Microenvironment Impact on Survival Rate, Growth and Stability Traits, in a Half-Sib Test of Pendula and Pyramidalis Varieties of Norway Spruce
Abstract
:1. Introduction
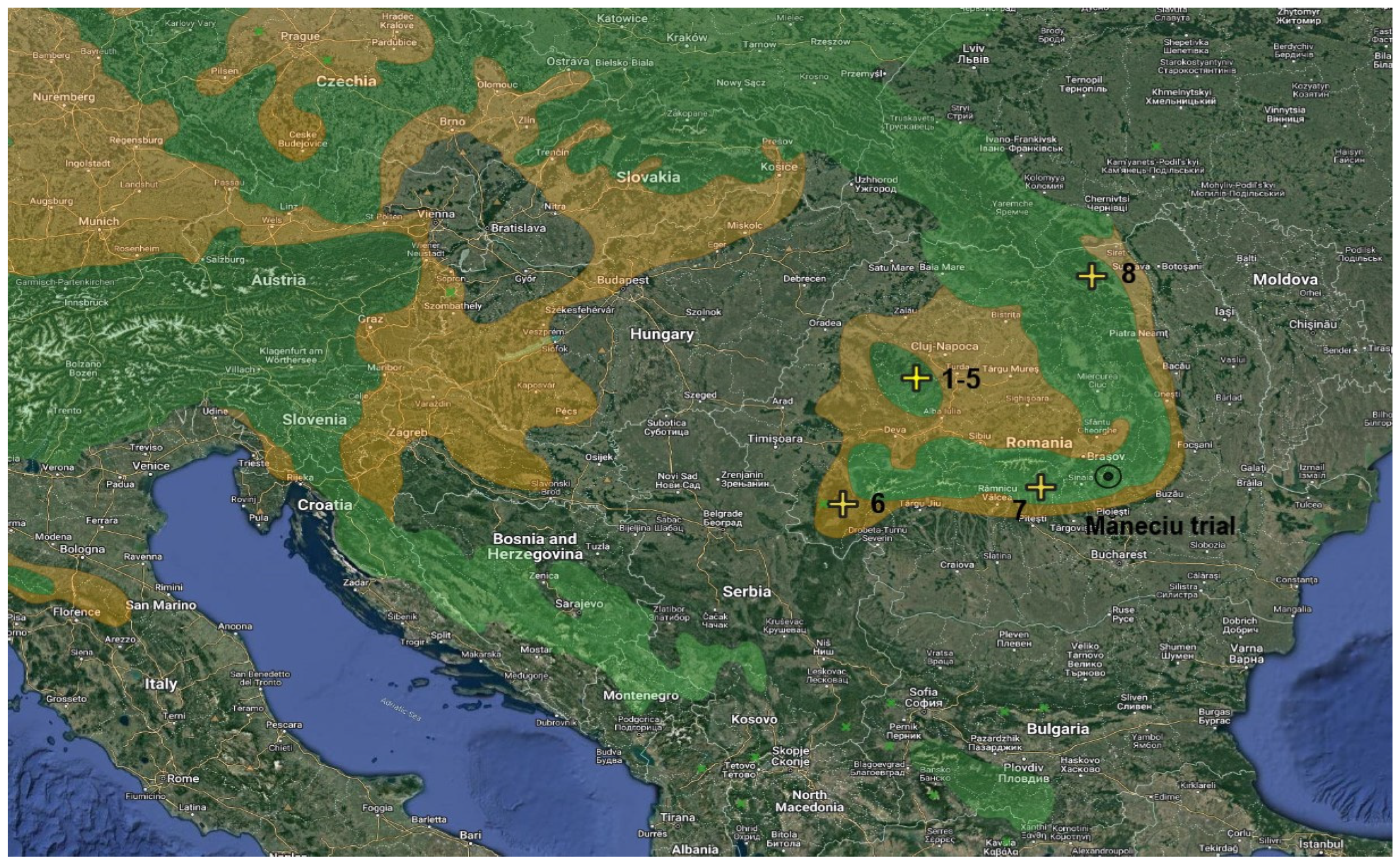
2. Materials and Methods
3. Results
3.1. Survival Rate
3.2. Edge Effect
3.3. Slope Position
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodriguez, Y.P.; Morales, L.; Willför, S.; Pulkkinen, P.; Peltola, H.; Pappinen, A. Wood decay caused by Heterobasidion parviporum in juvenile wood specimens from normal- and narrow-crowned Norway spruce. Scand. J. For. Res. 2013, 28, 331–339. [Google Scholar] [CrossRef]
- Kauppi, P.E.; Posch, M.; Pirinen, P. Large impacts of climatic warming on growth of boreal forests since 1960. PLoS ONE 2014, 9, e111340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keenan, R.J. Climate change impacts and adaptation in forest management: A review. Ann. For. Sci. 2015, 72, 145–167. [Google Scholar] [CrossRef] [Green Version]
- Boisvenue, C.; Running, S.W. Impacts of climate change on natural forest productivity–Evidence since the middle of the 20th century. Glob. Chang. Biol. 2016, 12, 862–882. [Google Scholar] [CrossRef]
- Pretzsch, H.; Biber, P.; Schütze, G.; Kemmerer, J.; Uhl, E. Wood density reduced while wood volume growth accelerated in Central European forests since 1870. For. Ecol. Manag. 2018, 429, 589–616. [Google Scholar] [CrossRef]
- Dincă, L.; Murariu, G.; Iticescu, C.; Budeanu, M.; Murariu, A. Norway spruce (Picea abies (L.) Karst.) smart forests from the southern Carpathians. Int. J. Conserv. Sci. 2019, 10, 781–790. [Google Scholar]
- Bouriaud, O.; Leban, J.M.; Bert, D.; Deleuze, C. Intra-annual variations in climate influence growth and wood density of Norway spruce. Tree Physiol. 2005, 25, 651–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschini, T.; Bontemps, J.D.; Gelhaye, P.; Rittie, D.; Herve, J.C.; Gegout, J.C.; Leban, J.M. Decreasing trend and fluctuations in the mean ring density of Norway spruce through the twentieth century. Ann. For. Sci. 2010, 67, 816. [Google Scholar] [CrossRef] [Green Version]
- Franceschini, T.; Bontemps, J.D.; Leban, J.M. Transient historical decrease in earlywood and latewood density and unstable sensitivity to summer temperature for Norway spruce in northeastern France. Can. J. For. Res. 2012, 42, 219–226. [Google Scholar] [CrossRef]
- Reyer, C.; Lasch-Born, P.; Suckow, F.; Gutsch, M.; Murawski, A.; Pilz, T. Projections of regional changes in forest net primary productivity for different tree species in Europe driven by climate change and carbon dioxide. Ann. For. Sci. 2014, 71, 211–225. [Google Scholar] [CrossRef] [Green Version]
- Semeniuc, A.I.; Popa, I. Comparative analysis of tree ring parameters Variation in four coniferous species: (Picea abies, Abies alba, Pinus sylvestris and Larix decidua). Int. J. Conserv. Sci. 2018, 9, 591–598. [Google Scholar]
- Mátyás, C. Climatic adaptation of trees: Rediscovering provenance tests. Euphytica 1996, 92, 45–54. [Google Scholar] [CrossRef]
