a valuable invasive species for the restoration of degraded croplands. Catena 2016, 137, 310–317. [Google Scholar] [CrossRef]
- Gentili, R.; Ferrè, C.; Cardarelli, E.; Montagnani, C.; Bogliani, G.; Citterio, S.; Comolli, R. Comparing negative impacts of Prunus serotina, Quercus rubra and Robinia pseudoacacia on native forest ecosystems. Forests 2019, 10, 842. [Google Scholar] [CrossRef]
- Gillespie, A.R.; Pope, P.E. Rhizosphere acidification increases phosphorus recovery of black locust: II. Model predictions and measured recovery. Soil Sci. Soc. Am. J. 1990, 54, 538–541. [Google Scholar] [CrossRef]
- Tateno, R.; Tokuchi, N.; Yamanaka, N.; Du, S.; Otsuki, K.; Shimamura, T.; Xue, Z.; Wang, S.; Hou, Q. Comparison of litterfall production and leaf litter decomposition between an exotic black locust plantation and an indigenous oak forest near Yan’an on the Loess Plateau. China. For. Ecol. Manag. 2007, 241, 84–90. [Google Scholar] [CrossRef]
- Wang, Y.L.; Liu, G.B.; Kume, T.; Otsuki, K.; Yamanaka, N.; Du, S. Estimating water use of a black locust plantation by the thermal dissipation probe method in the semiarid region of Loess Plateau, China. J. For. Res. 2010, 15, 241–251. [Google Scholar] [CrossRef]
- Mantovani, D.; Veste, M.; Böhm, C.; Vignudelli, M.; Freese, D. Spatial and temporal variation of drought impact on black locust (Robinia pseudoacacia L.) water status and growth. Forest 2015, 8, 743–747. [Google Scholar] [CrossRef]
- Quinkenstein, A.; Boehm, C.; da Silva Matos, E.; Freese, D.; Huettl, R.F. Assessing the carbon sequestration in short rotation coppices of Robinia pseudoacacia L. on marginal sites in Northeast Germany. In Carbon Sequestration Potential of Agroforestry Systems; Springer: Dordrecht, The Netherlands, 2011; pp. 201–216. [Google Scholar] [CrossRef]
- Wang, J.J.; Hu, C.X.; Bai, J.; Gong, C.M. Carbon sequestration of mature black locust stands on the Loess Plateau, China. Plant Soil Environ. 2015, 61, 116–121. [Google Scholar] [CrossRef]
- Cierjacks, A.; Kowarik, I.; Joshi, J.; Hempel, S.; Ristow, M.; von der Lippe, M.; Weber, E. Biological flora of the British Isles: Robinia pseudoacacia. J. Ecol. 2013, 101, 1623–1640. [Google Scholar] [CrossRef]
- Song, X.; Shi, S.; Lu, S.; Ren, R.; He, C.; Meng, P.; Zhang, J.; Yin, C.; Zhang, X. Changes in soil chemical properties following afforestation of cropland with Robinia pseudoacacia in the southeastern Loess Plateau of China. For. Ecol. Manag. 2021, 487, 118993. [Google Scholar] [CrossRef]
- Dupraz, C.; Liagre, F. Agroforesterie: Des Arbres et Des Cultures; France Agricole Editions: Paris, France, 2008. [Google Scholar]
- Barrio-Anta, M.; Sixto-Blanco, H.; Canellas-Rey, D.V.I.; Castedo-Dorado, F. Dynamic growth model for I-214 poplar plantations in the northern and central plateaux in Spain. For. Ecol. Manag. 2008, 255, 1167–1178. [Google Scholar] [CrossRef]
- Rytter, R.M. The potential of willow and poplar plantations as carbon sinks in Sweden. Biomass Bioenergy 2012, 36, 86–95. [Google Scholar] [CrossRef]
- Danielsen, L.; Lohaus, G.; Sirrenberg, A.; Karlovsky, P.; Bastien, C.; Pilate, G.; Polle, A. Ectomycorrhizal colonization and diversity in relation to tree biomass and nutrition in a plantation of transgenic poplars with modified lignin biosynthesis. PLoS ONE 2013, 8, e59207. [Google Scholar] [CrossRef]
- Habib, M.T.; Heller, T.; Polle, A. Molecular physiology of tree ectomycorrhizal interactions. In Plant Roots: The Hidden Half; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar] [CrossRef]
