Abstract
The installation of commercial stands with exotic forest species on low fertility soils originally covered by native pastures is an unusual situation worldwide. In recent years, the area occupied by forest systems designed for pulp or wood production with immediate replanting has increased strongly in the Pampean region of South America. In this context, the study of nutrient recycling from forest litter decomposition acquires particular relevance. This work seeks to evaluate and compare the nutrient release from the decomposition of forest litter produced by 14-year-old Eucalyptus grandis Hill ex Maiden and Pinus taeda L. stands and test the applicability of a new sampling methodology in the nutrient recycling assessment. For two years, the evaluation of N, P, K, Ca, Mg, Fe, Mn, Cu and Zn dynamics during litter decomposition was carried out. In general, K concentration decreased through decomposition, meanwhile, all other nutrients showed some degree of immobilization, but this was counteracted by biomass loss for most of them. This mainly resulted in net nutrient release from litter. A higher release rate of all nutrients from P. taeda forest litter compared to E. grandis, with the exception of Mn, was verified. Fe immobilization was observed in both species showing a higher immobilization rate in E. grandis compared to P. taeda. Finally, Zn exhibited immobilization processes in E. grandis and releases in P. taeda. This might suggest higher temporal and quantitative availability of nutrients in P. taeda, due their faster return to the soil. These findings could be relevant in the development of models for sustainable management, adapting the demand for nutrients to the supply during forest rotations.
1. Introduction
During the last years, the installation of commercial stands with different species of Eucalyptus and Pinus in sites originally covered by native pastures in the Pampean region of South America has experienced a relevant growth [1,2,3]. Normally, these systems are designed for pulp or wood production, in relatively short rotations, with immediate replanting, usually on soils with low natural fertility [3,4]. This change in coverage implies the incorporation of a new component to the soil–plant system, the forest litter [5]. In this sense, the nutrient leaching and forest litter decomposition constitute fundamental processes in nutrient recycling, acquiring relevance in this productive context [4,6,7,8,9].
Throughout the litter decomposition process, some of the absorbed nutrients to support the forest growth return to the soil, becoming gradually available for plant uptake [10,11]. The quantitative and temporal availability of these nutrients will fundamentally depend on whether they are directly released to the soil or whether, on the contrary, immobilization processes occur [1]. At the same time, differences in the availability of nutrients will be chiefly influenced by their mobility, as well as by the material quality and the environmental conditions [1,6,12].
The degree of control of the environmental conditions on litter decomposition influences mostly on a global or regional scale, but the effect of material quality is locally relevant [13,14,15]. In fact, the initial concentrations of total carbon (C), soluble C and some nutrients such as nitrogen (N) and phosphorus (P) are used as indicators of the decomposition potential of plant residues related to their quality [6,16]. Other characteristics, such as the concentration of soluble polyphenols and lignin, substances that produce slowdowns in the decomposition process, can be highly useful for understanding the process dynamics [17]. Proportions that involve different quality indicators, such as C:N, C:P and lignin-N (L:N) ratios, have been related with decomposition and nutrient release or immobilization [7,18,19].
In general, the litter of broadleaf species tends to decompose faster than that of coniferous species, releasing the nutrients at a higher rate [20,21]. This is linked to the lower quality of the litter of coniferous species, given by lower nutrient concentration and higher lignin content [20,22,23]. This condition can be highly variable depending on the considered forest species [24]. In this context, given that the nutrient recycling rate affects forest productivity, the study of nutrient immobilization/release through litter decomposition constitutes a fundamental factor for the sustainable management of these stands [6,25,26].
The present work seeks to evaluate and compare the nutrient release dynamics from the decomposition of litter produced by 14-year-old Eucalyptus grandis Hill ex Maiden and Pinus taeda L. stands. At the same time, the study aims to test the applicability of a new sampling methodology for the assessment of litter production and decomposition, that was previously proposed by the authors, in the evaluation of the forest litter nutrient dynamics during its decomposition process.
The investigation hypotheses were the following: (i) the dynamics of nutrient concentration during litter decomposition differs enough to alter the average trend evidenced for biomass loss; (ii) the nutrient release/immobilization rate varies according to the considered nutrient, species and forest litter layers, even in the absence of significant differences in the litter decomposition rate and; (iii) the mobility of the nutrients depends on the considered species, altering their return to the soil through the decomposition process.
2. Materials and Methods
2.1. Experimental Site Characterization
During the year 2003, two first-turn forest experiments were installed at Rivera Department, Uruguay (coordinates: 31°23′55.11″ S and 55°41′43.88″ W), one with E. grandis and the other with P. taeda. In this area, the original vegetation of native pastures dominated by perennial summer grasses and some winter grasses was substituted by a forest cover [2]. For each forest species, a stand density experiment with three treatments (816, 1111 and 2066 trees ha−1) was arranged in a randomized complete block design with three replications. For this study, the plots with the lowest density were considered because they represent a similar situation to stands for quality wood production. The study began in June 2017 (Southern Hemisphere) when the stands were 14 years old.
The soils under the experiment correspond to thermic Humic Hapludults [27]. A detailed soil characterization is available in previous studies [2,5]. The climate in the area is temperate, with an average annual temperature around 18.6 °C and an average annual rainfall of 1605 mm which distribution presents a main maximum during autumn and a main minimum in winter, showing high interannual variation [28].
2.2. Sampling Method and Decomposition Assessment
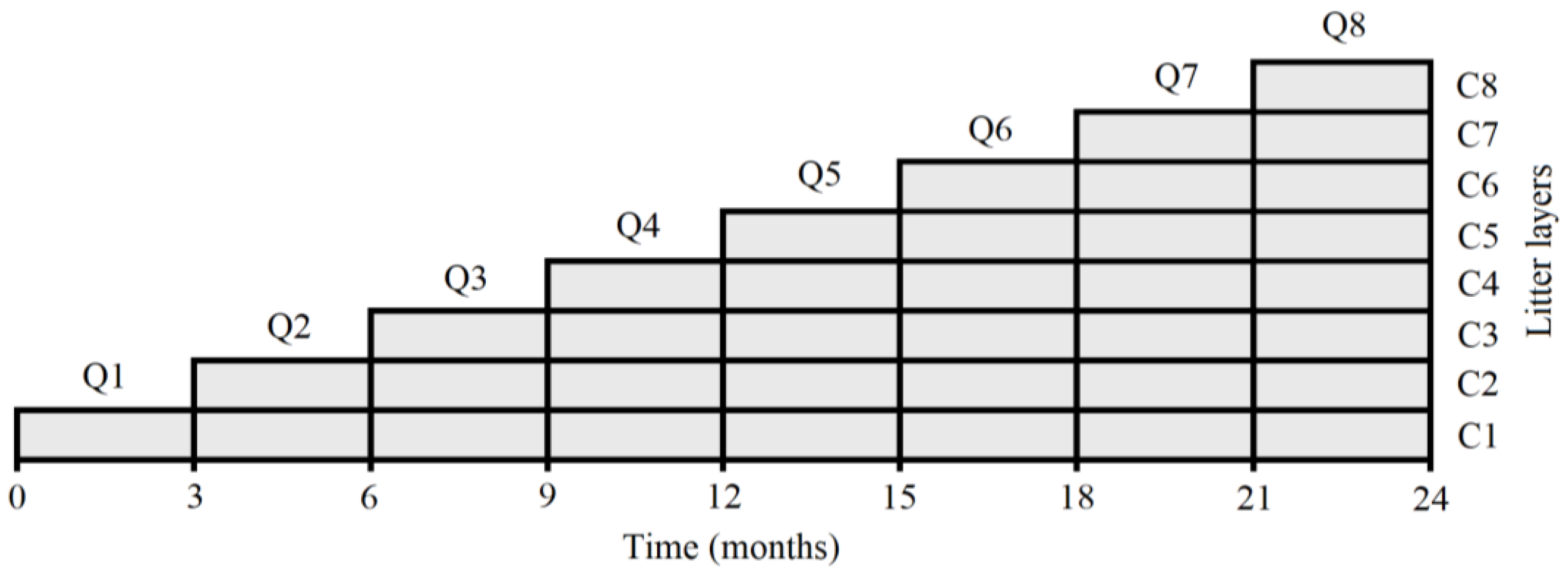
To carry out the experiment, 14-year-old E. grandis and P. taeda stands were considered with a density of 816 trees ha−1. In each of them, the three installed plots of 30 m × 30 m were used. At each plot, a subplot of 4 m × 2 m was arranged equidistant from the tree rows (spacing of 3.5 m × 3.5 m). In all cases, the canopy in the sampling area was homogeneous and totally closed, containing trees with average dimensions that were representative of the stands. In each subplot, the forest litter was carefully removed and a rectangular trap of the same dimensions of the subplot was installed, subdividing the area in eight sampling quadrants of 1 m2. At the soil surface, a layer of plastic mesh fabric (1 mm2 opening) was installed in each quadrant with the aim to collect the litter that fell during each sampling period, allowing the passage of air and water. At the first sampling moment (first season, three-month period), one of the eight quadrants (Q1) from each subplot was randomly removed, meanwhile, the fallen material in the remaining quadrants was not collected, placing a new mesh on each of them. This allowed us to separate the material that fell during the first season from that which was deposited during the second season. This procedure was continued for two years until the end of the study (Figure 1).
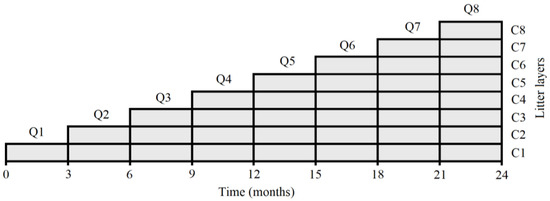
Figure 1.
Schematic representation of the sampling procedure used during the study. Q: sampling quadrant.
In the laboratory, the forest litter of each layer was separated and dried at 65 °C until constant weight (48 h) to determine its total dry weight. With the measured weight at the time of the formation of each layer and the value obtained from its replications at each sampling period, the remaining dry weight proportion was calculated. Then, exponential decay models [29] were fitted to the remaining biomass of each litter layer by species, individually. Statistically significant adjustments were achieved only for three of them, corresponding to three times of the beginning of the evaluation, C1 (winter year 1), C2 (spring year 1) and C3 (summer year 1). The obtained decomposition rate from the model for each layer was compared between species and layers by each species individually. A detailed explanation of the sampling methodology and the results of the decomposition dynamics are available in a related previous work [5].
