The Effect of Guttation on the Growth of Bamboo Shoots
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Observation and Collection of Guttation Samples
2.3. Water Transport Path Tracer Experiment
2.4. Anatomical Structure of Bamboo Shoot and Sheath
2.5. Bamboo Sheath Treatment
2.6. Statistical Analysis
3. Results
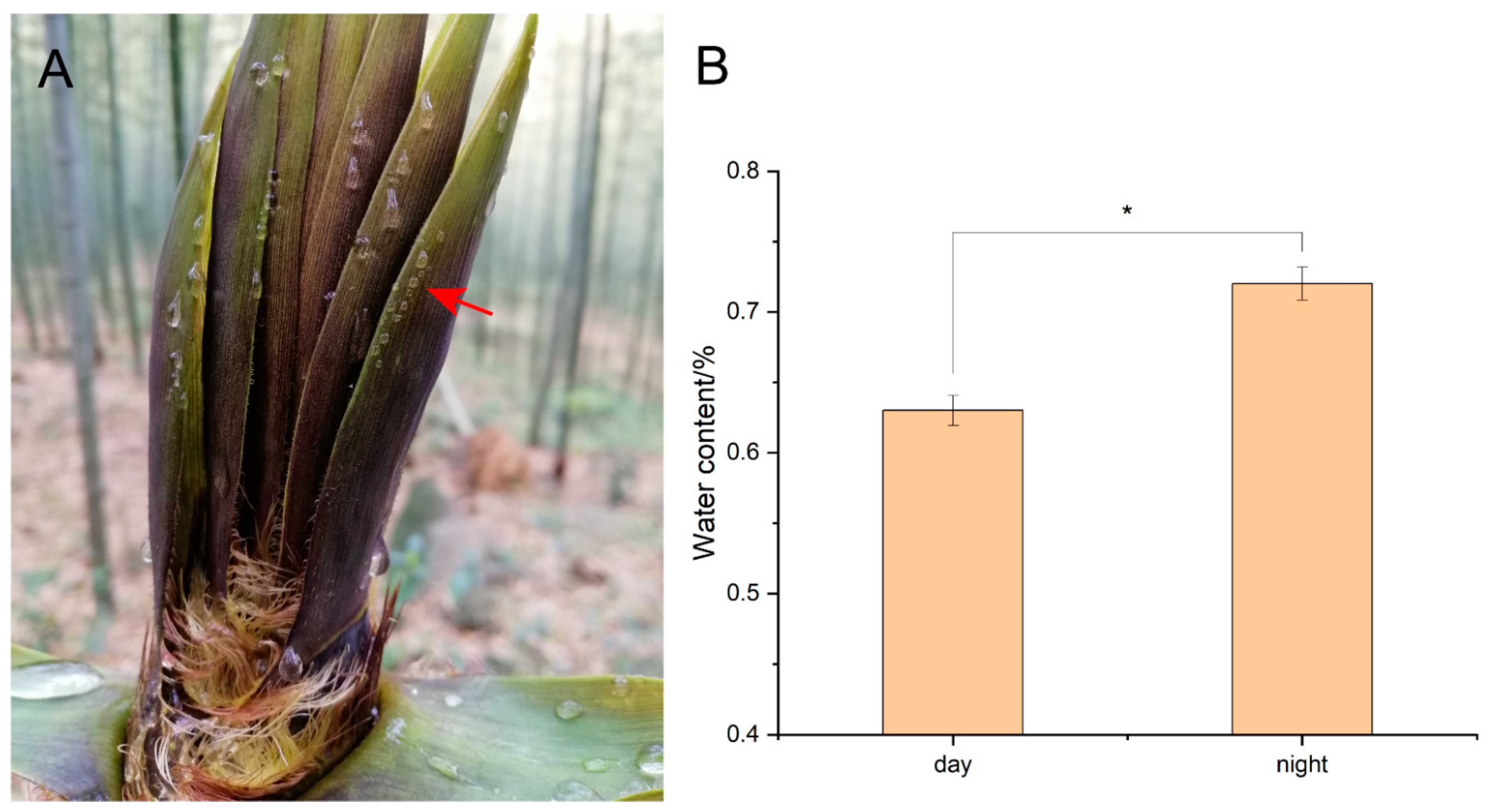
3.1. Guttation of Sheath Blades
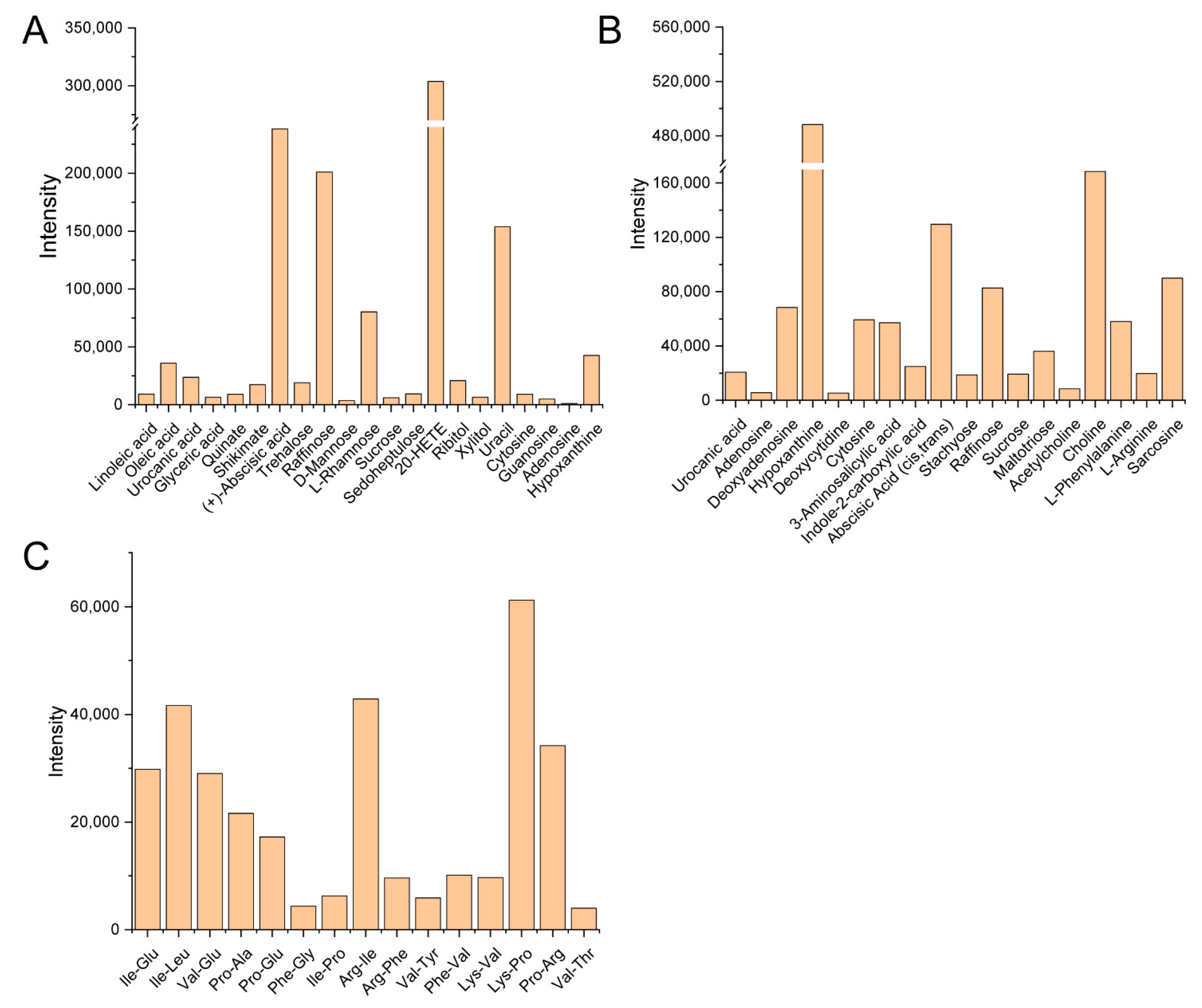
3.2. Composition Analysis of the Guttation Fluid
3.3. Water Transport in Bamboo Sheaths
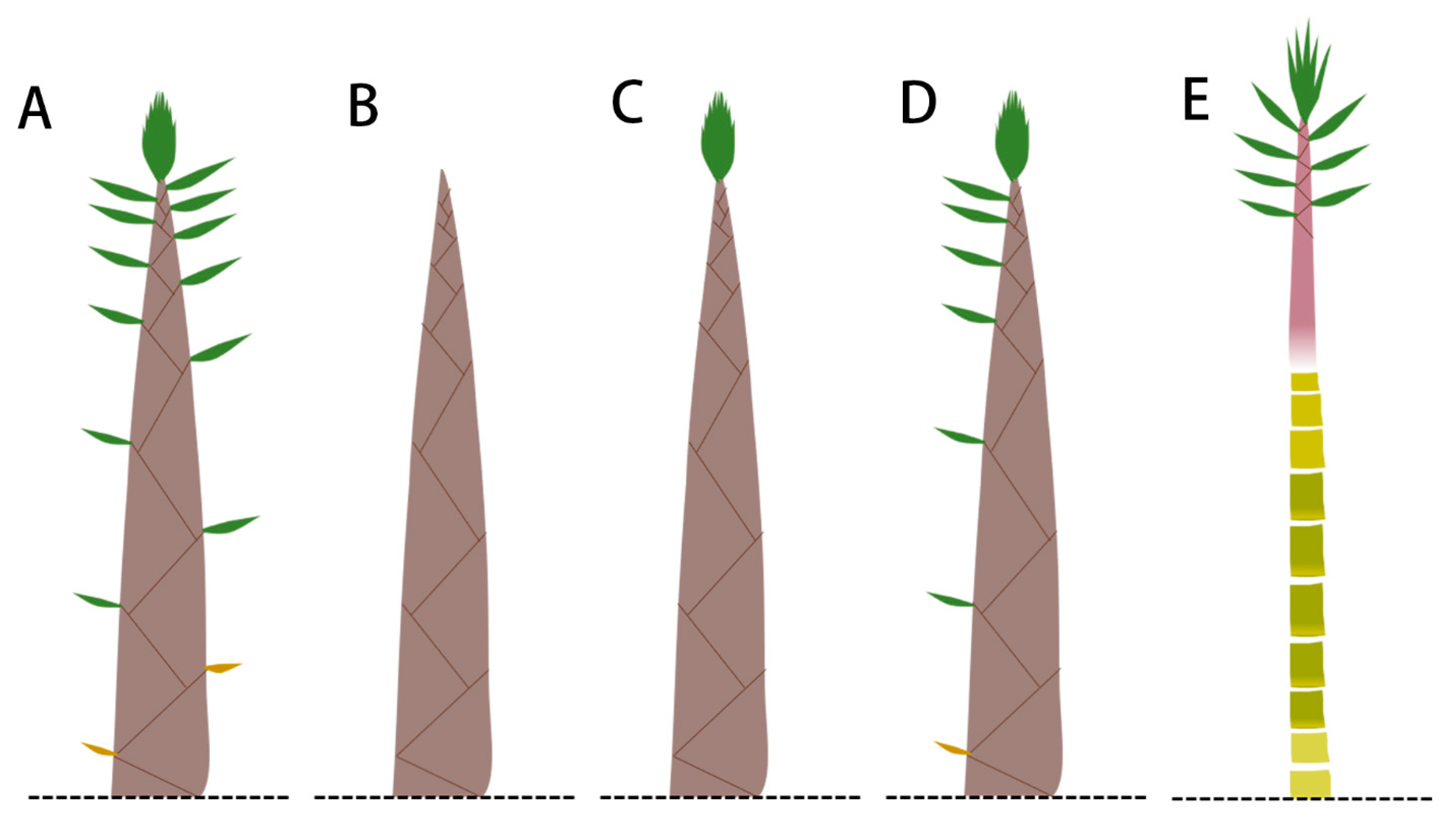
3.4. Effect of Sheath Blades and Culm Sheaths on the Growth of Bamboo Shoots
4. Discussion
4.1. Guttation Phenomenon of Sheath Blades of Bamboo Shoots
4.2. Various Components of the Guttation Fluid from Bamboo Sheath Blades
4.3. Water and Substance Transport Pathways in Bamboo Shoots
4.4. Bamboo Sheath Affects Internode Growth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Raleigh, G.J. The effect of various ions on guttation of the tomato. Plant Physiol. 1946, 21, 194–200. [Google Scholar] [CrossRef]
- Schenke, D.; Wirtz, I.P.; Lorenz, S.; Pistorius, J.; Heimbach, U. Two-year field data on neonicotinoid concentrations in guttation drops of seed treated maize (Zea mays). Data Brief. 2018, 21, 299–306. [Google Scholar] [CrossRef]
- Bailey, K.J.; Leegood, R.C. Nitrogen recycling from the xylem in rice leaves: Dependence upon metabolism and associated changes in xylem hydraulics. J. Exp. Bot. 2016, 67, 2901–2911. [Google Scholar] [CrossRef]
- Kovalskaya, N.; Owens, R.; Baker, C.J.; Deahl, K.; Hammond, R.W. Application of a modified EDTA-mediated exudation technique and guttation fluid analysis for Potato spindle tuber viroid RNA detection in tomato plants (Solanum lycopersicum). J. Virol. Methods 2014, 198, 75–81. [Google Scholar] [CrossRef][Green Version]
- Goatley, J.L.; Lewis, R.W. Composition of guttation fluid from rye, wheat, and barley seedlings. Plant Physiol. 1966, 41, 373–375. [Google Scholar] [CrossRef]
