Effects of Biochar Application Pyrolyzed at Different Temperatures on Soil Properties, Growth and Leaf Secondary Metabolite Accumulation in Cyclocarya paliurus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Wheel Wingnut-Based Biochar
2.2. Plant Material and Experimental Design
2.3. Data Collection and Measurement
2.3.1. Growth and Biomass Measurement
2.3.2. Nutrient Content Measurement
2.3.3. Secondary Metabolite Measurement
2.4. Statistical Analysis
3. Results
3.1. Effects of Pyrolysis Temperature on Biochar Properties
3.2. Effects of Biochar Additions on the Soil Properties
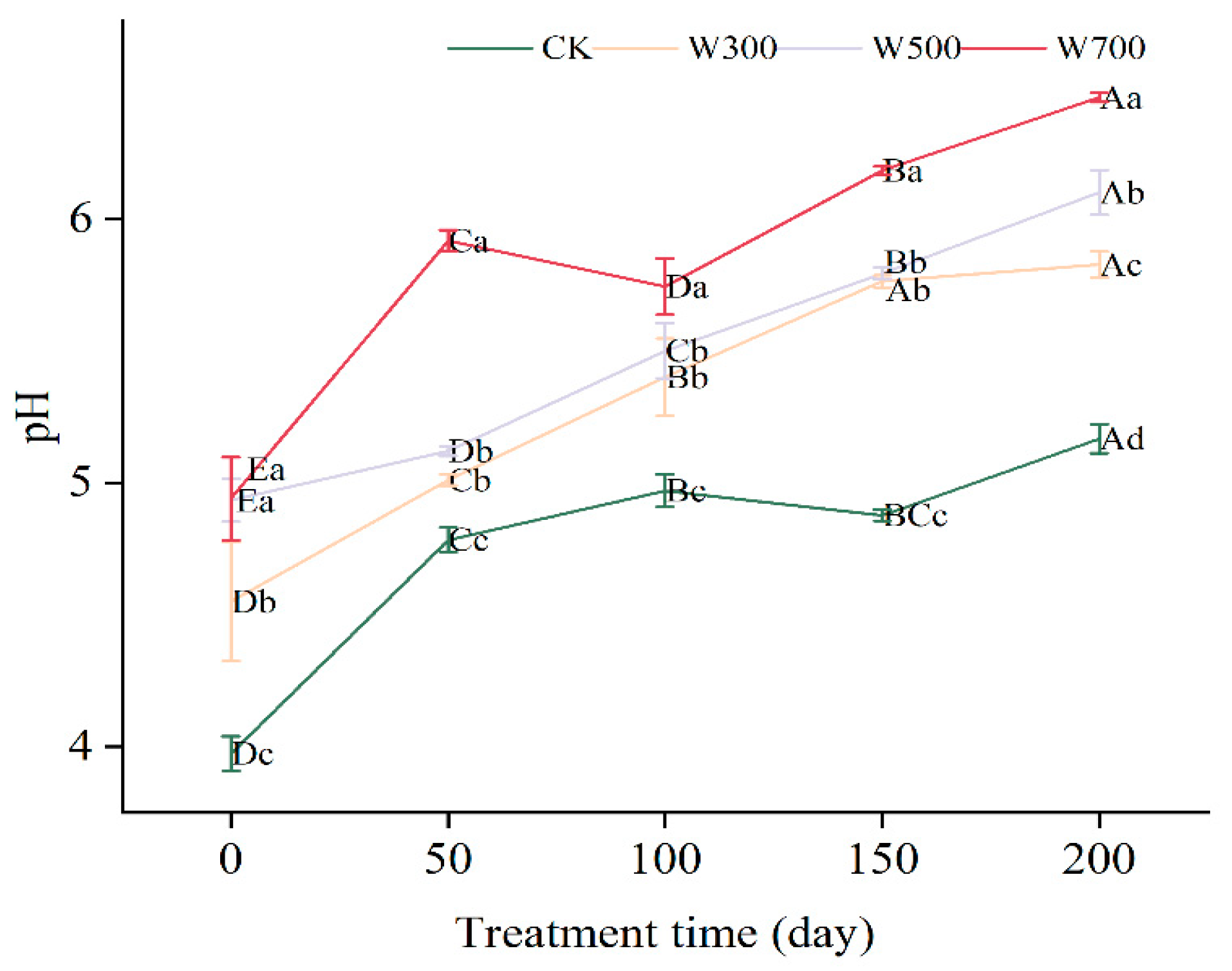
3.2.1. Soil pH
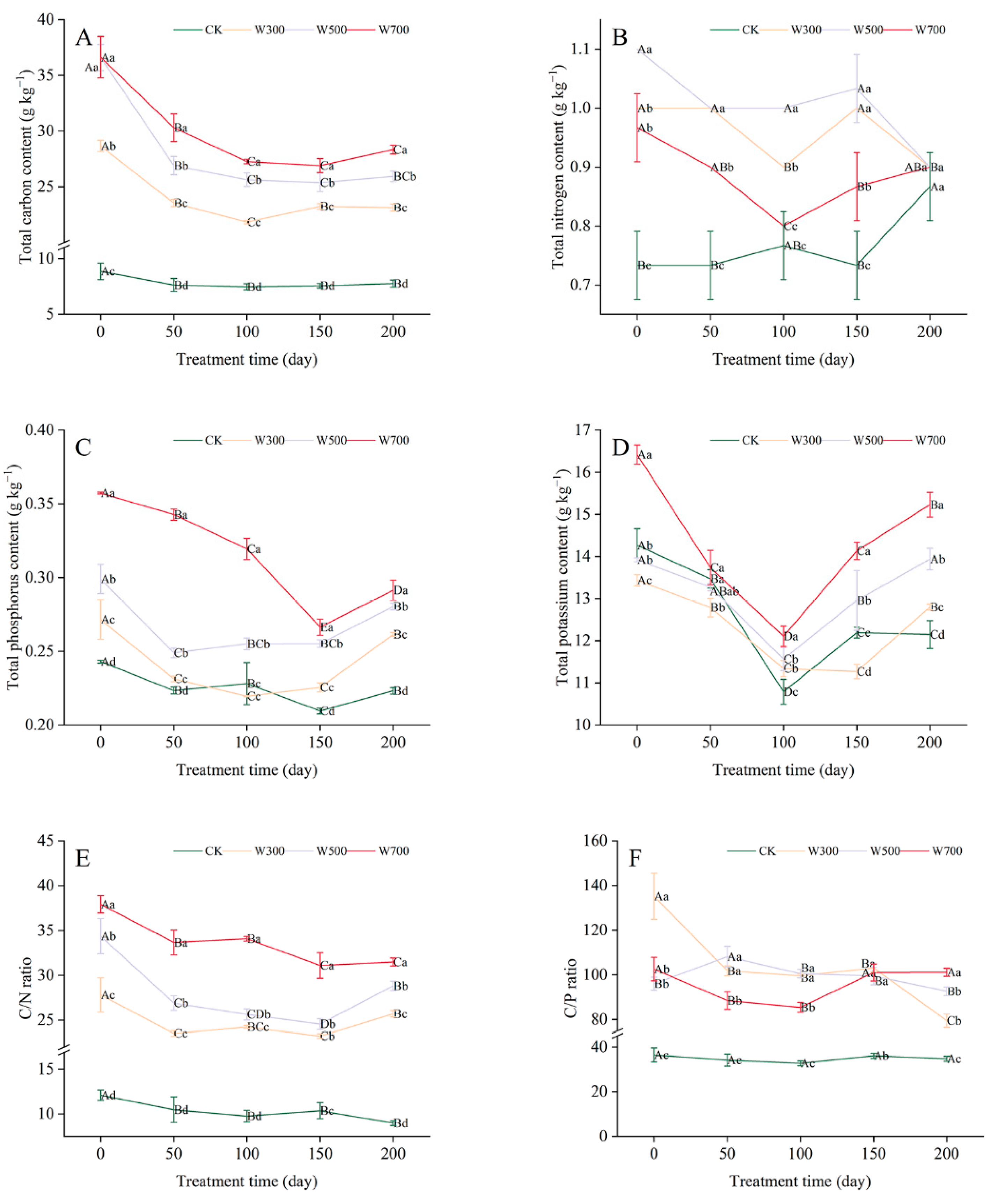
3.2.2. Total Nutrient Contents
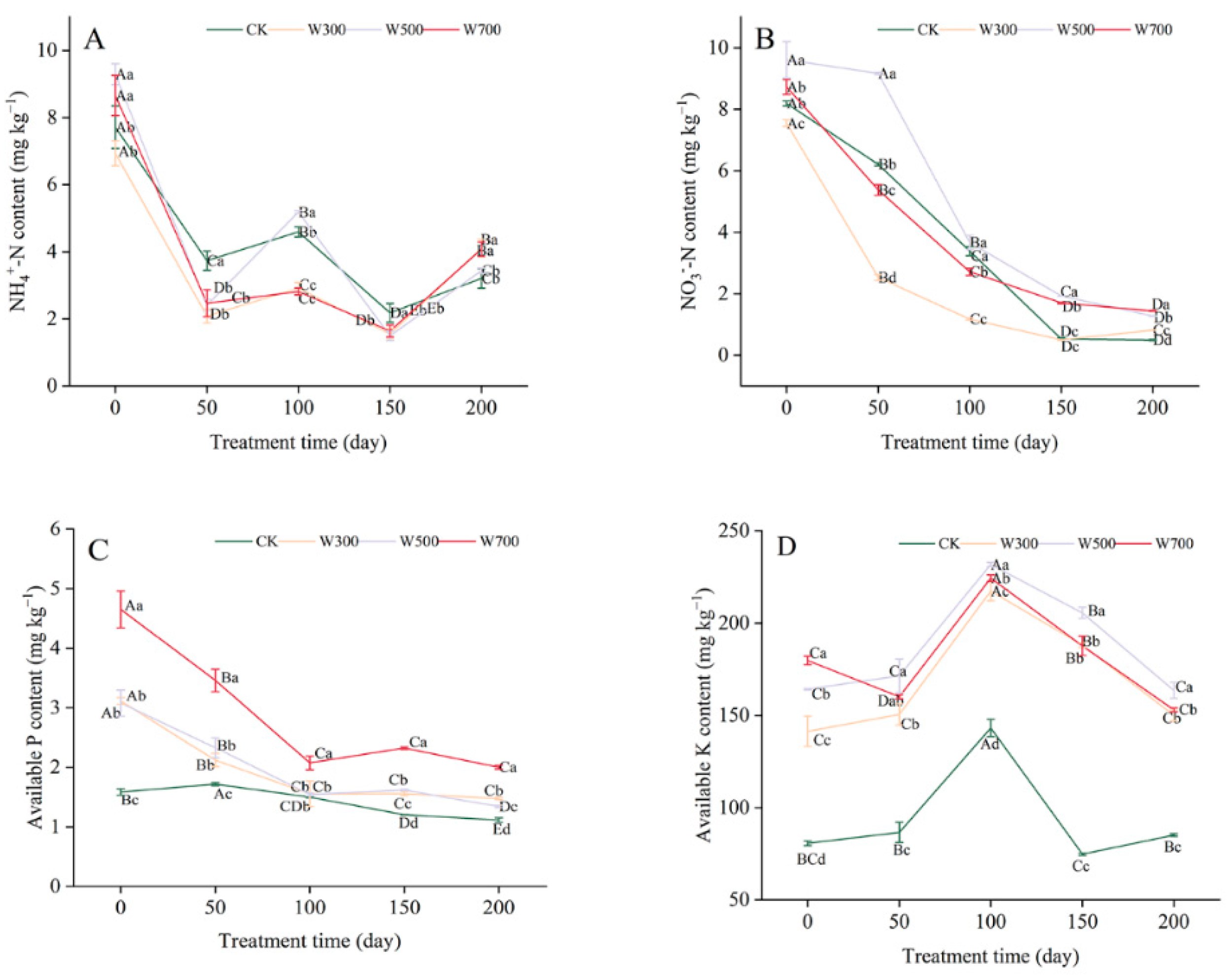
3.2.3. Available Nutrient Contents
3.3. Effects of Biochar Additions on Growth and Nutrient in C. paliurus
3.3.1. Growth and Biomass Production
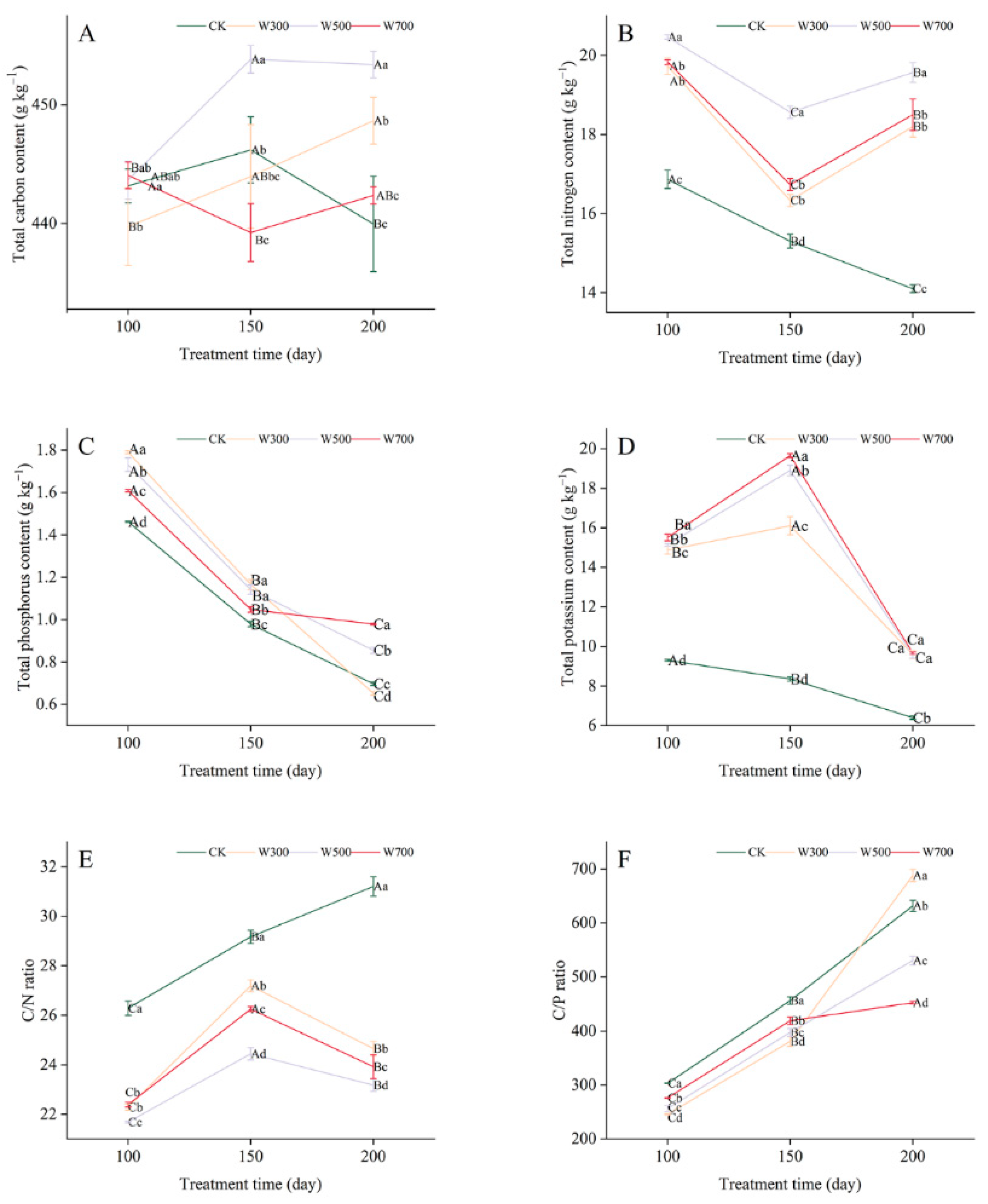
3.3.2. Leaf Nutrient Contents
3.4. Effects of Biochar Additions on Leaf Secondary Metabolite Accumulations
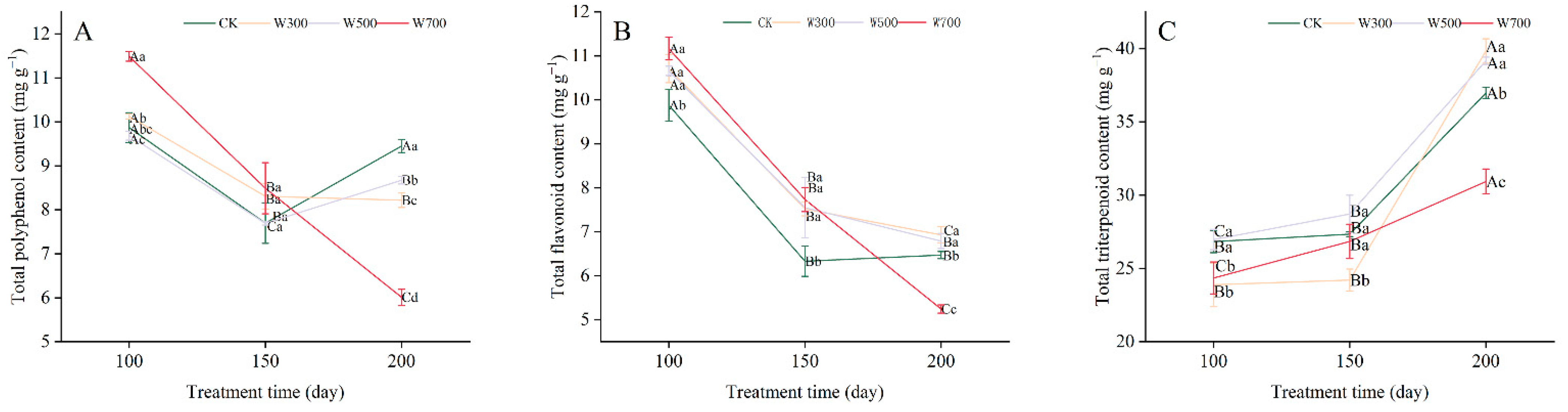
3.4.1. Secondary Metabolite Contents
3.4.2. Secondary Metabolite Accumulations
3.5. Correlation Analysis
4. Discussion
4.1. Effects of Pyrolysis Temperatures on Biochar Properties
4.2. Effects of Biochar Addition on Soil Property
4.3. Effects of Biochar Addition on Plant Growth and Secondary Metabolite Accumulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Welfle, A.; Gilbert, P.; Thornley, P. Increasing biomass resource availability through supply chain analysis. Biomass Bioenergy 2014, 70, 249–266. [Google Scholar] [CrossRef]
- Carnaje, N.P.; Talagon, R.B.; Peralta, J.P.; Shah, K.; Paz-Ferreiro, J. Development and characterisation of charcoal briquettes from water hyacinth (Eichhornia crassipes)-molasses blend. PLoS ONE 2018, 13, e0207135. [Google Scholar] [CrossRef] [PubMed]
- Romero-García, J.M.; Niño, L.; Martínez-Patiño, C.; Álvarez, C.; Castro, E.; Negro, M.J. Biorefinery based on olive biomass. State of the art and future trends. Bioresour. Technol. 2014, 159, 421–432. [Google Scholar] [CrossRef]