2.3. Chemical Analyses
The dried material corresponding to C1, C2 and C3 layers from each evaluation moment and considered species was ground to a particle size < 0.5 mm. Subsequently, the concentration of nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), iron (Fe), manganese (Mn), copper (Cu) and zinc (Zn) was determined. To analyze P, K, Ca, Mg, Fe, Mn, Cu and Zn, 1 g of this material was placed in a porcelain crucible and mineralized in a muffle at 550 °C for 5 h. Then, the obtained ashes were dissolved in 10% HCl. In the resulting extract, P was determined by colorimetry [30]. Ca, Mg, Fe, Mn, Cu and Zn concentrations were determined by atomic absorption spectrophotometry and K by emission spectrophotometry [31]. N concentration was determined by Kjeldahl distillation, after a mineralization with H2SO4 at 350 °C with a mixture of catalysts (CuSO4 and K2SO4) for 90 min [32].
In the initial samples of each considered forest litter layer, determinations related with residue quality were carried out. The lignin determination was made by acid hydrolysis [33]. For soluble polyphenols concentration, an extraction with H2O during 2 h at 100 °C with a subsequent determination by colorimetry using Folin–Ciocalteu reagent was carried out [34,35]. The soluble C content was determined in the same extract by oxidation with K2Cr2O7 and H2SO4 at 150 °C during 30 min followed by colorimetric determination [36]. Finally, the forest litter samples were ground again to a particle size < 0.149 mm and, subsequently, the total C concentration was determined using the elemental analyzer integrated into Integra 2 EA-IRMS equipment (Sercon Ltd., Cheshire, UK).
2.4. Initial Material Characterization and Nutrient Release Dynamics Evaluation
Based on the chemical determinations, an initial characterization of the nutrient concentration of each considered layer by species was obtained, including residue quality indicators such as the concentration of lignin, polyphenols and soluble C. At the same time, the following ratios were determined: C:N, C:P and L:N. Further, the initial content of all nutrients for each considered layer was obtained.
In order to evaluate the nutrient dynamics during the decomposition process, two main variables were determined as a proportion of the initial records, the remaining nutrient concentration and the remaining nutrient content. The remaining nutrient concentration was determined as follows:
where R[c] is the remaining nutrient concentration for a particular layer and sampling moment; Ci the initial nutrient concentration of the layer at its formation time and Ct the nutrient concentration of each replication collected at each sampling moment. The remaining nutrient content was calculated through the following expression:
where Rc is the remaining nutrient content for a particular layer and sampling moment; Ci the initial nutrient concentration of the layer at its formation time; Mi the initial dry weight of the layer at its formation time; Ct the nutrient concentration of each replication collected at each sampling moment and; Mt the dry weight of each replication collected at each sampling moment.
2.5. Statistical Analysis
The response variables remaining nutrient content (Rc) and remaining nutrient concentration (R[c]) were analyzed by the adjustment of generalized linear mixed models (GLMM) for each nutrient, litter layer and species individually, through the following statistical model:
where Yij is the response variable; e the exponential number; µ the general mean; αi the fixed effect of decomposition time; Zj the random effect of the subplot and εij the experimental error.
For each model, the slope represents the rate of change of the dependent variable. In order to interpret the tendency, a significant positive slope denotes an increase of the remaining nutrient concentration with decomposition time (i.e., nutrient concentration increases) as well as for the remaining nutrient content (i.e., nutrient immobilization). On the other hand, a negative slope represents a diminution of the remaining nutrient concentration with decomposition time (i.e., nutrient concentration decreases) as well as for the remaining nutrient content (i.e., nutrient release). In those cases, in which a significant relationship between the response variable and the decomposition time was not found, a stable behavior with oscillations was assumed. For the remaining nutrient content, this situation indicates the occurrence of immobilization and nutrient release periods through decomposition time.
In order to compare the slopes between species for each dependent variable, there were considered situations in which a significant relationship was found with the decomposition time for a particular nutrient in both species. For this, the adjustment of GLMM to the data of both species for each considered nutrient was carried out using the following statistical model:
where Yijk is the response variable; e the exponential number; µ the general mean; αi the fixed effect of decomposition time; βj the fixed effect of forest species; (αβ)ij the fixed effect of the interaction between decomposition time and species; Zk the random effect of the subplot and; εijk the experimental error. In these models, a significant interaction term indicates significant differences between species.
A similar strategy was used for the comparison of slopes between litter layers for each species and nutrient individually. A set of three individual models was adjusted for each nutrient and forest species combining the data from C1 + C2, C1 + C3 and C2 + C3 layers for each model, respectively. This procedure was carried out only in the cases in which the response variables presented a significant trend with decomposition time for all evaluated layers. The considered statistical model was the following:
where Yijk is the response variable; e the exponential number; µ the general mean; αi the fixed effect of decomposition time; βj the fixed effect of litter layer; (αβ)ij the fixed effect of the interaction between decomposition time and litter layer; Zk the random effect of the subplot and; εijk the experimental error. For these models, a significant interaction term indicates significant differences between layers.
In all cases a p < 0.05 was considered significant. The specified family distribution for all adjusted models was gamma with log as link function. Finally, in order to represent the proportion of the variability of the remaining nutrient concentration and the remaining nutrient content explained by decomposition time considering the subplot random effect, the conditional pseudo-R squared () was determined. The for the adjusted models for each nutrient, layer and species individually was calculated according to the following equation [37]:
where represents the proportion of the variance explained by both fixed and random effects; the variance explained by fixed effects; the variance explained by random effects and the observation-level variance.
In this case, the lognormal approximation for gamma distribution was used where the observation-level variance was determined as follows [37]:
where corresponds to the observation-level variance and v to the shape parameter of gamma distribution. All analyses were carried out using R (version 4.0.3, R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Dry Weight and Chemical Characterization of the Initial Material
The initial dry weight and the chemical characterization for each litter layer by species are presented in Table 1. The initial nutrient stock by species and litter layer is shown in Supplementary Table S1. P. taeda produced a higher average amount of litter (3.23 Mg ha−1) than E. grandis (2.98 Mg ha−1). The registered dry weight in C1 and C3 layers followed the same trend, with the opposite occurring for the C2 layer. Regarding nutrient concentration, the litter layers of E. grandis showed a higher average record for K (2.15 g kg−1), Mg (1.56 g kg−1), Ca (7.89 g kg−1), Fe (88 mg kg−1), Mn (360 mg kg−1) and Cu (5 mg kg−1) with respect to P. taeda (K: 1.40 g kg−1; Mg: 0.87 g kg−1; Ca: 3.85 g kg−1; Fe: 60 mg kg−1; Mn: 299 mg kg−1; Cu: 3 mg kg−1). In individual terms, all analyzed litter layers followed the same response. P. taeda litter layers presented a higher average concentration of N (8.14 g kg−1), P (0.48 g kg−1) and Zn (19 mg kg−1) compared to E. grandis (N: 7.99 g kg−1; P: 0.30 g kg−1; Zn: 11 mg kg−1). The concentrations of P and Zn of all considered litter layers were higher in P. taeda than in E. grandis, following the response demonstrated by the average results. For N, C2 and C3 layers showed the same tendency, with the opposite occurring for the C1 layer.

Table 1.
Initial dry weight and chemical composition of each considered E. grandis and P. taeda litter layer.
The average C concentration for E. grandis (460.79 g kg−1) was higher than that verified for P. taeda litter (434.69 g kg−1), obtaining the same trend for each litter layer. Regarding soluble polyphenols, the results presented the same behavior characterized by higher average concentration in E. grandis (68.01 g kg−1) compared to P. taeda (18.03 g kg−1). For each litter layer, the results followed the same tendency as occurred for C concentration.
Lignin showed an average amount per mass unit slightly higher in P. taeda (464.98 g kg−1) compared to E. grandis (461.56 g kg−1). In line with this result, the soluble C concentration presented a slightly higher average record in P. taeda (108.56 g kg−1) with respect to E. grandis (105.63 g kg−1). For lignin, C2 and C3 layers followed the same average trend, meanwhile, the C1 layer exhibited an opposite response with higher values in E. grandis compared to P. taeda. Regarding soluble C concentration, only the C2 layer had the same tendency obtained for the average results. For C1 and C3 layers the variable was higher in E. grandis forest litter.
The C:N, C:P and L:N ratios demonstrated higher average values in E. grandis (62, 1587 and 61, respectively) than in P. taeda litter (55, 946 and 58, respectively). The C:P ratio for each considered layer followed the same obtained behavior for the average values. The C:N and L:N ratios showed higher records for the C1 litter layer in P. taeda compared to E. grandis.
Regarding the differences in dry weight, concentration of nutrients and C of the analyzed layers for each species individually, the C1 layer presented the highest value for N, Ca, Fe, Zn and C in E. grandis; meanwhile, for dry weight, K, Mg, Mn and Cu this occurred in the C2 layer. For P concentration, C1 and C2 layers shared the highest registered value. P. taeda showed the highest concentration of nutrients and C in general in the C2 layer, with the exception of Fe, which presented the highest value in the C1 layer. For dry weight, the highest record was verified in the C3 layer.
E. grandis exhibited the highest record of soluble polyphenols in the C3 layer, as well as for soluble C, meanwhile, the highest value for lignin was registered in the C1 layer. Considering P. taeda litter, the highest record for lignin and soluble polyphenols occurred in the C3 layer and the highest soluble C concentration in the C2 layer. Regarding the plant residue quality indexes, the C3 layer presented the highest C:N, C:P and L:N ratios in E. grandis. Finally, P. taeda showed higher C:N and L:N ratios in the C3 layer and the highest C:P value in the C1 layer.