- Singh, S. Guttation: Path, principles and functions. Aust. J. Bot. 2013, 61, 497–515. [Google Scholar] [CrossRef]
- Singh, S. Guttation: Mechanism, momentum and modulation. Bot. Rev. 2016, 82, 149–182. [Google Scholar] [CrossRef]
- Komarnytsky, S.; Borisjuk, N.V.; Borisjuk, L.G.; Alam, M.Z.; Raskin, I. Production of recombinant proteins in tobacco guttation fluid. Plant Physiol. 2000, 124, 927–934. [Google Scholar] [CrossRef]
- Singh, S.; Singh, T.N.; Chauhan, J.S. Guttation in rice: Occurrence, regulation, and significance in varietal improvement. J. Crop Improv. 2009, 23, 351–365. [Google Scholar] [CrossRef]
- Li, L.; Cheng, Z.; Ma, Y.; Bai, Q.; Li, X.; Cao, Z.; Wu, Z.; Gao, J. The association of hormone signalling genes, transcription and changes in shoot anatomy during moso bamboo growth. Plant Biotechnol. J. 2017, 16, 72–85. [Google Scholar] [CrossRef]
- Li, L.; Mu, S.; Cheng, Z.; Cheng, Y.; Zhang, Y.; Miao, Y.; Hou, C.; Li, X.; Gao, J. Characterization and expression analysis of the WRKY gene family in moso bamboo. Sci. Rep. 2017, 7, 6675. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, C.; Zhang, Y.; Hu, T.; Mu, S.; Li, X.; Gao, J. Transcriptome sequencing and analysis of the fast growing shoots of moso bamboo (Phyllostachys edulis). PLoS ONE 2013, 8, e78944. [Google Scholar] [CrossRef]
- Liese, W.; Tang, T.K.H. Properties of the bamboo culm. In Bamboo; Springer: Cham, Switzerland, 2015; pp. 227–256. [Google Scholar]
- Plumb, R.S.; Granger, J.H.; Stumpf, C.L.; Johnson, K.A.; Smith, B.W.; Gaulitz, S.; Wilson, I.D.; Castro-Perez, J. A rapid screening approach to metabonomics using UPLC and oa-TOF mass spectrometry: Application to age, gender and diurnal variation in normal/Zucker obese rats and black, white and nude mice. Analyst 2005, 130, 844–849. [Google Scholar] [CrossRef]
- Xie, Z.; Bondada, B.; Li, B.; Ding, J. Visualization of axial water transport pathways in grapevines using dye-tracing technique. Plant Physiol. J. 2016, 52, 1159–1168. [Google Scholar] [CrossRef]