- Jesus, M.; Romani, A.; Mata, F.; Domingues, L. Current options in the valorisation of vine pruning residue for the production of biofuels, biopolymers, antioxidants, and bio-composites following the concept of biorefinery: A Review. Polymers 2022, 14, 1640. [Google Scholar] [CrossRef]
- Chen, W.F.; Meng, J.; Han, X.R.; Lan, Y.; Zhang, W.M. Past, present, and future of biochar. Biochar 2019, 1, 75–87. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K.; Avasthe, R.K.; Sinha, K. Compositional heterogeneity of different biochar: Effect of pyrolysis temperature and feedstocks. J. Environ. Manag. 2021, 278, 111501. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokolowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Asai, H.; Samson, B.K.; Stephan, H.M.; Songyikhangsuthor, K.; Homma, K.; Kiyono, Y.; Inoue, Y.; Shiraiwa, T.; Horie, T. Biochar amendment techniques for upland rice production in Northern Laos1. Soil physical properties, leaf SPAD and grain yield. Field Crops Res. 2009, 111, 81–84. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration in terrestrial ecosystems—A review. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Deng, L.F.; Dong, G.; Cai, X.X.; Tang, J.J.; Yuan, H.R. Biochar derived from the inner membrane of passion fruit as cathode catalyst of microbial fuel cells in neutral solution. J. Fuel Chem. Technol. 2018, 46, 120–128. [Google Scholar]
- Wang, H.; Lin, K.; Hou, Z.; Richardson, B.; Gan, J. Sorption of the herbicide terbuthylazine in two New Zealand forest soils amended with biosolids and biochars. J. Soils Sediments 2010, 10, 283–289. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, P.K.; Zhang, H.B.; Yuan, W.Q. Biochar production and applications in agro and forestry systems: A review. Sci. Total. Environ. 2020, 723, 137775. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.C.; Pawłowski, A. Life cycle assessment of two emerging sewage sludge-to-energy systems: Evaluating energy and greenhouse gas emissions implications. Bioresour. Technol. 2013, 127, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Chintala, R.; Mollinedo, J.; Schumacher, T.E.; Malo, D.D.; Julson, J. Effect of biochar on chemical properties of acidic soil. Arch. Agron. Soil. Sci. 2014, 60, 393–404. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Van Zwieten, L.; Singh, B.P.; Jefery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Vu, N.T.; Do, K.U. Insights into adsorption of ammonium by biochar derived from low temperature pyrolysis of coffee husk. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Uchimiya, M.; Wartelle, L.H.; Lima, I.M.; Klasson, K.T. Sorption of deisopropylatrazine on broiler litter biochars. J. Agric. Food. Chem. 2010, 58, 12350–12356. [Google Scholar] [CrossRef]
- Yuan, J.H.; Xu, R.K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef]
- Hanger, M.; Kemppainen, R.; Jauhiainen, L.; Tiilikkala, K.; Setala, H. The effects of birch (Betula spp.) biochar and pyrolysis temperature on soil properties and plant growth. Soil Tillage Res. 2016, 163, 224–234. [Google Scholar] [CrossRef]
- Fang, S.Z.; Yang, W.X.; Chu, X.L.; Shang, X.L.; She, C.Q.; Fu, X.X. Provenance and temporal variations in selected flavonoids in leaves of Cyclocarya paliurus. Food Chem. 2011, 124, 382–1386. [Google Scholar] [CrossRef]
- Xie, J.H.; Xie, M.Y.; Nie, S.P.; Shen, M.Y.; Wang, Y.X.; Li, C. Isolation, chemical composition and antioxidant activities of a water-soluble polysaccharide from Cyclocarya paliurus (Batal.) Iljinskaja. Food Chem. 2010, 119, 1626–1632. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y.N.; Fang, S.Z.; Wang, T.L.; Yin, Z.Q.; Shang, X.L.; Yang, W.X.; Fu, X.X. Antidiabetic effect of Cyclocarya paliurus leaves depends on the contents of antihyperglycemic flavonoids and antihyperlipidemic triterpenoids. Molecules 2018, 23, 1042. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.M.; Chen, P.; Lin, P.; Fang, S.Z.; Shang, X.L. A comprehensive assessment of bioactive metabolites, antioxidant and antiproliferative activities of Cyclocarya paliurus (Batal.) Iljinskaja leaves. Forests 2019, 10, 625. [Google Scholar] [CrossRef]
- Xie, J.H.; Wang, Z.J.; Shen, M.Y.; Nie, S.P.; Gong, B.; Li, H.S.; Zhao, Q.; Li, W.J.; Xie, M.Y. Sulfated modifification, characterization and antioxidant activities of polysaccharide from Cyclocarya paliurus. Food Hydrocolloids 2016, 53, 7–15. [Google Scholar] [CrossRef]