3.2. Remaining Nutrient Concentration Dynamics
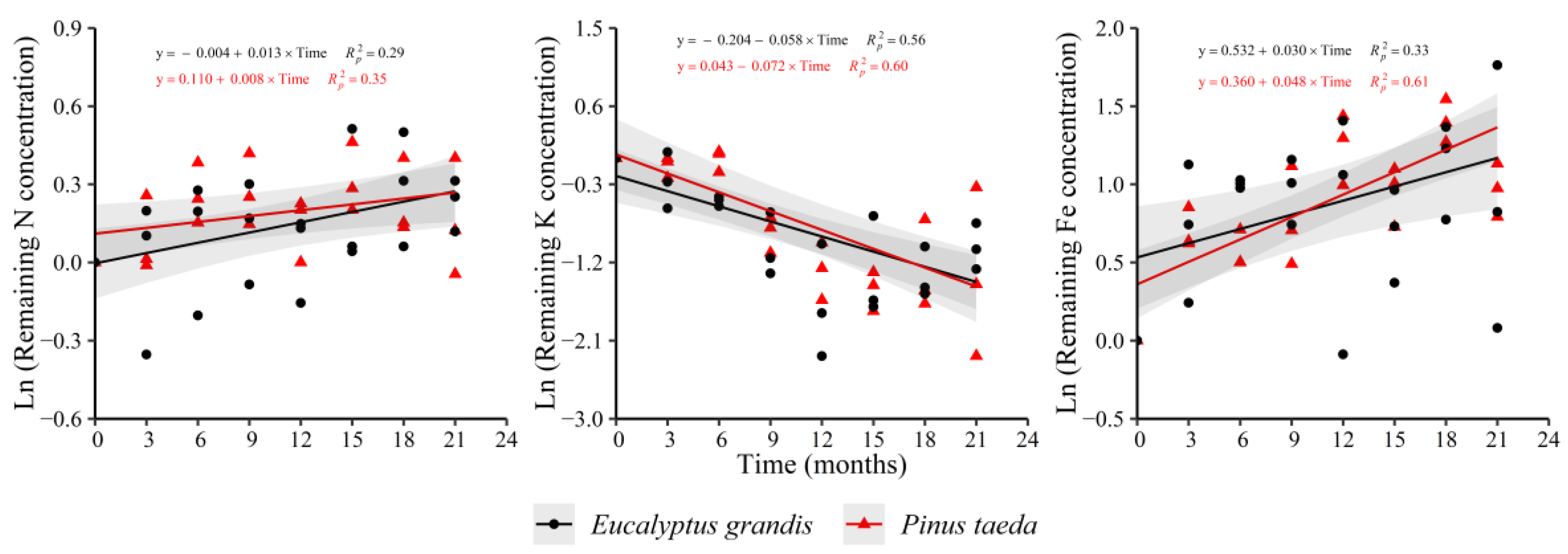
In the C1 layer, an increase in the remaining concentration of N and Fe as function of time in both species was observed, with the opposite occurring for K (Figure 2). For E. grandis, the remaining Zn concentration showed increases with respect to decomposition time (y = 0.248 + 0.037 × Time, = 0.56) without a clear trend in P. taeda. This species exhibited increases in the variable for Mn (y = 0.136 + 0.022 × Time, = 0.51) and decreases for Ca (y = 0.174 − 0.018 × Time, = 0.18) and Cu (y = 0.748 − 0.032 × Time, = 0.24).
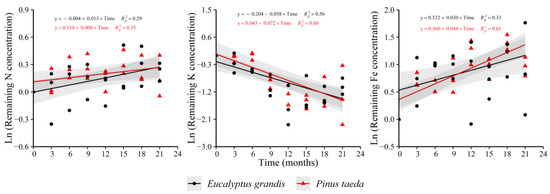
Figure 2.
Remaining nutrient concentration of E. grandis (black symbols) and P. taeda (red symbols) litter for the different sampling moments (C1 layer). The gray bands around the models represent the 95% confidence interval.
In E. grandis, it was not possible to detect a significant trend during the study for these nutrients. For the other nutrients-species combinations (Mg and P), the remaining concentration did not show a significant behavior with decomposition time. Regarding the rate of change of the variable with time, no significant differences were observed between species (Supplementary Table S2).
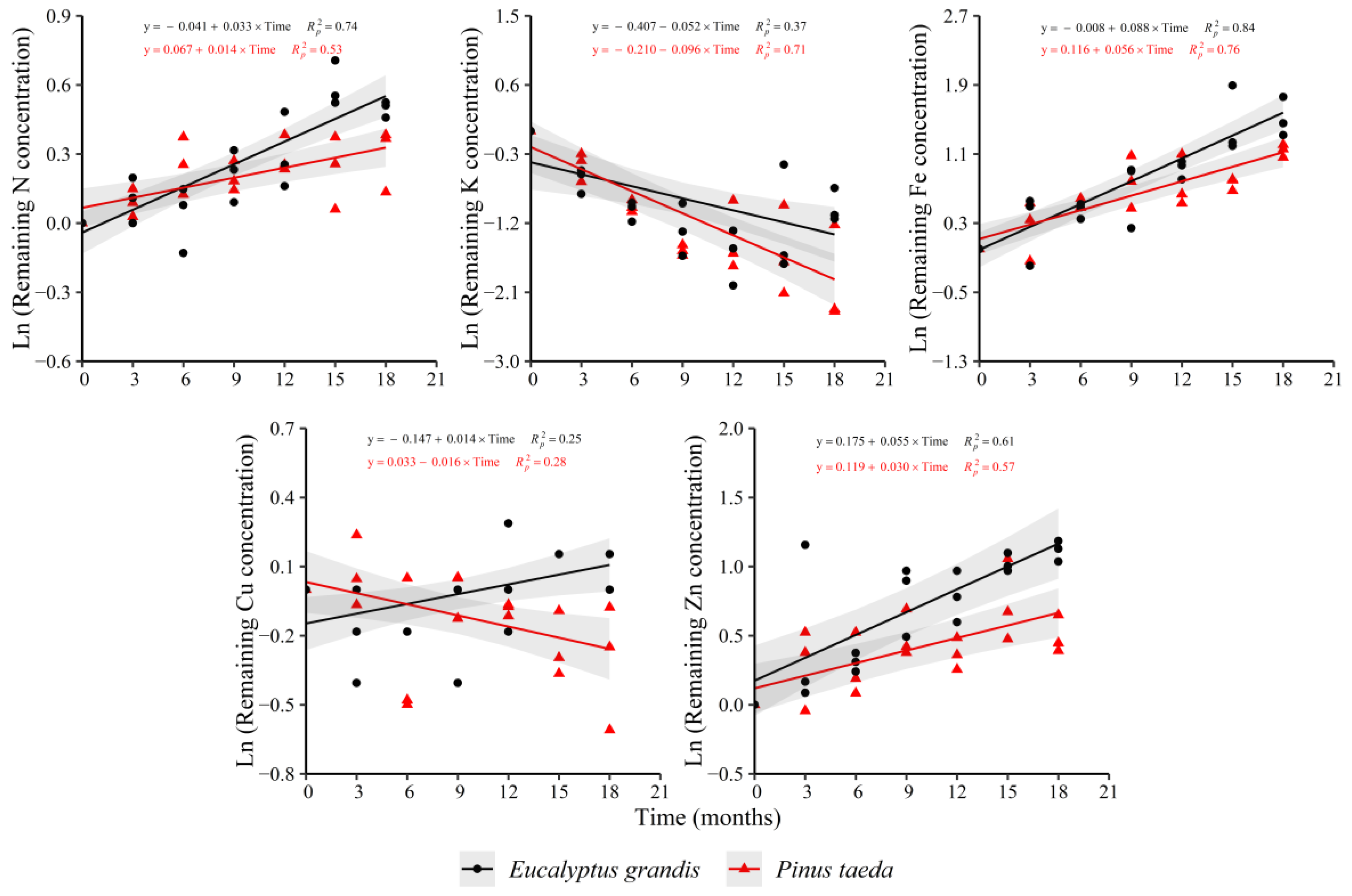
In the C2 layer, the behavior of the remaining nutrient concentration was similar to the C1 layer (Figure 3). For N and Fe, increases in the variable were observed in both species, with the opposite occurring for K. The remaining Zn concentration showed an increase in both species, which had occurred only for E. grandis in the C1 layer. The behavior of the remaining Cu concentration between species was different, showing increases in E. grandis and decreases in P. taeda (as occurred in the C1 layer for this species). Ca (y = −0.010 + 0.020 × Time, = 0.59) and P (y = 0.009 + 0.017 × Time, = 0.42) presented increases only for E. grandis without trends in P. taeda. For Mn, increases were evidenced in P. taeda (y = 0.026 + 0.012 × Time, = 0.32) without a clear response in E. grandis. Finally, the remaining Mg concentration did not show clear trends with decomposition time in both species.
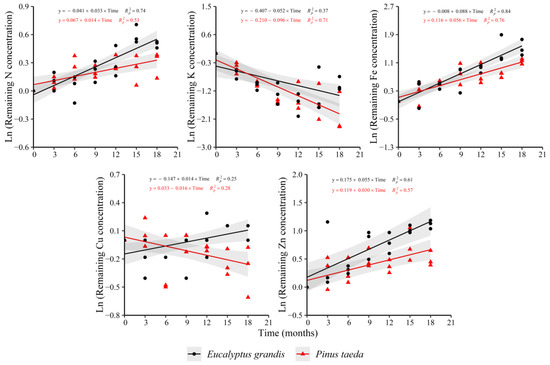
Figure 3.
Remaining nutrient concentration of E. grandis (black symbols) and P. taeda (red symbols) litter for the different sampling moments (C2 layer). The gray bands around the models represent the 95% confidence interval.
Regarding the rate of change in the remaining nutrient concentration (Supplementary Table S3), higher values were observed for N, Fe and Zn in E. grandis compared to P. taeda, meanwhile, there was a faster diminution for K in P. taeda. Finally, Cu showed an increasing trend in E. grandis, with the opposite occurring in P. taeda.