- Luo, Z.B.; Wu, C.; Zhang, C.; Li, H.; Lipka, U.; Polle, A. The role of ectomycorrhizas in heavy metal stress tolerance of host plants. Environ. Exp. Bot. 2014, 108, 47–62. [Google Scholar] [CrossRef]
- Jeffries, P.; Gianinazzi, S.; Perotto, S.; Turnau, K.; Barea, J.M. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils 2003, 37, 1–16. [Google Scholar] [CrossRef]
- Toljander, J. Interactions between Soil Bacteria and Arbuscular Mycorrhizal Fungi; Swedish University of Agriculture Sciences, Acta Universitatis Agriculturae Sueciae: Uppsala, Sweden, 2006. [Google Scholar]
- Barman, J.; Samanta, A.; Saha, B.; Datta, S. Mycorrhiza. Resonance 2016, 21, 1093–1104. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Zhang, X.; Tang, M. Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis, water status, and K+/Na+ homeostasis. Front. Plant Sci. 2017, 8, 1739. [Google Scholar] [CrossRef]
- He, F.; Sheng, M.; Tang, M. Effects of Rhizophagus irregularis on photosynthesis and antioxidative enzymatic system in Robinia pseudoacacia L. under drought stress. Front. Plant Sci. 2017, 8, 183. [Google Scholar] [CrossRef] [PubMed]
- Dagher, D.J.; de la Providencia, I.E.; Pitre, F.E.; St-Arnaud, M.; Hijri, M. Arbuscular mycorrhizal fungal assemblages significantly shifted upon bacterial inoculation in non-contaminated and petroleum-contaminated environments. Microorganisms 2020, 8, 602. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.E.; White, J.R.; Wafula, D.; Athar, R.; Dickerson, T.; Williams, H.N.; Chauhan, A. Soil functional diversity analysis of a bauxite-mined restoration Chronosequence. Microb. Ecol. 2010, 59, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Diagne, N.; Ngom, M.; Djighaly, P.I.; Fall, D.; Hocher, V.; Svistoonoff, S. Roles of arbuscular mycorrhizal fungi on plant growth and performance: Importance in biotic and abiotic stressed regulation. Diversity 2020, 12, 370. [Google Scholar] [CrossRef]
- Liu, J.; Ha, V.N.; Shen, Z.; Zhu, H.; Zhao, F.; Zhao, Z. Characteristics of bulk and rhizosphere soil microbial community in an ancient Platycladus orientalis forest. Appl. Soil Ecol. 2018, 132, 91–98. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, N.; Zhao, Y.; Wang, L.; Zhang, C.; Li, X.; Cao, K.; Gao, Y. A consecutive 4-year elevated air temperature shaped soil bacterial community structure and metabolic functional groups in the rhizosphere of black locust seedlings exposed to lead pollution. Sci. Total Environ. 2020, 732, 139273. [Google Scholar] [CrossRef]
- Giannoulis, K.D.; Skoufogianni, E.; Bartzialis, D.; Solomou, A.D.; Danalatos, N.G. Growth and productivity of Salvia officinalis L. under Mediterranean climatic conditions depends on biofertilizer, nitrogen fertilization, and sowing density. Ind. Crop. Prod. 2021, 160, 113136. [Google Scholar] [CrossRef]
- Hungarian Meteorological Service. Available online: https://www.met.hu/eghajlat/magyarorszag_eghajlata/ (accessed on 29 August 2021).
- Escudie, F.; Auer, L.; Bernard, M.; Mariadassou, M.; Cauquil, L.; Vidal, K.; Maman, S.; Hernandez-Raquet, G.; Combes, S.; Pascal, G. FROGS: Find, Rapidly, OTUs with Galaxy Solution. Bioinformatics 2018, 34, 1287–1294. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Mahé, F.; Rognes, T.; Quince, C.; de Vargas, C.; Dunthorn, M. Swarm: Robust and fast clustering method for amplicon-based studies. PeerJ 2014, 2, e593. [Google Scholar] [CrossRef]
- McGinnis, S.; Madden, T.L. BLAST: At the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Res. 2004, 32 (Suppl. S2), W20–W25. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. Available online: https://www.r-project.org/ (accessed on 10 February 2020).