- Qin, J.; Yue, X.L.; Fang, S.Z.; Qian, M.Y.; Zhou, S.T.; Shang, X.L.; Yang, W.X. Responses of nitrogen metabolism, photosynthetic parameter and growth to nitrogen fertilization in Cyclocarya paliurus. For. Ecol. Manag. 2021, 502, 119715. [Google Scholar] [CrossRef]
- El-Azazy, M.; Nabil, I.; Hassan, S.S.; El-Shafie, A.S. Adsorption characteristics of pristine and magnetic olive stones biochar with respect to clofazimine. Nanomaterials 2021, 11, 963. [Google Scholar] [CrossRef]
- Fang, S.Z.; Wang, J.Y.; Wei, Z.Y.; Zhu, Z.X. Methods to break seed dormancy in Cyclocarya paliurus (Batal.) Iljinskaja. Sci. Hortic. 2006, 110, 305–309. [Google Scholar] [CrossRef]
- Xi, J.G.; Li, H.; Xi, J.M.; Tan, S.B.; Zheng, J.L.; Tan, Z.X. Effect of returning biochar from different pyrolysis temperatures and atmospheres on the growth of leaf-used lettuce. Environ. Sci. Pollut. Res. 2020, 27, 35802–35813. [Google Scholar] [CrossRef]
- Steiner, C.; Teixeira, W.G.; Lehmann, J.; Nehls, T.; de Macedo, J.L.V.; Blum, W.E.H.; Zech, W. Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant Soil 2007, 291, 275–290. [Google Scholar] [CrossRef]
- Lu, R.K. Analytical Methods of Soil Agricultural Chemistry; Agricultural Science Press: Beijing, China, 1999; pp. 190–191. [Google Scholar]
- Li, T.T.; Li, Y.Q.; Sun, Z.J.; Xi, X.L.; Sha, G.L.; Ma, C.Q.; Tian, Y.K.; Wang, C.H.; Zheng, X.D. Resveratrol alleviates the KCl salinity stress of Malus hupehensis rhed. Front. Plant Sci. 2021, 12, 650485. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Guo, M.J.; Wang, Y.G.; Yuan, X.Y.; Dong, S.Q.; Song, X.E.; Guo, P.Y. An investigation into the beneficial effects and molecular mechanisms of humic acid on foxtail millet under drought conditions. PLoS ONE 2020, 15, e0234029. [Google Scholar] [CrossRef] [PubMed]
- Sims, G.K.; Ellsworth, T.R.; Mulvaney, R.L. Microscale determination of inorganic nitrogen in water and soil extracts. Commun. Soil Sci. Plant Anal. 2008, 26, 303–316. [Google Scholar] [CrossRef]
- Guo, L.Z.; Li, J.H.; He, W.; Liu, L.; Huang, D.; Wang, K. High nutrient uptake efficiency and high water use efficiency facilitate the spread of Stellera chamaejasme L. in degraded grasslands. BMC Ecol. 2019, 19, 50. [Google Scholar] [CrossRef]