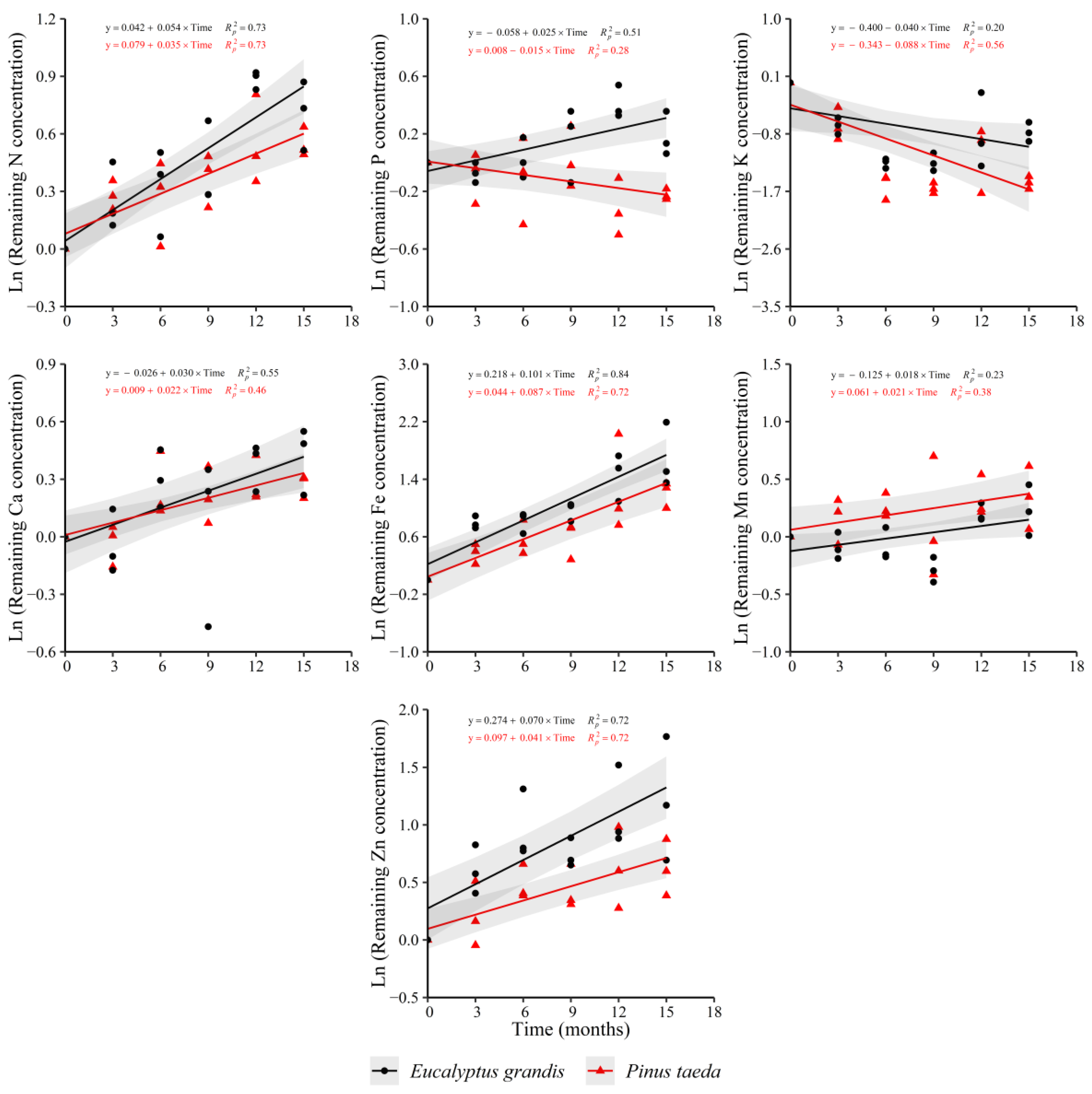
The evidenced trends in the C3 layer were similar to the other analyzed layers (Figure 4). Increases in N, Ca, Fe, Mn and Zn in both species were verified. The remaining K concentration showed decreases in both species as occurred in C1 and C2 layers. For P, the behaviors were different between the considered species, with increases in E. grandis and decreases in P. taeda. Mg (y = −0.105 + 0.016 × Time, = 0.36) and Cu (y = −0.046 + 0.024 × Time, = 0.46) showed an increasing trend only for E. grandis without a clear response in P. taeda. For the rate of change in the remaining nutrient concentration, no differences were observed between species for all nutrients, except Zn, which presented a higher increase in E. grandis, and P, with contrasting trends between species (Supplementary Table S4).
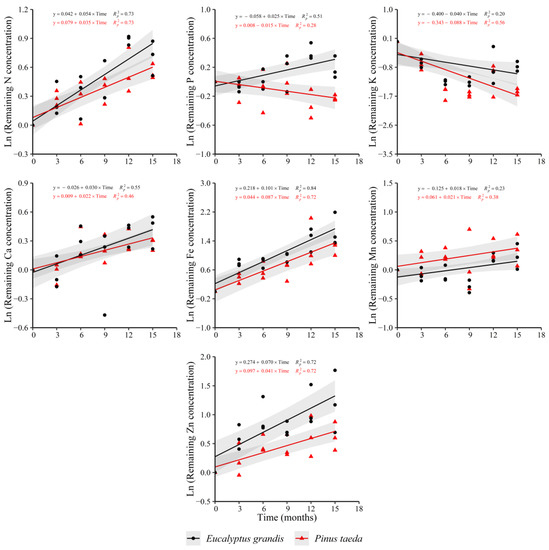
Figure 4.
Remaining nutrient concentration of E. grandis (black symbols) and P. taeda (red symbols) litter for the different sampling moments (C3 layer). The gray bands around the models represent the 95% confidence interval.
Regarding the comparison for the rate of change in the variable between forest litter layers for each species individually, some differences were found depending on the considered nutrient. E. grandis presented significant interaction effects (L × T) for N, Fe and Zn (Table 2). According to these results and the rates obtained from the adjusted models for each layer and nutrient individually (see the coefficients of the adjusted models by layer presented in this section), the highest rate of increase of N concentration was verified in the C3 layer, an intermediate value in the C2 layer and a minimum in the C1 layer. For Fe, the rate of increase was higher in the C2 and C3 layers compared to the C1 layer. Zn concentration increased faster in the C3 layer than in the C1 layer with an intermediate value, similar from these records, in the C2 layer. Finally, the rate of decrease of the remaining K concentration did not show differences between the considered layers.

Table 2.
Coefficients obtained from the adjustment of the generalized linear mixed models for the remaining nutrient concentration of E. grandis forest litter according to the comparison set.
P. taeda showed significant interactions between L and T (Table 3) only for N and Fe. Based on these results, the highest rate of increment on the remaining N and Fe concentration occurred in the C3 layer, meanwhile, C1 and C2 litter layers exhibited the lowest value. Finally, regarding K and Mn, no differences were found between layers.

Table 3.
Coefficients obtained from the adjustment of the generalized linear mixed models for the remaining nutrient concentration of P. taeda forest litter according to the comparison set.
3.3. Remaining Nutrient Content Dynamics
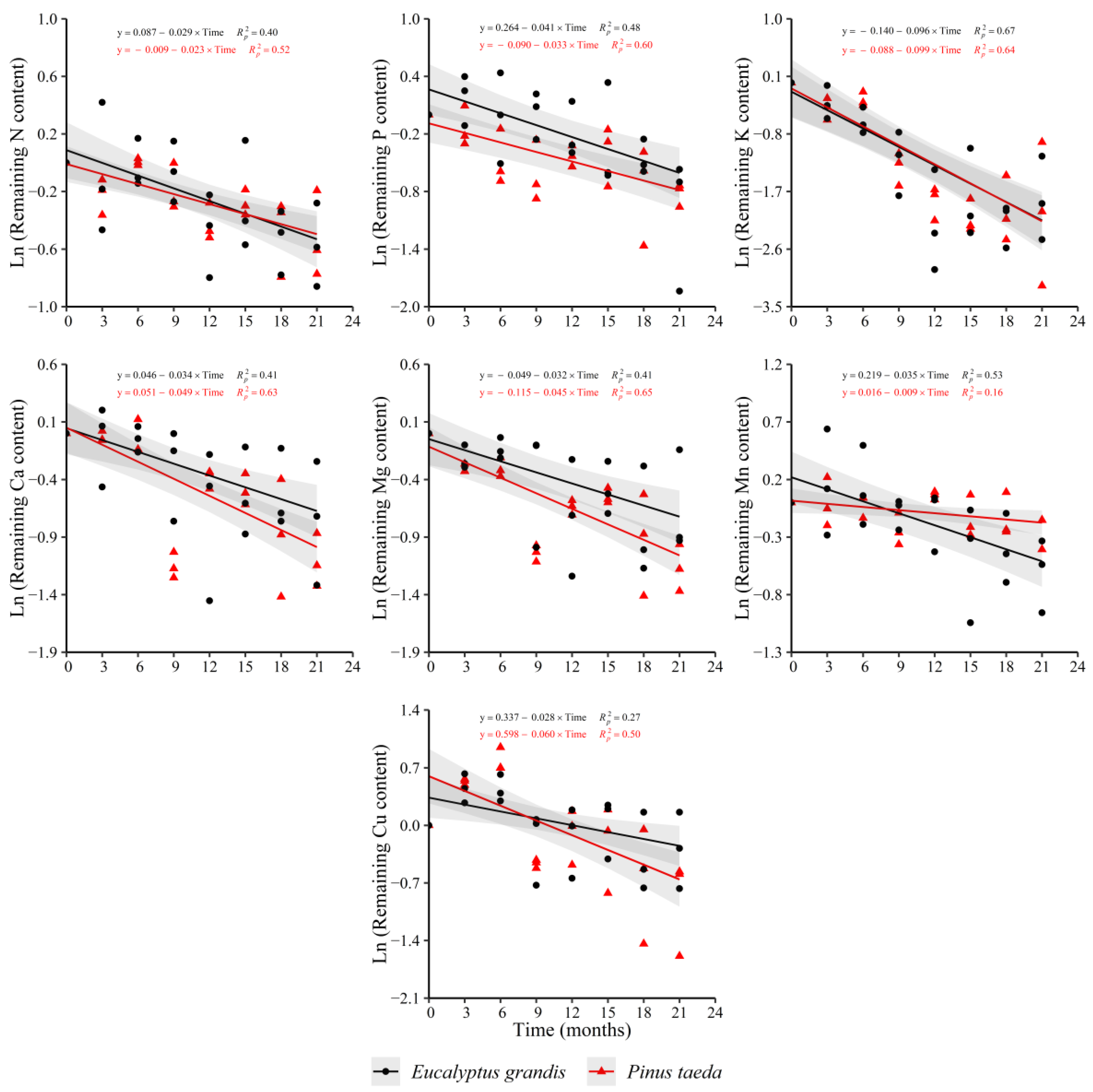
The results for the remaining content of nutrients in the C1 layer showed a decreasing trend in both species for N, P, K, Ca, Mg, Mn and Cu (Figure 5). For Fe (y = 0.228 + 0.017 × Time, = 0.25) and Zn (y = 0.089 − 0.022 × Time, = 0.30), a significant effect of decomposition time was obtained only for P. taeda. The remaining Fe content evidenced an increment, with the opposite occurring for Zn, which showed a clear reduction in the variable.
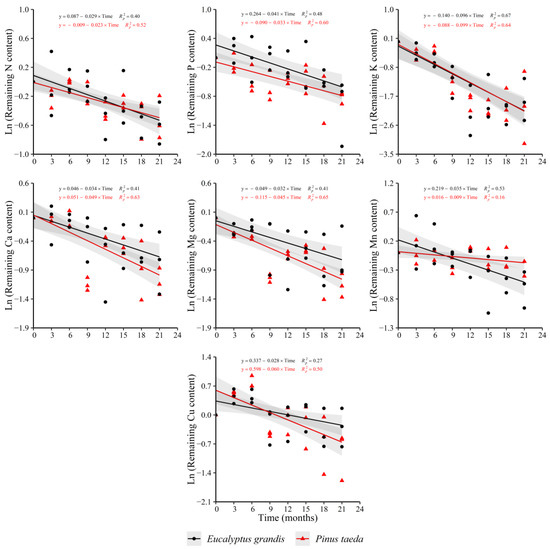
Figure 5.
Remaining nutrient content of E. grandis (black symbols) and P. taeda (red symbols) litter for the different sampling moments (C1 layer). The gray bands around the models represent the 95% confidence interval.