- Bzdyk, R.M.; Olchowik, J.; Studnicki, M.; Nowakowska, J.A.; Oszako, T.; Urban, A.; Hilszczańska, D. Ectomycorrhizal Colonisation in Declining Oak Stands on the Krotoszyn Plateau, Poland. Forests 2019, 10, 30. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z. Assessing bacterial diversity in soil. J. Soils Sediments 2008, 8, 379–388. [Google Scholar] [CrossRef]
- Wei, H.; Peng, C.; Yang, B.; Song, H.; Li, Q.; Jiang, L.; Wei, G.; Wang, K.; Wang, H.; Liu, S.; et al. Contrasting Soil Bacterial Community, Diversity, and Function in Two Forests in China. Front. Microbiol. 2018, 9, 1693. [Google Scholar] [CrossRef]
- Wu, S.J.; Deng, J.; Yin, Y.; Qin, S.J.; Zhu, W.X.; Zhou, Y.B.; Wang, B.; Ruan, H.; Jin, L. Bacterial Community Changes Associated with Land Use Type in the Forest Montane Region of Northeast China. Forests 2019, 11, 40. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Jangid, K.; Williams, M.A.; Franzluebbers, A.J.; Sanderlin, J.S.; Reeves, J.H.; Jenkins, M.B.; Endale, D.M.; Coleman, D.C.; Whitman, W.B. Relative impacts of land- use, management intensity and fertilization upon soil microbial community structure in agricultural systems. Soil Biol. Biochem. 2008, 40, 2843–2853. [Google Scholar] [CrossRef]
- Gottel, N.R.; Castro, H.F.; Kerley, M.; Yang, Z.; Pelletier, D.A.; Podar, M.; Karpinets, T.; Uberbacher, E.; Tuskan, G.A.; Vilgalys, R.; et al. Distinct microbial communities within the endosphere and rhizosphere of Populus deltoides roots across contrasting soil types. Appl. Environ. Microbiol. 2011, 77, 5934–5944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, G.; Xue, S.; Wang, G. Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the Loess Plateau. Soil Biol. Biochem. 2016, 97, 40–49. [Google Scholar] [CrossRef]
- Castañeda, L.E.; Barbosa, O. Metagenomic analysis exploring taxonomic and functional diversity of soil microbial communities in Chilean vineyards and surrounding native forests. PeerJ 2017, 5, e3098. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, L.; Wang, E.T.; Liu, Y.; Zhang, L.; Zhao, K.; Gu, Y.; Yu, X.; Ma, M.; Penttinen, P.; et al. Combined microbial consortium inoculation and black locust planting is effective in the bioremediation of waste drill cuttings. Front. Microbiol. 2020, 11, 536787. [Google Scholar] [CrossRef]
- Cederlund, H.; Wessén, E.; Enwall, K.; Jones, C.M.; Juhanson, J.; Pell, M.; Philippot, L.; Hallin, S. Soil carbon quality and nitrogen fertilization structure bacterial communities with predictable responses of major bacterial phyla. Appl. Soil Ecol. 2014, 84, 62–68. [Google Scholar] [CrossRef]
- Park, M.K.; Park, Y.J.; Kim, M.; Kim, M.C.; Ibal, J.C.; Kang, G.U.; Lee, G.Y.-D.; Tagele, S.B.; Kwon, H.-J.; Kang, M.-S.; et al. Complete genome sequence of a plant growth-promoting bacterium Pseudarthrobacter sp. NIBRBAC000502772, isolated from shooting range soil in the Republic of Korea. The Microbiol. Soc. Korea 2020, 56, 390–393. [Google Scholar] [CrossRef]
- Evtushenko, L.I.; Ariskina, E.V. Nocardioidaceae. Bergey’s Man. Syst. Archaea Bact. 2015, 14, 1–18. [Google Scholar] [CrossRef]
- De Vries, H.J.; Beyer, F.; Jarzembowska, M.; Lipińska, J.; van den Brink, P.; Zwijnenburg, A.; Timmers, P.H.A.; Stams, A.J.M.; Plugge, C.M. Isolation and characterization of Sphingomonadaceae from fouled membranes. npj Biofilms Microbiomes 2019, 5, 1–9. [Google Scholar] [CrossRef]
- Trujillo, M.E.; Hong, K.; Genilloud, O. The family Micromonosporaceae. The Prokaryotes 2014, 499–569. [Google Scholar] [CrossRef]
- Mayer, Z.; Sasvári, Z.; Szentpéteri, V.; Pethőné Rétháti, B.; Vajna, B.; Posta, K. Effect of long-term cropping systems on the diversity of the soil bacterial communities. Agronomy 2019, 9, 878. [Google Scholar] [CrossRef]