- Nagai, M.; Ohnishi, M.; Uehara, T.; Yamagami, M.; Miura, E.; Kamakura, M.; Kitamura, A.; Sakaguchi, S.; Sakamoto, W.; Shimmen, T.; et al. Ion gradients in xylem exudate and guttation fluid related to tissue ion levels along primary leaves of barley. Plant Cell Environ. 2013, 36, 1826–1837. [Google Scholar] [CrossRef]
- Mortl, M.; Takacs, E.; Klatyik, S.; Szekacs, A. Appearance of thiacloprid in the guttation liquid of coated maize seeds. Int. J. Environ. Res. Public Health 2020, 17, 3290. [Google Scholar] [CrossRef]
- Hoffmann, E.J.; Castle, S.J. Imidacloprid in melon guttation fluid: A potential mode of exposure for pest and beneficial organisms. J. Econ. Entomol. 2012, 105, 67–71. [Google Scholar] [CrossRef]
- Wang, S.; He, W.; Zhan, H. Culm sheaths affect height growth of bamboo shoots in Fargesia yunnanensis. Braz. J. Bot. 2018, 41, 255–266. [Google Scholar] [CrossRef]
- Shapira, O.R.; Israeli, Y.; Shani, U.; Schwartz, A. Salt stress aggravates boron toxicity symptoms in banana leaves by impairing guttation. Plant Cell Environ. 2013, 36, 275–287. [Google Scholar] [CrossRef]
- Li, D.; Sun, D.; Sun, W.; Yu, C. Nutrient components in fresh bamboo shoots of eight Phyllostachys species. J. Bamboo Res. 2018, 37, 14–19. [Google Scholar] [CrossRef]
- Zhou, W.; He, Q.; Ye, C.; Xu, R.; Tong, X.; Wang, B.; Hua, X.; Zhou, L.; Lu, J. Comparative analysis of nutrients in bamboo shoot at different seasons. J. Zhejiang For. Sci. Technol. 2013, 33, 64–67. [Google Scholar]
- Huang, X.; Li, J.; Zhang, W.; Peng, S.; Zhu, Y.; Lin, L. Nutritional components and active ingredient analysis of different parts of winter moso bamboo shoots. Food Sci. Technol. 2014, 39, 59–63. [Google Scholar] [CrossRef]
- Dahiya, A.; Saini, R.; Saini, H.S.; Devi, A. Sucrose metabolism: Controls the sugar sensing and generation of signalling molecules in plants. J. Pharmacogn. Phytochem. 2017, 6, 1563–1572. [Google Scholar]