There were no significant differences in the release rate of N, P, K, Ca, Mg and Cu between species. For Mn, differences marked by a higher release rate in E. grandis with respect to P. taeda were found (Supplementary Table S5).
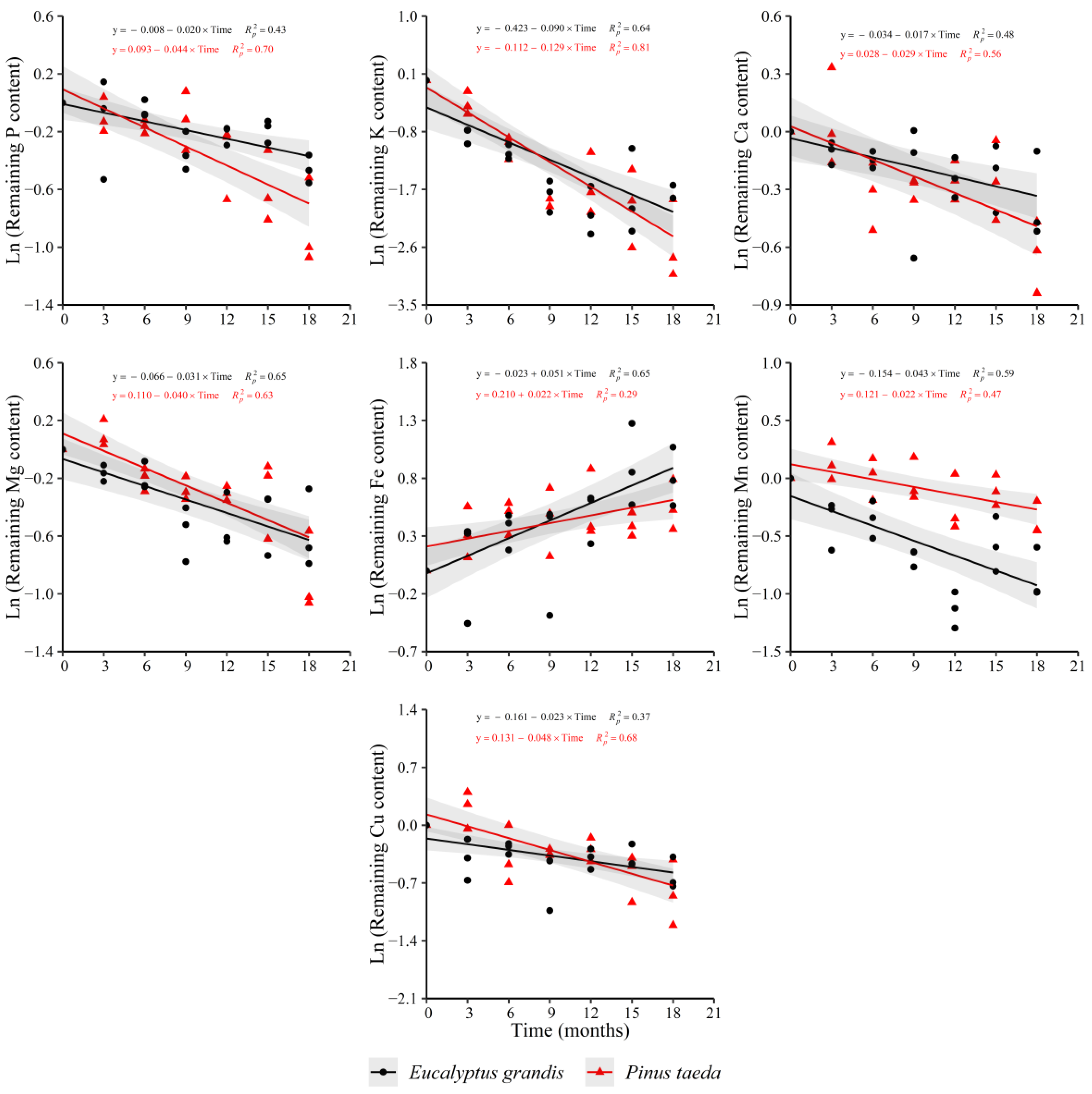
The remaining content of P, K, Ca, Mg, Mn and Cu showed a decreasing trend in the C2 layer for both species. Nevertheless, following the obtained results in C1 layer for P. taeda, the remaining Fe content increased with decomposition time in both species (Figure 6). For N, a decreasing behavior was verified only for P. taeda (y = 0.170 − 0.019 × Time, = 0.34), showing the same trend obtained in the C1 layer. The remaining Zn content increased with decomposition time only for E. grandis (y = 0.136 + 0.019 × Time, = 0.33) without a clear response in P. taeda.
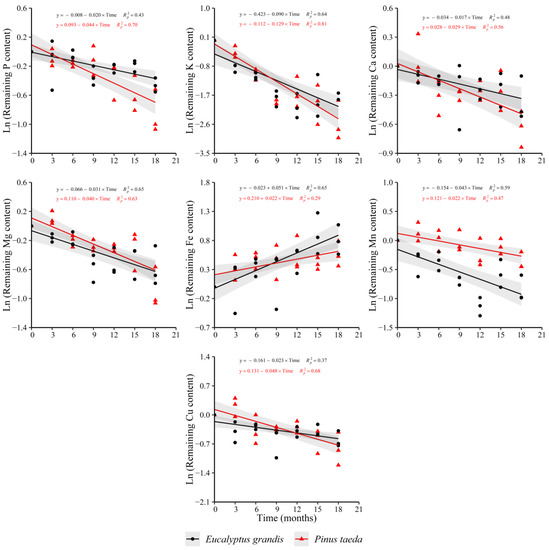
Figure 6.
Remaining nutrient content of E. grandis (black symbols) and P. taeda (red symbols) litter for the different sampling moments (C2 layer). The gray bands around the models represent the 95% confidence interval.
When comparing the release rate between species, no differences were found for K, Ca and Mg (Supplementary Table S6). The obtained results for Mn were similar to those found in the C1 layer, with a higher release rate in E. grandis compared to P. taeda. The decrease in the remaining content of P and Cu was greater in P. taeda with respect to E. grandis. For Fe, the increase in its remaining content showed a higher rate in E. grandis compared to P. taeda.
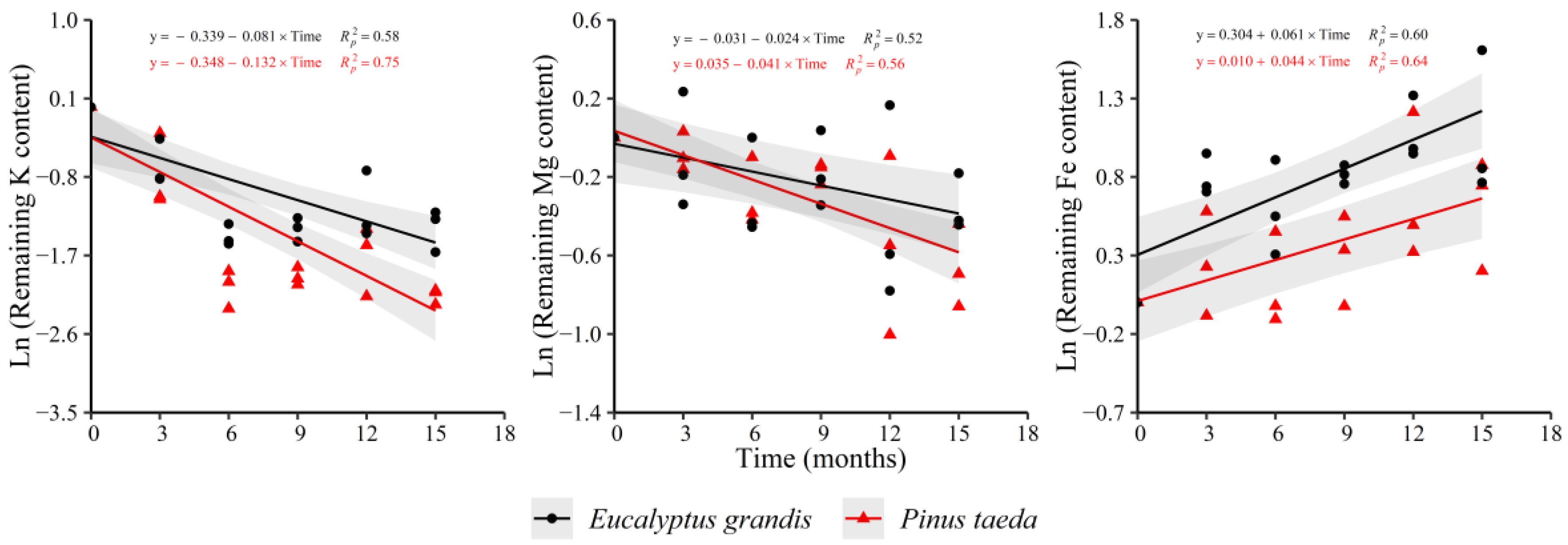
The remaining K and Mg content decreased in both species in the C3 litter layer, as occurred for C1 and C2 layers (Figure 7). Regarding Fe, an increase in both species was evidenced. Ca (y = −0.033 − 0.018 × Time, = 0.26), P (y = −0.022 − 0.055 × Time, = 0.58) and Cu (y = 0.100 − 0.039 × Time, = 0.46) presented diminutions with decomposition time only for P. taeda. E. grandis showed a decrease in the remaining Mn content (y = −0.057 − 0.021 × Time, = 0.33) and an increase for Zn (y = 0.345 + 0.031 × Time, = 0.44), without clear trends in P. taeda. For the remaining N content, no trend was found with decomposition time in both species. Regarding the nutrient release rate, no differences were observed between species for Mg and Fe. Finally, a higher rate of loss of K was verified in P. taeda (Supplementary Table S7).
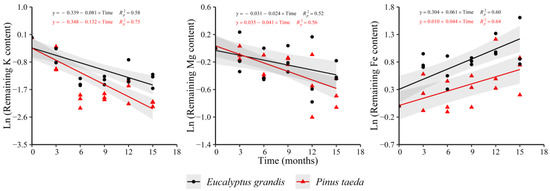
Figure 7.
Remaining nutrient content of E. grandis (black symbols) and P. taeda (red symbols) litter for the different sampling moments (C3 layer). The gray bands around the models represent the 95% confidence interval.
The comparison of the nutrient release between forest litter layers did not show significant differences for E. grandis. These results are explained by the absence of significant interaction effects for the models presented in Table 4.

Table 4.
Coefficients obtained from the adjustment of the generalized linear mixed models for the remaining nutrient content of E. grandis forest litter according to the comparison set.