- Bobay, L.M.; Ochman, H. The evolution of bacterial genome architecture. Front. Genet. 2017, 8, 72. [Google Scholar] [CrossRef]
- Brewer, T.E.; Handley, K.M.; Carini, P.; Gilbert, J.A.; Fierer, N. Genome reduction in an abundant and ubiquitous soil bacterium ‘Candidatus Udaeobacter copiosus’. Nat. Microbiol. 2016, 2, 1–7. [Google Scholar] [CrossRef]
- Lin, Y.T.; Jangid, K.; Whitman, W.B.; Coleman, D.C.; Chiu, C.Y. Soil bacterial communities in native and regenerated perhumid montane forests. Appl. Soil Ecol. 2011, 47, 111–118. [Google Scholar] [CrossRef]
- Slabbert, E.; Jacobs, S.M.; Jacobs, K. The soil bacterial com- munities of South African fynbos riparian ecosystems invaded by Australian Acacia species. PLoS ONE 2014, 9, e86560. [Google Scholar] [CrossRef]
- Le Roux, J.J.; Ellis, A.G.; van Zyl, L.M.; Hosking, N.D.; Keet, J.H.; Yannelli, F.A. Importance of soil legacy effects and successful mutualistic interactions during Australian Acacia invasions in nutrient-poor environments. J. Ecol. 2018, 106, 2071–2081. [Google Scholar] [CrossRef]
- Yu, X.Y.; Zhu, Y.J.; Wang, B.; Liu, D.; Bai, H.; Jin, L.; Wang, B.T.; Ruan, H.H.; Mao, L.; Jin, F.J.; et al. Effects of nitrogen addition on rhizospheric soil microbial communities of poplar plantations at different ages. For. Ecol. Manag. 2021, 494, 119328. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, X.; Zhou, B.; Zhao, B.; Ma, M.; Guan, D.; Li, J.; Chen, S.; Cao, F.; Shen, D.; et al. Thirty four years of nitrogen fertilization decreases fungal diversity and alters fungal community composition in black soil in northeast China. Soil Biol. Biochem. 2016, 95, 135–143. [Google Scholar] [CrossRef]
- Aleklett, K.; Hart, M. The root microbiota—a fingerprint in the soil? Plant Soil 2013, 370, 671–686. [Google Scholar] [CrossRef]
- Bzdyk, R.M.; Olchowik, J.; Studnicki, M.; Oszako, T.; Sikora, K.; Szmidla, H.; Hilszczańska, D. The Impact of Effective Microorganisms (EM) and Organic and Mineral Fertilizers on the Growth and Mycorrhizal Colonization of Fagus syl-vatica and Quercus robur Seedlings in a Bare-Root Nursery Experiment. Forests 2018, 9, 597. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Han, X.; Deng, Y. Higher precipitation strengthens the microbial interactions in semi-arid grassland soils. Glob. Ecol. Biogeogr. 2018, 7, 1–11. [Google Scholar] [CrossRef]
- Ma, M.; Jiang, X.; Wang, Q.; Ongena, M.; Wei, D.; Ding, J.; Guan, J.; Cao, F.; Zhao, B.; Li, J. Responses of fungal community composition to long-term chemical and organic fertilization strategies in Chinese Mollisols. MicrobiologyOpen 2018, 7, e00597. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, H.; Dou, Y.; An, S. Plant and soil traits driving soil fungal community due to tree plantation on the Loess Plateau. Sci. Total. Environ. 2020, 708, 134560. [Google Scholar] [CrossRef]
- Wu, N.; Li, Z.; Meng, S.; Wu, F. Soil properties and microbial community in the rhizosphere of Populus alba var. pyramidalis along a chronosequence. Microbiol. Res. 2021, 250, 126812. [Google Scholar] [CrossRef]
- James, T.Y.; Stajich, J.E.; Hittinger, C.T.; Rokas, A. Toward a fully resolved fungal tree of life. Annu. Rev. Microbiol. 2020, 74, 291–313. [Google Scholar] [CrossRef]
- Yang, Y.; Song, Y.; Scheller, H.V.; Ghosh, A.; Ban, Y.; Chen, H.; Tang, M. Community structure of arbuscular mycorrhizal fungi associated with Robinia pseudoacacia in uncontaminated and heavy metal contaminated soils. Soil Biol. Biochem. 2015, 86, 146–158. [Google Scholar] [CrossRef]
- He, F.; Tang, M.; Zhong, S.L.; Yang, R.; Huang, L.; Zhang, H.Q. Effects of soil and climatic factors on arbuscular mycorrhizal fungi in rhizosphere soil under Robinia pseudoacacia in the Loess Plateau, China. Eur. J. Soil Sci. 2016, 67, 847–856. [Google Scholar] [CrossRef]