- Zhang, D.X.; Pan, G.X.; Wu, G.; Kibue, G.W.; Li, L.Q.; Zhang, X.H.; Zheng, J.W.; Zheng, J.F.; Cheng, K.; Joseph, S.; et al. Biochar helps enhance maize productivity and reduce greenhouse gas emissions under balanced fertilization in a rainfed low fertility inceptisol. Chemosphere 2016, 142, 106–113. [Google Scholar] [CrossRef]
- Cao, Y.N.; Fang, S.Z.; Yin, Z.Q.; Fu, X.X.; Shang, X.L.; Yang, W.X.; Yang, H.M. Chemical fingerprint and multicomponent quantitative analysis for the quality evaluation of Cyclocarya paliurus leaves by HPLC–Q–TOF–MS. Molecules 2017, 22, 1927. [Google Scholar] [CrossRef]
- Xie, P.J.; Huang, L.X.; Zhang, C.H.; Zhang, Y.L. Phenolic compositions, and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure-activity relationships. J. Funct. Foods 2015, 16, 460–471. [Google Scholar] [CrossRef]
- Li, F.M.; Tan, J.; Nie, S.P.; Dong, C.Y.; Li, C. The study on determination methods of total flavonoids in Cyclocarya paliurus. Food Sci. 2006, 4, 34–37. (In Chinese) [Google Scholar] [CrossRef]
- Fan, J.P.; He, C.H. Simultaneous quantification of three major bioactive triterpene acids in the leaves of Diospyros kaki by high-performance liquid chromatography method. J. Pharm. Biomed. 2006, 41, 950–956. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R. A Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef]
- Mendez, A.; Terradillos, M.; Gasco, G. Physicochemical and agronomic properties of biochar from sewage sludge pyrolysed at different temperatures. J. Anal. Appl. Pyrol. 2013, 102, 124–130. [Google Scholar] [CrossRef]
- Yang, C.D.; Liu, J.J.; Lu, S.G. Pyrolysis temperature affects pore characteristics of rice straw and canola stalk biochars and biochar-amended soils. Geoderma 2021, 397, 115097. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.D.; Masek, O.; Zimmerman, A. Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J. Hazard. Mater. 2013, 256, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.; Lopes, H.; Coser, T.; Vale, A.; Busato, J.; Aguiar, N.; Novotny, E.; Canellas, L. Influence of pyrolysis temperature on chemical and physical properties of biochar from sewage sludge. Arch. Argon. Soil Sci. 2018, 64, 881–889. [Google Scholar] [CrossRef]