For P. taeda litter, although no differences between layers were observed for the release of P, K, Mg and Cu, there were differences between C1 and C3 layers for Ca and Fe (Table 5). A higher release rate of Ca occurred in the C1 layer, a minimum in the C3 layer and an intermediate record in the C2 layer that was similar to these values. A higher Fe immobilization rate was verified in the C3 layer, a minimum in the C1 layer and an intermediate value in the C2 layer which was similar to these records (see the coefficients of the adjusted models by layer presented in this section).

Table 5.
Coefficients obtained from the adjustment of the generalized linear mixed models for the remaining nutrient content of P. taeda forest litter according to the comparison set.
4. Discussion
According to the results from a previous study, there were not significant differences in the litter decomposition rate obtained from exponential decay models between the considered species and layers [5]. In this context, this study found that the nutrient dynamics differs enough to alter the average trend evidenced for biomass loss. Considering each litter layer and species individually, despite the verified variations in the remaining nutrient concentration and in the remaining nutrient content, it was possible to obtain a general pattern of behavior of these variables.
In all layers of both species, the concentration and the remaining K content followed a decreasing trend. This is usually attributed to the high mobility of this nutrient, which does not constitute organic structures in plant tissues [38]. Its loss mainly occurs through leaching from the decomposing residues [9,39], being easily removed even in early stages of the process [40]. Releases of this nutrient have been evidenced in litter of different species of Eucalyptus and Pinus [3,41,42,43].
For Mg, the remaining concentration kept on mostly stable in both species with decreases in its remaining content in all layers. This indicates that Mg dynamics is closely related to biomass loss [1,4,26]. This type of response has been verified in different species of both genera [1,4,15,21].
Regarding Ca, the remaining concentration mainly showed increases in E. grandis, being variable in P. taeda. When considering the remaining content, there was a predominance of decreases in both species. The largest proportion of this nutrient is found in cell walls and only a small part is present as soluble salts or ions in plants [38,44]. This implies that its release mainly depends on decomposition through biotic activity instead of direct leaching [6,39]. This was linked to the increases in concentration evidenced in E. grandis and to the relatively slow release during the decomposition, a response reported in previous works in Eucalyptus sp. [4,6]. For P. taeda, the reductions in the remaining content showed a similar result to that previously obtained for Pinus sp. [12,22,41,45].
The remaining N concentration exhibited increases in all layers for both forest species, meanwhile, the remaining N content was mostly stable in E. grandis and presented a predominantly downward trend in P. taeda litter. The increases in concentration can be linked to two main factors that act individually or in combination: immobilization of soil N by microbial population decomposing the forest litter, and a lower release rate with respect to the rate of biomass loss [46,47]. Increases in N concentration with decomposition time have been reported in different Eucalyptus species [6,24,42]. This is usually associated with immobilization processes during the litter decomposition [4,7,48], alternation of immobilization and release [1,49] or nutrient release with remaining content diminutions [3,6,42]. Although this study showed clear increases in N concentration, the observed stability for the remaining content in E. grandis would indicate that the rate of change in the remaining concentration was similar to that verified for biomass loss, oscillating between mineralization and immobilization periods. The reduction in the remaining content together with the increases in N concentration in P. taeda has been previously reported for other species of Pinus [11,21,50,51]. This indicates that the rate of increase in concentration was not enough to offset the biomass loss. The contrasting behavior between species could be related to the lower initial quality that was evidenced for E. grandis forest litter (higher C:N and L:N ratios and lower initial N concentration), promoting some N immobilization moments through the decomposition [10,35,52].
The remaining P concentration mainly showed increases in E. grandis, meanwhile, in P. taeda the response was fundamentally stable. Regarding the remaining P content, decreases were observed in the litter of both species in general. The increases in concentration observed in E. grandis litter during the study were counteracted by the biomass loss, resulting in decreases in the remaining content. Responses in the same line have been previously recorded for several Eucalyptus species [3,42]. In P. taeda, the stability in the remaining concentration with decreases in the remaining content indicates a close relationship between the release of this nutrient and the biomass loss, a similar result to that recorded for the litter of other species of the genus [53,54].
Regarding Fe, increases in the remaining concentration were obtained in all analyzed layers for both species with increases in the remaining Fe content in general terms. This is in line with previous reports in different species of Eucalyptus and Pinus, indicating immobilization processes [1,3,42,45,55,56]. This enrichment could be linked to the Fe adsorption from the surrounding soil on the surface of the decomposing litter [57,58,59,60].
Mn showed increases in its concentration for P. taeda, meanwhile, in E. grandis it remained fundamentally stable, presenting decreases in the remaining content in both species in general. The evidenced response in E. grandis would indicate a link between the release of the nutrient and the biomass loss from the litter, making this process dependent on its decomposition, situation that has been reported in the same species on sandy soils in Argentina [1]. In P. taeda, this response would indicate that the rate of increase did not compensate for the biomass loss. This result is in line with that previously reported for different species of Pinus [21,51].
Increases in Cu concentration in E. grandis with the decomposition time were mainly observed. In P. taeda, there were decreases in this variable in general. Regarding the remaining content, the average behavior in both species was characterized by decreases with decomposition time. Viera et al. [42], for Eucalyptus dunnii in Brazil, verified increases in the concentration of this nutrient with decreases in the remaining content, which indicates that the increases in concentration were not capable of counteracting the biomass loss. For P. taeda, the results showed releases of Cu from the decomposing forest litter, contrasting with those previously reported that showed immobilization processes in litter of Pinus sp. [21,55,58], however, the available literature of the dynamics of this nutrient during litter decomposition is still limited [61].
Finally, Zn presented increases in the concentration in the analyzed litter layers of both species in general, a situation which has been previously reported [3,21,42,51,55,62]. Also, the remaining content in E. grandis mostly showed increases that could be related with immobilization processes, response previously verified in Eucalyptus dunnii in Brazil [3]. For P. taeda litter, on average, a stable behavior with oscillations between release and immobilization was observed which indicates that the rate of concentration increment compensated the rate of biomass loss. Similar results were obtained for forest litter of Pinus halepensis in Spain [55].
When considering the rate of change of the remaining nutrient concentration, most of the evaluated cases did not present significant differences between species. The differences did not make it possible to identify a general pattern of behavior, however, it allowed to verify a higher rate of enrichment in Zn for E. grandis in C2 and C3 layers. Although comparative studies about the dynamics of this metal during decomposition process are scarce, higher Zn enrichments have been previously identified for angiosperm compared to gymnosperm forest litter [60]. This situation allows to verify a more consistent trend, which is characterized by a greater enrichment of this metal in E. grandis. This context would explain the Zn immobilization processes identified in E. grandis and the oscillations between release and immobilization periods obtained in P. taeda, since there were no significant differences in the decomposition rate between species [5].
Regarding the comparison in the rate of change of the remaining nutrient content, in most of the considered nutrients, there were no significant differences between species. In those cases in which significant differences were found, the results did not allow obtaining a general pattern of behavior, however, it was possible to detect a higher rate of Mn loss from E. grandis in C1 and C2 layers. This result makes it possible to identify a trend towards a greater loss of Mn in E. grandis compared to P. taeda. Considering that there were no significant differences in the decomposition rate between species [5], the differences in the nutrient release rate would be linked to the stability evidenced in the concentration of Mn in E. grandis and the increases of the variable in P. taeda. The higher initial Mn concentration evidenced for E. grandis forest litter could promote the higher release rate of this nutrient. In coincidence, increases in Mn releases have been reported for residues with higher initial concentration of this nutrient [61,63].
With respect to the evidenced variations between litter layers of each species for the remaining nutrient concentration and for the remaining nutrient content, only some small differences were found. Differences in the rate of increase of the N and Fe concentration were verified between the analyzed litter layers of E. grandis and P. taeda. In turn, the Zn concentration evidenced a similar response only for E. grandis. For the remaining nutrient content, differences in the release rate of Ca and in the immobilization rate of Fe were obtained only for P. taeda. The differences in the rate of increase of N concentration observed between litter layers of both species were possibly related to lower residue quality. In concordance, higher C:N and L:N ratios and lower initial N concentration have been associated with N immobilization, behavior that has been previously evidenced in different forest species [6,7]. The obtained results for Fe were associated with forest litter layers with higher initial concentrations of this nutrient, where this variable constitutes a regulator of Fe dynamics [64]. For Zn, a similar behavior was observed, with greater increases in those litter layers of E. grandis with a lower initial concentration of the nutrient. This situation has been previously observed for some heavy metals during the litter decomposition process [64,65,66]. Likewise, P. taeda showed some variations in Ca release rate, presenting a minor value in the layer with the lowest initial concentration of this nutrient. In this sense, positive correlations have been evidenced between the initial Ca concentration and the release of this nutrient during forest litter decomposition [6,57].
Considering the obtained average nutrient release/immobilization rates through the models for each species and litter layer with respect to the remaining content, it was possible to establish a hierarchical order of the mobility of the nutrients. For E. grandis, the order was: K > Mn > Mg > P > Cu = Ca > N. For Fe and Zn, immobilization processes were verified, with a higher rate for the first compared to the second. The average values indicate a monthly release rate of 8.5% for K, 3.2% for Mn, 2.9% for Mg, 2.0% for P, 1.7% for Cu and Ca and 1.0% for N. The annual rates were 66%, 33%, 29%, 22%, 18%, 18% and 11%, respectively. Regarding Zn and Fe, a monthly immobilization rate of 1.7% and 3.8% (22% and 57% in annual rate) was obtained for each nutrient, respectively. For Eucalyptus globulus litter in Portugal, an order of mobility of type K > Mg > Ca > P > N was reported [6], being similar to that evidenced in the present study, although with a lower relative mobility of Ca. For E. grandis litter in Brazil, a similar order has been verified following the arrangement: K > Mg > Ca > N [26]. Metals such as Cu have lower mobility and are generally released slowly during the decomposition [42]. Zn and Fe immobilization processes are frequently reported in Eucalyptus sp., with higher responses for Fe [1,3,42], which are consistent with the findings obtained in the present study. Finally, Mn showed an average mobility close to that obtained for Mg, which release was related to the biomass loss, a situation previously verified in the same species in Argentina [1].