- Calvo-Polanco, M.; Armada, E.; Zamarreno, A.M.; Garcia-Mina, J.M.; Aroca, R. Local root ABA/cytokinin status and aquaporins regulate poplar responses to mild drought stress independently of the ectomycorrhizal fungus Laccaria bicolor. J. Exp. Bot. 2019, 70, 6437–6446. [Google Scholar] [CrossRef]
- Yunta, C.; Martinez-Ripoll, M.; Zhu, J.K.; Albert, A. The structure of Arabidopsis thaliana OST1 provides insights into the kinase regulation mechanism in response to osmotic stress. J. Mol. Biol. 2011, 414, 135–144. [Google Scholar] [CrossRef]
- Gamuyao, R.; Nagai, K.; Ayano, M.; Mori, Y.; Minami, A.; Kojima, M.; Suzuki, T.; Sakakibara, H.; Higashiyama, T.; Ashikari, M.; et al. Hormone distribution and transcriptome profiles in bamboo shoots provide insights on bamboo stem emergence and growth. Plant Cell Physiol. 2017, 58, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Urbaneja-Bernat, P.; Tena, A.; Gonzalez-Cabrera, J.; Rodriguez-Saona, C. Plant guttation provides nutrient-rich food for insects. Proc. Biol. Sci. 2020, 287, 20201080. [Google Scholar] [CrossRef]
- Pospíšilová, J.; Zimmermann, M.H. Xylem structure and the ascent of sap. Struct. Chem. 2012, 23, 1831–1836. [Google Scholar]
- Fang, D.; Mei, T.; Roll, A.; Holscher, D. Water transfer between bamboo culms in the period of sprouting. Front. Plant Sci. 2019, 10, 786. [Google Scholar] [CrossRef]
- Mei, T.; Fang, D.; Röll, A.; Hölscher, D. Bamboo water transport assessed with deuterium tracing. Forests 2019, 10, 623. [Google Scholar] [CrossRef]
- Zhao, X.H.; Zhao, P.; Zhang, Z.Z.; Zhu, L.W.; Niu, J.F.; Ni, G.Y.; Hu, Y.T.; Lei, O.Y. Sap flow-based transpiration in Phyllostachys pubescens: Applicability of the TDP methodology, age effect and rhizome role. Trees 2016, 31, 765–779. [Google Scholar] [CrossRef]
- Suzuki, S.; Burnell, J.N. The pck1 promoter from Urochloa panicoides (a C4 plant) directs expression differently in rice (a C3 plant) and maize (a C4 plant). Plant Sci. 2003, 165, 603–611. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Du, J.; Zhao, Y.; Lu, X.; Wen, W.; Gu, S.; Fan, J.; Wang, C.; Wu, S.; et al. Dissecting the phenotypic components and genetic architecture of maize stem vascular bundles using high-throughput phenotypic analysis. Plant Biotechnol. J. 2020, 19, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Fei, C.; Geng, X.; Xu, Z.; Xu, Q. Multiple areas investigation reveals the genes related to vascular bundles in rice. Rice 2019, 12, 17. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, L.; Pan, X.; Wang, J.; Guo, X.; Du, J. Micron-scale phenotyping techniques of maize vascular bundles based on X-ray microcomputed tomography. J. Vis. Exp. 2018, 140, e58501. [Google Scholar] [CrossRef]
- Deng, L.; Li, P.; Chu, C.; Ding, Y.; Wang, S. Symplasmic phloem unloading and post-phloem transport during bamboo internode elongation. Tree Physiol. 2020, 40, 391–412. [Google Scholar] [CrossRef] [PubMed]
- Canessa Fortuna, A.; Zerbetto De Palma, G.; Aliperti Car, L.; Armentia, L.; Vitali, V.; Zeida, A.; Estrin, D.A.; Alleva, K. Gating in plant plasma membrane aquaporins: The involvement of leucine in the formation of a pore constriction in the closed state. FEBS J. 2019, 286, 3473–3487. [Google Scholar] [CrossRef]
- Cochard, H.; Venisse, J.S.; Barigah, T.S.; Brunel, N.; Herbette, S.; Guilliot, A.; Tyree, M.T.; Sakr, S. Putative role of aquaporins in variable hydraulic conductance of leaves in response to light. Plant Physiol. 2007, 143, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.X.; Steudle, E. Gating of aquaporins by light and reactive oxygen species in leaf parenchyma cells of the midrib of Zea mays. J. Exp. Bot. 2009, 60, 547–556. [Google Scholar] [CrossRef]
- Chen, Z.H.; Chen, G.; Dai, F.; Wang, Y.; Hills, A.; Ruan, Y.L.; Zhang, G.; Franks, P.J.; Nevo, E.; Blatt, M.R. Molecular evolution of grass stomata. Trends Plant Sci. 2017, 22, 124–139. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Dong, H. Plant aquaporins in infection by and immunity against pathogens—A critical review. Front. Plant Sci. 2019, 10, 632. [Google Scholar] [CrossRef]