- Bagreev, A.; Bandosz, T.J.; Locke, D.C. Pore structure and surface chemistry of adsorbents obtained by pyrolysis of sewage sludge-derived fertilizer. Carbon 2001, 39, 1971–1979. [Google Scholar] [CrossRef]
- Li, B.; Wang, S.C.; Zhang, Y.; Qiu, D.W. Acid Soil Improvement Enhances Disease Tolerance in Citrus Infected by Candidatus Liberibacter asiaticus. Int. J. Mol. Sci. 2020, 21, 3614. [Google Scholar] [CrossRef]
- Chang, Y.B.; Liu, W.G.; Mao, Y.Q.; Yang, T.; Chen, Y.G. Biochar Addition Alters C: N: P Stoichiometry in Moss Crust-Soil Continuum in Gurbantunggut Desert. Plants 2022, 11, 814. [Google Scholar] [CrossRef]
- Lasota, J.; Babiak, T.; Blonska, E. C: N: P stoichiometry associated with biochar in forest soils at historical charcoal production sites in Poland. Geoderma Reg. 2022, 28, e00482. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, G.; Lv, Y.C.; Shao, W.B. Effects of biochar amendment on soil physical and chemical properties: Current status and knowledge gaps. Adv. Earth Sci. 2014, 29, 68–79. [Google Scholar]
- Abujabhah, I.S.; Doyle, R.; Bound, S.A.; Bowman, J.P. The effect of biochar loading rates on soil fertility, soil biomass, potential nitrification, and soil community metabolic profiles in three different soils. J. Soil Sediments 2016, 16, 2211–2222. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.; Alburquerque, J.A.; Sanchez-Monedero, M.A.; Roig, A.; Cayuela, M.L. Biochar accelerates organic matter degradation and enhances N mineralisation during composting of poultry manure without a relevant impact on gas emissions. Bioresour. Technol. 2015, 192, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Riza, M.; Liu, B.; Xia, H.; El-desouki, Z.; Jiang, C.C. Two-year study of biochar: Achieving excellent capability of potassium supply via alter clay mineral composition and potassium-dissolving bacteria activity. Sci. Total Environ. 2020, 717, 137286. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.S.; Saggar, S.; Luo, J.F.; Bhandral, R.; Singh, J. Gaseous emissions of nitrogen from grazed pastures: Processes, measurements and modelling, environmental implications, and mitigation. Adv. Agron. 2004, 84, 37–120. [Google Scholar] [CrossRef]