For P. taeda, the mobility order was: K > Cu > P > Mg > Ca > N > Mn > Zn. Immobilization processes were observed only for Fe. On average, the monthly release rate reached a value of 11.3% for K, 4.8% for Cu, 4.3% for P, 4.1% for Mg, 3.1% for Ca, 1.4% for N, 1.0% for Mn and 0.7% in the case of Zn (76%, 44%, 41%, 40%, 32%, 15%, 12% and 8% in annual rate, respectively). Fe showed an immobilization at an average monthly rate of 2.8% (39% in annual rate). In decomposition studies in the Southeastern United States with forest litter of P. taeda, the following hierarchical order has been identified: K > Mg > Ca > N > P [12,22]. For the considered nutrients, the order in both studies was similar to that obtained in the present work, except for the greater relative mobility of P. This situation would be related to the high rates of decomposition of the litter obtained in this study [5], since the release of this nutrient was closely linked to biomass loss. Heavy metals such as Zn and Mn were slowly released from the decomposing litter, a similar behavior to that obtained in Pinus sp. in various regions [21,51]. Cu exhibited a significant release, being a contrary response to which was previously evidenced in different species of Pinus [21,55,58]. For Fe, immobilization processes were verified, which have been reported in litter of different species of this genus [45,55,56]. Despite the absence of significant differences in the forest litter decomposition rate between species [5], when comparing the average results for the nutrient release rates, P. taeda showed greater records than E. grandis, with the exception of Mn. Further, Zn presented releases in P. taeda and immobilization processes in E. grandis and the Fe immobilization rate was superior in E. grandis compared to P. taeda. These differences could promote a higher temporal and quantitative availability of nutrients in P. taeda, due to their faster return to the soil through litter decomposition [1,6,12].
Finally, considering the obtained results, the previously proposed sampling methodology [5] allowed us to compare the nutrient dynamics between the species and litter layers. The use of a larger sample size could increase the accuracy of the forest litter inputs estimations [67], promoting an improvement in the adjustment of the nutrient dynamics models. In this sense, following studies should determine a minimum sample size according to temporal and spatial variations on litterfall for each considered forest cover. Nevertheless, these results constitute a first approach to understanding the nutrients dynamics during the decomposition and litter accumulation.
5. Conclusions
The variation in the concentration of the evaluated nutrients during the decomposition process is dependent on the considered nutrient and can significantly alter the evidenced behavior for the biomass loss. The average results of the E. grandis litter layers showed decreases in the remaining content of all nutrients, except for Fe and Zn, which presented increases with decomposition time. For P. taeda, increases were only observed for Fe, with decreases for the rest of the nutrients. For nutrients in which a rise of the remaining content was evidenced, the increases in their remaining concentration were able to compensate and counteract the litter biomass loss, resulting in an opposite trend to that observed for this variable. These results are useful to identify a group of nutrients with which the forest litter is capable of making direct contributions to the soil and another group which promotes immobilization processes producing temporal reductions in nutrient availability for plant uptake.
It is possible to affirm that nutrient recycling depends on the forest species, even in the absence of significant differences between species in the litter decomposition rate. In P. taeda there was a higher release rate of all nutrients compared to E. grandis, except for Mn, which showed superior values for E. grandis. The Zn remaining content exhibited immobilization processes in E. grandis and releases in P. taeda forest litter. Further, the Fe immobilization rate was higher in E. grandis compared to P. taeda. These results were partially verified in the comparisons for each litter layer individually. This situation suggests a greater mobility of the nutrients in P. taeda during the decomposition process and, therefore, a faster return of them to the soil, where the contribution made by the different litter layers deposited during its formation may differ significantly. These findings could be relevant in the development of models for sustainable management, adapting the demand for nutrients to the supply during forest rotations.
Finally, according to the obtained results, the employed sampling methodology allowed us to evaluate and compare the nutrient dynamics between the species and the forest litter layers. In this sense, it is necessary to establish long-term experiments in which a greater stratification in the decomposition status of the litter layers can be identified. This could allow capturing possible variations in the nutrient dynamics due to the continuous incorporation of material through litterfall as well as interactions between forest litter layers during the decomposition process.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/f12091227/s1: Table S1. Initial nutrient stocks of each considered E. grandis and P. taeda litter layer; Table S2. Generalized linear mixed models adjusted for data of both forest species for the remaining nutrient concentration (C1 layer); Table S3. Generalized linear mixed models adjusted for data of both forest species for the remaining nutrient concentration (C2 layer); Table S4. Generalized linear mixed models adjusted for data of both forest species for the remaining nutrient concentration (C3 layer); Table S5. Generalized linear mixed models adjusted for data of both forest species for the remaining nutrient content (C1 layer); Table S6. Generalized linear mixed models adjusted for data of both forest species for the remaining nutrient content (C2 layer); Table S7. Generalized linear mixed models adjusted for data of both forest species for the remaining nutrient content (C3 layer).
Author Contributions
A.B. carried out the sampling activities, statistical and laboratory analyses and led the article writing process with revision from all authors. A.H. was involved in data organization, statistical analyses and in the revision process. J.H. participated in the development of the methodology, litter sampling activities and in the revision process. A.d.P. contributed in the development of the sampling methodology, chemical analyses and in the article review. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Lumin S.A. and the Agencia Nacional de Investigación e Innovación (ANII) [POS_NAC_2016_1_130479].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request to corresponding author.
Acknowledgments
The authors wish to thank the technicians and operational personnel of Lumin S.A. for the logistical support for the evaluations.
Conflicts of Interest
No author is involved in competing interests.
References
- Goya, J.F.; Frangi, J.L.; Pérez, C.; Tea, F.D. Decomposition and nutrient release from leaf litter in Eucalyptus grandis plantations on three different soils in Entre Ríos, Argentina. Bosque Valdivia 2008, 29, 217–226. [Google Scholar] [CrossRef]
- Hernández, J.; del Pino, A.; Vance, E.D.; Califra, Á.; Del Giorgio, F.; Martínez, L.; González-Barrios, P. Eucalyptus and Pinus stand density effects on soil carbon sequestration. For. Ecol. Manag. 2016, 368, 28–38. [Google Scholar] [CrossRef]
- Momolli, D.R.; Schumacher, M.V.; Dick, G.; Viera, M.; De Souza, H.P. Decomposição da serapilheira foliar e liberação de nutrientes em Eucalyptus dunnii no Bioma Pampa. Sci. For. 2018, 46. [Google Scholar] [CrossRef]
- Hernández, J.; del Pino, A.; Salvo, L.; Arrarte, G. Nutrient export and harvest residue decomposition patterns of a Eucalyptus dunnii Maiden plantation in temperate climate of Uruguay. For. Ecol. Manag. 2009, 258, 92–99. [Google Scholar] [CrossRef]
- Baietto, A.; Hernández, J.; del Pino, A. Comparative Dynamics of Above-Ground Litter Production and Decomposition from Eucalyptus grandis Hill ex Maiden and Pinus taeda L., and Their Contribution to Soil Organic Carbon. Forests 2021, 12, 349. [Google Scholar] [CrossRef]
- Ribeiro, C.; Madeira, M.; Araújo, M. Decomposition and nutrient release from leaf litter of Eucalyptus globulus grown under different water and nutrient regimes. For. Ecol. Manag. 2002, 171, 31–41. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, J.; Boyd, S.E.; Xu, Z.; Zhou, Q. Effects of litter quality and quantity on chemical changes during Eucalyptus litter decomposition in subtropical Australia. Plant Soil 2019, 442, 65–78. [Google Scholar] [CrossRef]
- Tukey, H.B. The Leaching of Substances from Plants. Annu. Rev. Plant Physiol. 1970, 21, 305–324. [Google Scholar] [CrossRef]
- Bessaad, A.; Korboulewsky, N. How much does leaf leaching matter during the pre-drying period in a whole-tree harvesting system? For. Ecol. Manag. 2020, 477, 118492. [Google Scholar] [CrossRef]
- Guo, L.; Sims, R. Litter decomposition and nutrient release via litter decomposition in New Zealand eucalypt short rotation forests. Agric. Ecosyst. Environ. 1999, 75, 133–140. [Google Scholar] [CrossRef]
- Polyakova, O.; Billor, N. Impact of deciduous tree species on litterfall quality, decomposition rates and nutrient circulation in pine stands. For. Ecol. Manag. 2007, 253, 11–18. [Google Scholar] [CrossRef]
- Sanchez, F.G. Loblolly pine needle decomposition and nutrient dynamics as affected by irrigation, fertilization, and substrate quality. For. Ecol. Manag. 2001, 152, 85–96. [Google Scholar] [CrossRef]
- Aerts, R. Climate, Leaf Litter Chemistry and Leaf Litter Decomposition in Terrestrial Ecosystems: A Triangular Relationship. Oikos 1997, 79, 439. [Google Scholar] [CrossRef]
- Hoorens, B.; Aerts, R.; Stroetenga, M. Does initial litter chemistry explain litter mixture effects on decomposition? Oecologia 2003, 137, 578–586. [Google Scholar] [CrossRef]
- Berg, B.; Davey, M.P.; De Marco, A.; Emmett, B.; Faituri, M.; Hobbie, S.; Johansson, M.-B.; Liu, C.; McClaugherty, C.; Norell, L.; et al. Factors influencing limit values for pine needle litter decomposition: A synthesis for boreal and temperate pine forest systems. Biogeochemistry 2010, 100, 57–73. [Google Scholar] [CrossRef]
- Mary, B.; Recous, S.; Darwis, D.; Robin, D. Interactions between decomposition of plant residues and nitrogen cycling in soil. Plant Soil 1996, 181, 71–82. [Google Scholar] [CrossRef]