- Wang, S.; Zhan, H.; Li, P.; Chu, C.; Li, J.; Wang, C. Physiological mechanism of internode bending growth after the excision of shoot sheath in Fargesia yunnanensis and its implications for understanding the rapid growth of bamboos. Front. Plant Sci. 2020, 11, 418. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Ding, Y.; Fei, Z.; Jiao, C.; Fan, M.; Yao, B.; Xin, P.; Chu, J.; Wei, Q. Cellular and molecular characterization of a thick-walled variant reveal a pivotal role of shoot apical meristem in transverse development of bamboo culm. J. Exp. Bot. 2019, 70, 3911–3926. [Google Scholar] [CrossRef]
- Wei, Q.; Guo, L.; Jiao, C.; Fei, Z.; Chen, M.; Cao, J.; Ding, Y.; Yuan, Q. Characterization of the developmental dynamics of the elongation of a bamboo internode during the fast growth stage. Tree Physiol. 2019, 39, 1201–1214. [Google Scholar] [CrossRef]
- Cao, K.F.; Yang, S.J.; Zhang, Y.J.; Brodribb, T.J. The maximum height of grasses is determined by roots. Ecol. Lett. 2012, 15, 666–672. [Google Scholar] [CrossRef]




| Time | Guttation/mL | Temperature/°C | Humidity/% |
|---|---|---|---|
| 22:00 | 14.962 a | 14.4 | 64.5 |
| 2:00 | 24.044 b | 16.6 | 43.8 |
| 6:00 | 18.148 ab | 13.7 | 60.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Cai, M.; Bai, Y.; Xu, J.; Xie, Y.; Song, H.; Li, J.; Gao, J. The Effect of Guttation on the Growth of Bamboo Shoots. Forests 2022, 13, 31. https://doi.org/10.3390/f13010031
Zheng H, Cai M, Bai Y, Xu J, Xie Y, Song H, Li J, Gao J. The Effect of Guttation on the Growth of Bamboo Shoots. Forests. 2022; 13(1):31. https://doi.org/10.3390/f13010031
Chicago/Turabian StyleZheng, Huifang, Miaomiao Cai, Yucong Bai, Junlei Xu, Yali Xie, Huajian Song, Juan Li, and Jian Gao. 2022. "The Effect of Guttation on the Growth of Bamboo Shoots" Forests 13, no. 1: 31. https://doi.org/10.3390/f13010031
APA StyleZheng, H., Cai, M., Bai, Y., Xu, J., Xie, Y., Song, H., Li, J., & Gao, J. (2022). The Effect of Guttation on the Growth of Bamboo Shoots. Forests, 13(1), 31. https://doi.org/10.3390/f13010031