- Kavitha, B.; Reddy, P.V.L.; Kim, B.; Lee, S.S.; Paddey, S.K.; Kim, K.H. Benefits and limitations of biochar amendment in agricultural soils: A review. J. Environ. Manag. 2018, 227, 146–154. [Google Scholar] [CrossRef]
- Abideen, Z.; Koyro, H.W.; Huchzermeyer, B.; Ansari, R.; Zulfiqar, F.; Gul, B. Ameliorating effects of biochar on photosynthetic efficiency and antioxidant defence of Phragmites karka under drought stress. Plant Biol. 2020, 22, 259–266. [Google Scholar] [CrossRef]
- Naeem, M.A.; Khalid, M.; Aon, M.; Abbas, G.; Tahir, M.; Amjad, M.; Murtaza, B.; Yang, A.Z.; Akhtar, S.S. Effect of wheat and rice straw biochar produced at different temperatures on maize growth and nutrient dynamics of a calcareous soil. Arch. Agron. Soil Sci. 2017, 63, 2048–2061. [Google Scholar] [CrossRef]
- Saha, A.; Basak, B.B.; Gajbhiye, N.A.; Kalariya, K.A.; Manivel, P. Sustainable fertilization through co-application of biochar and chemical fertilizers improves yield, quality of Andrographis paniculata and soil health. Ind. Crops Prod. 2019, 140, 111607. [Google Scholar] [CrossRef]
- Petruccelli, R.; Bonetti, A.; Traversi, M.L.; Faraloni, C.; Valagussa, M.; Pozzi, A. Influence of biochar application on nutritional quality of tomato (Lycopersicon esculentum). Crop Pasture Sci. 2015, 66, 747–755. [Google Scholar] [CrossRef] [Green Version]


| Biochar Type | BET Surface Area (m2·g−1) | Total Pore Volume (cm3·g−1) | t-Plot Micropore Area (m2·g−1) | t-Plot Micropore Volume (cm3·g−1) |
|---|---|---|---|---|
| W300 | 2.05 | 2.50 × 10−3 | 0 | 0 |
| W500 | 19.60 | 1.86 × 10−2 | 1.41 | 6.2 × 10−4 |
| W700 | 54.85 | 5.29 × 10−2 | 37.08 | 1.96 × 10−2 |
| Biochar Type | pH | Total Carbon (g·kg−1) | Total Nitrogen (g·kg−1) | Total Phosphorus (g·kg−1) | Total Potassium (g·kg−1) | C/N | C/P |
|---|---|---|---|---|---|---|---|
| W300 | 6.71 ± 0.02 c | 681.30 ± 3.95 c | 11.27 ± 0.12 a | 2.21 ± 0.03 c | 3.75 ± 0.13 c | 60.47 ± 0.72 c | 308.90 ± 2.45 a |
| W500 | 9.38 ± 0.01 b | 754.23 ± 4.63 b | 10.00 ± 0.10 b | 2.56 ± 0.04 b | 5.52 ± 0.08 b | 75.43 ± 0.91 b | 294.85 ± 2.99 b |