- Mungai, N.W.; Motavalli, P.P. Litter quality effects on soil carbon and nitrogen dynamics in temperate alley cropping systems. Appl. Soil Ecol. 2006, 31, 32–42. [Google Scholar] [CrossRef]
- Chae, H.M.; Choi, S.H.; Lee, S.H.; Cha, S.; Yang, K.C.; Shim, J.K. Effect of Litter Quality on Needle Decomposition for Four Pine Species in Korea. Forests 2019, 10, 371. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, X.; Geng, Q.; Shi, Z.; Luo, Y.; Xu, X. Leaf litter traits predominantly control litter decomposition in streams worldwide. Glob. Ecol. Biogeogr. 2019, 28, 1469–1486. [Google Scholar] [CrossRef]
- Gholz, H.L.; Wedin, D.A.; Smitherman, S.M.; Harmon, M.E.; Parton, W.J. Long-term dynamics of pine and hardwood litter in contrasting environments: Toward a global model of decomposition. Glob. Chang. Biol. 2000, 6, 751–765. [Google Scholar] [CrossRef]
- Gurlevik, N.; Kelting, D.L.; Allen, H. The effects of vegetation control and fertilization on net nutrient release from decomposing loblolly pine needles. Can. J. For. Res. 2003, 33, 2491–2502. [Google Scholar] [CrossRef]
- Piatek, K.B.; Allen, H.L. Are forest floors in mid-rotation stands of loblolly pine (Pinus taeda) a sink for nitrogen and phosphorus? Can. J. For. Res. 2001, 31, 1164–1174. [Google Scholar] [CrossRef]
- Rahman, M.M.; Tsukamoto, J.; Rahman, M.; Yoneyama, A.; Mostafa, K.M. Lignin and its effects on litter decomposition in forest ecosystems. Chem. Ecol. 2013, 29, 540–553. [Google Scholar] [CrossRef]
- Demessie, A.; Singh, B.R.; Lal, R.; Strand, L.T. Leaf litter fall and litter decomposition under Eucalyptus and coniferous plantations in Gambo District, southern Ethiopia. Acta Agric. Scand. Sect. B Plant Soil Sci. 2011, 1–10. [Google Scholar] [CrossRef]
- Swift, M.J.; Heal, O.W.; Anderson, J.M. Decomposition in Terrestrial Ecosystems; University of California Press: Berkeley, CA, USA, 1979; ISBN 0-520-04001-5. [Google Scholar]
- Costa, G.S.; Da Gama-Rodrigues, A.C.; Cunha, G.D.M. Decomposição e liberação de nutrientes da serapilheira foliar em povoamentos de Eucalyptus grandis no norte fluminense. Revista Árvore 2005, 29, 563–570. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Castaño, J.P.; Giménez, A.; Ceroni, M.; Furest, J.; Aunchayna, R.; Bidegain, M. Caracterización Agroclimática del Uruguay 1980-2009; INIA Serie Técnica; Instituto de Investigaciones Agropecuarias: Montevideo, Uruguay, 2011; ISBN 978-9974-38-330-2. [Google Scholar]
- Olson, J.S. Energy Storage and the Balance of Producers and Decomposers in Ecological Systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Isaac, R.A.; Kerber, J.D. Atomic Absorption and Flame Photometry: Techniques and Uses in Soil, Plant, and Water Analysis. In Instrumental Methods for Analysis of Soils and Plant Tissue; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1971; pp. 17–37. ISBN 978-0-89118-876-6. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen—Total. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1983; pp. 595–624. ISBN 978-0-89118-977-0. [Google Scholar]
- Schwanninger, M.; Hinterstoisser, B. Klason Lignin: Modifications to Improve the Precision of the Standardized Determination. Holzforschung 2002, 56, 161–166. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Corbeels, M.; O’Connell, A.; Grove, T.; Mendham, D.; Rance, S. Nitrogen release from eucalypt leaves and legume residues as influenced by their biochemical quality and degree of contact with soil. Plant Soil 2003, 250, 15–28. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. Methods of Soil Analysis Part 3—Chemical Methods; SSSA Book Series; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; ISBN 978-0-89118-866-7. [Google Scholar]
- Nakagawa, S.; Johnson, P.C.D.; Schielzeth, H. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 2017, 14, 20170213. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK; Waltham, MA, USA, 2011; ISBN 978-0-12-384906-9. [Google Scholar]
- Staaf, H. Release of plant nutrients from decomposing leaf litter in a South Swedish beech forest. Ecography 1980, 3, 129–136. [Google Scholar] [CrossRef]
- O’Connell, A.M.; Grove, T.S. Biomass production, nutrient uptake and nutrient cycling in the jarrah (Eucalyptus marginata) and karri (Eucalyptus diversicolor) forests of south-western Australia. In Nutrition of Eucalypts; CSIRO Publishing: Collingwood, Australia, 1996; pp. 155–189. ISBN 978-0-643-10522-5. [Google Scholar]
- Kiser, L.C.; Fox, T.R.; Carlson, C.A. Foliage and Litter Chemistry, Decomposition, and Nutrient Release in Pinus taeda. Forests 2013, 4, 595. [Google Scholar] [CrossRef]
- Viera, M.; Schumacher, M.; Araújo, E.F. Disponibilização de nutrientes via decomposição da serapilheira foliar em um plantio de Eucalyptus urophylla × Eucalyptus globulus. Floresta Ambient. 2014, 21, 307–315. [Google Scholar] [CrossRef][Green Version]
- Liechty, H.O.; Reinke, M. The influence of repeated prescribed fire on decomposition and nutrient release in uneven-aged loblolly–shortleaf pine stands. Fire Ecol. 2020, 16. [Google Scholar] [CrossRef]
- Demarty, M.; Morvan, C.; Thellier, M. Calcium and the cell wall. Plant Cell Environ. 1984, 7, 441–448. [Google Scholar] [CrossRef]
- Xu, X. Nutrient dynamics in decomposing needles of Pinus luchuensis after typhoon disturbance in a subtropical environment. Ann. For. Sci. 2006, 63, 707–713. [Google Scholar] [CrossRef]
- Berg, B.; Staaf, H. Leaching, Accumulation and Release of Nitrogen in Decomposing Forest Litter. Ecol. Bull. 1981, 33, 163–178. [Google Scholar]
- Titus, B. The long-term decomposition of Sitka spruce needles in brash. Forestry 1999, 72, 207–221. [Google Scholar] [CrossRef]
- Parsons, S.A.; Congdon, R.A. Plant litter decomposition and nutrient cycling in north Queensland tropical rain-forest communities of differing successional status. J. Trop. Ecol. 2008, 24, 317–327. [Google Scholar] [CrossRef]
- Adams, M.; Attiwill, P.M. Nutrient cycling and nitrogen mineralization in eucalypt forests of south-eastern Australia. Plant Soil 1986, 92, 319–339. [Google Scholar] [CrossRef]
- Jorgensen, J.R.; Wells, C.G.; Metz, L.J. Nutrient Changes in Decomposing Loblolly Pine Forest Floor. Soil Sci. Soc. Am. J. 1980, 44, 1307–1314. [Google Scholar] [CrossRef]
- Will, G.M.; Hodgkiss, P.D.; Madgwick, H.A.I. Nutrient Losses from Litterbags Containing Pinus radiata Litter: Influences of Thinning, Clear-Felling and Urea Fertilizer. N. Z. J. For. Sci. 1983, 13, 291–304. [Google Scholar]
- Berg, B.; McClaugherty, C. Nitrogen and phosphorus release from decomposing litter in relation to the disappearance of lignin. Can. J. Bot. 1989, 67, 1148–1156. [Google Scholar] [CrossRef]
- Kim, C.; Sharik, T.L.; Jurgensen, M.F. Canopy cover effects on mass loss, and nitrogen and phosphorus dynamics from decomposing litter in oak and pine stands in northern Lower Michigan. For. Ecol. Manag. 1996, 80, 13–20. [Google Scholar] [CrossRef]
- Kim, C.; Son, Y.; Lee, W.-K.; Jeong, J.; Noh, N.-J.; Kim, S.-R.; Yang, A.-R. Litter decomposition and nutrient dynamics following forest tending (Soopkakkugi) works in a Pinus densiflora stand. For. Sci. Technol. 2012, 8, 99–104. [Google Scholar] [CrossRef]
- Bueis, T.; Bravo, F.; Pando, V.; Turrión, M.-B. Local basal area affects needle litterfall, nutrient concentration, and nutrient release during decomposition in Pinus halepensis Mill. plantations in Spain. Ann. For. Sci. 2018, 75, 21. [Google Scholar] [CrossRef]
- He, W.; Lei, L.; Ma, Z.; Teng, M.; Wang, P.; Yan, Z.; Huang, Z.; Zeng, L.; Xiao, W. Nonadditive effects of decomposing mixed foliar litter on the release of several metallic elements in a Pinus massoniana Lamb. forest. Ann. For. Sci. 2020, 77, 1–12. [Google Scholar] [CrossRef]
- Rustad, L.E.; Cronan, C.S. Element loss and retention during litter decay in a red spruce stand in Maine. Can. J. For. Res. 1988, 18, 947–953. [Google Scholar] [CrossRef]
- Bockheim, J.G.; Jepsen, E.A.; Heisey, D.M. Nutrient dynamics in decomposing leaf litter of four tree species on a sandy soil in northwestern Wisconsin. Can. J. For. Res. 1991, 21, 803–812. [Google Scholar] [CrossRef]
- Gonzalez, G.; Lodge, D.J.; Richardson, B.A.; Richardson, M. A canopy trimming experiment in Puerto Rico: The response of litter decomposition and nutrient release to canopy opening and debris deposition in a subtropical wet forest. For. Ecol. Manag. 2014, 332, 32–46. [Google Scholar] [CrossRef]
- Pourhassan, N.; Bruno, S.; Jewell, M.D.; Shipley, B.; Roy, S.; Bellenger, J.-P. Phosphorus and micronutrient dynamics during gymnosperm and angiosperm litters decomposition in temperate cold forest from Eastern Canada. Geoderma 2016, 273, 25–31. [Google Scholar] [CrossRef]
- Berg, B. Foliar Litter Decomposition: A Conceptual Model with Focus on Pine (Pinus) Litter—A Genus with Global Distribution. ISRN For. 2014, 2014, 1–22. [Google Scholar] [CrossRef]
- Mubarak, A.R.; Elbashir, A.A.; Elamin, L.A.; Daldoum, D.M.A.; Steffens, D.; Benckiser, G. Decomposition and Nutrient Release from Litter Fall in the Semi-arid Tropics of Sudan. Commun. Soil Sci. Plant Anal. 2008, 39, 2359–2377. [Google Scholar] [CrossRef]
- Berg, B.; Erhagen, B.; Johansson, M.-B.; Vesterdal, L.; Faituri, M.; Sanborn, P.; Nilsson, M. Manganese dynamics in decomposing needle and leaf litter—A synthesis. Can. J. For. Res. 2013, 43, 1127–1136. [Google Scholar] [CrossRef]
- De Marco, A.; Vittozzi, P.; Rutigliano, F.A.; Virzo de Santo, A. Nutrient dynamics during decomposition of four different pine litters. In Proceedings of the International Workshop MEDPINE 3: Conservation, Regeneration and Restoration of Mediterranean Pines and Their Ecosystems, 26–30 September 2005; Leone, V., Lovreglio, R., Eds.; Options Méditerranéennes: Série A. Séminaires Méditerranéens; CIHEAM: Bari, Italy, 2007; Volume 75, pp. 73–77. ISBN 2-85352-356-X. [Google Scholar]
- De Santo, A.V.; Fierro, A.; Berg, B.; Rutigliano, F.A.; De Marco, A. Heavy metals and litter decomposition in coniferous forests. In Developments in Soil Science; Violante, A., Huang, P.M., Bollag, J.-M., Gianfreda, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 28, pp. 63–78. ISBN 0166-2481. [Google Scholar]
- Scheid, S.; Günthardt-Goerg, M.S.; Schulin, R.; Nowack, B. Accumulation and solubility of metals during leaf litter decomposition in non-polluted and polluted soil. Eur. J. Soil Sci. 2009, 60, 613–621. [Google Scholar] [CrossRef]
- Finotti, R.; Freitas, S.R.; Cerqueira, R.; Vieira, M.V. A Method to Determine the Minimum Number of Litter Traps in Litterfall Studies1. Biotropica 2003, 35, 419–421. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).