- Perry, B.A.; Hansen, K.; Pfisterm, D.H. A phylogenetic overview of the family Pyronemataceae (Ascomycota, Pezizales). Mycol. Res. 2007, 111, 549–571. [Google Scholar] [CrossRef]
- Fujimura, K.F.; Smith, J.E.; Horton, T.R.; Weber, N.S.; Spatafora, J.W. Pezizalean mycorrhizas and sporocarps in ponder- osa pine (Pinus ponderosa) after prescribed fires in eastern Oregon, USA. Mycorrhiza 2005, 15, 79–86. [Google Scholar] [CrossRef]
- Tedersoo, L.; Hansen, K.; Perry, B.A.; Kjoller, R. Molecular and morphological diversity of pezizalean ectomycorrhiza. New Phytol. 2006, 170, 581–596. [Google Scholar] [CrossRef]
- Tedersoo, L.; May, T.W.; Smith, M.E. Ectomycorrhizal lifestyle in fungi: Global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 2010, 20, 217–263. [Google Scholar] [CrossRef]
- Sheng, M.; Chen, X.; Zhang, X.; Hamel, C.; Cui, X.; Chen, J.; Chen, H.; Tang, M. Changes in arbuscular mycorrhizal fungal attributes along a chronosequence of black locust (Robinia pseudoacacia) plantations can be attributed to the plantation-induced variation in soil properties. Sci. Total. Environ. 2017, 599, 273–283. [Google Scholar] [CrossRef]
- Yang, T.; Adams, J.M.; Shi, Y.; He, J.S.; Jing, X.; Chen, L.; Tedersoo, L.; Chu, H. Soil fungal diversity in natural grasslands of the Tibetan Plateau: Associations with plant diversity and productivity. New Phytol. 2017, 215, 756–765. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, D.; Cai, X.W.; Xia, L.; Luo, Y.Q.; Cheng, X.L.; An, S. Significant alterations in soil fungal communities along a chronosequence of Spartina alterniflora in a Chinese Yellow Sea coastal wetland. Sci. Total. Environ. 2019, 693, 133548. [Google Scholar] [CrossRef] [PubMed]

| Treatment 1 | Observed Species (OTU) | Chao1 Index | se.chao1 | Shannon Index | |
|---|---|---|---|---|---|
| Black locust bacterial diversity | I | 1864 | 1881.14 | 11.47 | 6.79 |
| II | 1784 | 1814.19 | 10.68 | 6.61 | |
| III | 1828 | 1848.65 | 10.48 | 6.65 | |
| Black locust fungal diversity | I | 529 | 562.11 | 13.44 | 4.87 |
| II | 508 | 525.44 | 7.36 | 4.62 | |
| III | 477 | 505.74 | 11.16 | 4.70 | |
| Poplar bacterial diversity | A | 1830 | 1848.70 | 8.51 | 6.57 |
| B | 1882 | 1883.67 | 2.04 | 6.82 | |
| C | 1860 | 1863.797 | 2.97 | 6.76 | |
| Poplar fungal diversity | A | 460 | 482.88 | 10.16 | 4.98 |
| B | 500 | 544.38 | 14.67 | 4.69 | |
| C | 510 | 536.10 | 12.16 | 5.04 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayer, Z.; Csorbainé, A.G.; Juhász, Á.; Ombódi, A.; Pápai, A.; Némethné, B.K.; Posta, K. Impact of Soil-Applied Microbial Inoculant and Fertilizer on Fungal and Bacterial Communities in the Rhizosphere of Robinia sp. and Populus sp. Plantations. Forests 2021, 12, 1218. https://doi.org/10.3390/f12091218
Mayer Z, Csorbainé AG, Juhász Á, Ombódi A, Pápai A, Némethné BK, Posta K. Impact of Soil-Applied Microbial Inoculant and Fertilizer on Fungal and Bacterial Communities in the Rhizosphere of Robinia sp. and Populus sp. Plantations. Forests. 2021; 12(9):1218. https://doi.org/10.3390/f12091218
Chicago/Turabian StyleMayer, Zoltán, Andrea Gógán Csorbainé, Ákos Juhász, Attila Ombódi, Antal Pápai, Boglárka Kisgyörgy Némethné, and Katalin Posta. 2021. "Impact of Soil-Applied Microbial Inoculant and Fertilizer on Fungal and Bacterial Communities in the Rhizosphere of Robinia sp. and Populus sp. Plantations" Forests 12, no. 9: 1218. https://doi.org/10.3390/f12091218
APA StyleMayer, Z., Csorbainé, A. G., Juhász, Á., Ombódi, A., Pápai, A., Némethné, B. K., & Posta, K. (2021). Impact of Soil-Applied Microbial Inoculant and Fertilizer on Fungal and Bacterial Communities in the Rhizosphere of Robinia sp. and Populus sp. Plantations. Forests, 12(9), 1218. https://doi.org/10.3390/f12091218