| W700 | 9.51 ± 0.06 a | 784.60 ± 4.22 a | 7.37 ± 0.06 c | 3.15 ± 0.02 a | 8.65 ± 0.08 a | 106.51 ± 1.17 a | 248.88 ± 1.84 c |
| Treatment | Seedling Height (cm) | Ground Diameter (mm) | Biomass (g·Plant−1) | |||
|---|---|---|---|---|---|---|
| Leaf | Shoot | Root | Total | |||
| CK | 44.56 ± 0.38 b | 9.82 ± 0.09 b | 1.53 ± 0.11 b | 6.36 ± 0.18 b | 4.33 ± 0.15 b | 12.35 ± 0.19 b |
| W300 | 43.40 ± 0.62 c | 9.70 ± 0.22 b | 1.51 ± 0.07 b | 6.65 ± 0.17 b | 4.52 ± 0.17 b | 12.68 ± 0.35 b |
| W500 | 49.93 ± 0.60 a | 9.78 ± 0.27 b | 1.74 ± 0.12 a | 7.41 ± 0.23 a | 5.23 ± 0.19 a | 14.37 ± 0.38 a |
| W700 | 42.33 ± 0.40 d | 10.48 ± 0.32 a | 1.17 ± 0.07 c | 4.14 ± 0.39 c | 3.79 ± 0.07 c | 9.09 ± 0.43 c |
| Treatment | Accumulation (mg·Plant−1) | ||
|---|---|---|---|
| Total Polyphenol | Total Flavonoid | Total Triterpenoid | |
| CK | 14.43 ± 1.26 a | 9.88 ± 0.73 b | 56.46 ± 4.65 b |
| W300 | 12.44 ± 0.79 b | 10.49 ± 0.54 ab | 60.28 ± 2.82 b |
| W500 | 15.07 ± 1.04 a | 11.80 ± 1.09 a | 68.05 ± 4.96 a |
| W700 | 7.03 ± 0.23 c | 6.13 ± 0.23 c | 36.16 ± 1.22 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, R.; Lan, Z.; Shang, X.; Fang, S. Effects of Biochar Application Pyrolyzed at Different Temperatures on Soil Properties, Growth and Leaf Secondary Metabolite Accumulation in Cyclocarya paliurus. Forests 2022, 13, 1572. https://doi.org/10.3390/f13101572
Deng R, Lan Z, Shang X, Fang S. Effects of Biochar Application Pyrolyzed at Different Temperatures on Soil Properties, Growth and Leaf Secondary Metabolite Accumulation in Cyclocarya paliurus. Forests. 2022; 13(10):1572. https://doi.org/10.3390/f13101572
Chicago/Turabian StyleDeng, Rui, Ziyu Lan, Xulan Shang, and Shengzuo Fang. 2022. "Effects of Biochar Application Pyrolyzed at Different Temperatures on Soil Properties, Growth and Leaf Secondary Metabolite Accumulation in Cyclocarya paliurus" Forests 13, no. 10: 1572. https://doi.org/10.3390/f13101572
APA StyleDeng, R., Lan, Z., Shang, X., & Fang, S. (2022). Effects of Biochar Application Pyrolyzed at Different Temperatures on Soil Properties, Growth and Leaf Secondary Metabolite Accumulation in Cyclocarya paliurus. Forests, 13(10), 1572. https://doi.org/10.3390/f13101572