- Das, A.; Battles, J.; van Mantgem, P.J.; Stephenson, N.L. Spatial elements of mortality risk in old-growth forests. Ecology 2008, 89, 1744–1756. [Google Scholar] [CrossRef] [Green Version]
- Adame, P.; Del Río, M.; Cañellas, I. Modeling individual-tree mortality in Pyrenean oak (Quercus pyrenaica Willd.) stands. Ann. For. Sci. 2010, 67, 810. [Google Scholar] [CrossRef] [Green Version]
- Jutras, S.; Hökkä, H.; Alenius, V.; Salminen, H. Modeling mortality of individual trees in drained peatland sites in Finland. Silva Fenn. 2003, 37, 235–251. [Google Scholar] [CrossRef] [Green Version]
- Fraver, S.; D’Amato, A.W.; Bradford, J.B.; Jonsson, B.G.; Jönsson, M.; Esseen, P.A. Tree growth and competition in an old-growth Picea abies forest of boreal Sweden: Influence of tree spatial patterning. J. Veg. Sci. 2014, 25, 374–385. [Google Scholar] [CrossRef]
- Franklin, J.F.; Shugart, H.H.; Harmon, M.E. Tree death as an ecological process. BioScience 1987, 37, 550–556. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Battles, J.; Stephenson, N.L.; van Mantgem, P.J. The contribution of competition to tree mortality in old-growth coniferous forests. For. Ecol. Manag. 2011, 261, 1203–1213. [Google Scholar] [CrossRef]
- Wang, X.; Comita, L.S.; Hao, Z.; Davies, S.J.; Ye, J.; Lin, F.; Yuan, Z. Local-scale drivers of tree survival in a temperate forest. PLoS ONE 2012, 7, e29469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo-Oviedo, A.; Sterba, H.; del Río, M.; Bravo, F. Competition-induced mortality for Mediterranean Pinus pinaster Ait. and P. sylvestris L. For. Ecol. Manag. 2006, 222, 88–98. [Google Scholar] [CrossRef]
- Castagneri, D.; Lingua, E.; Vacchiano, G.; Nola, P.; Motta, R. Diachronic analysis of individual-tree mortality in a Norway spruce stand in the eastern Italian Alps. Ann. For. Sci. 2010, 67, 304. [Google Scholar] [CrossRef] [Green Version]
- Siipilehto, J.; Mäkinen, H.; Andreassen, K.; Peltoniemi, M. Models for integrating and identifying the effect of senescence on individual tree survival probability for Norway spruce. Silva Fenn. 2021, 55, 10496. [Google Scholar] [CrossRef]
- Lännenpää, A.; Aakala, T.; Kauhanen, H.; Kuuluvainen, T. Tree mortality agents in pristine Norway spruce forests in northern Fennoscandia. Silva Fenn. 2008, 42, 151–163. [Google Scholar] [CrossRef] [Green Version]
- Sims, A.; Mändma, R.; Laarmann, D.; Korjus, H. Assessment of tree mortality on the Estonian Network of Forest Research Plots. For. Stud. 2014, 60, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Popa, I. Doborâturi produse de vânt-factor de risc în ecosistemele forestiere montane. Windthrows–risk factors if moutainous forest ecosystems. An. ICAS 2005, 48, 3–28. [Google Scholar]
- Pârnuţă, G. Variabilitatea Genetică și Ameliorarea Arborilor de Molid cu Coroană Îngustă în România (Genetic Variability and Breeding of Narrow-Crown Spruce Trees in Romania); Silvică Publishing House: Bucharest, Romania, 2008; 181p, (In Romanian with English Abstract). [Google Scholar]
- Valkonen, S.; Aulus Giacosa, L.; Heikkinen, J. Tree mortality in the dynamics and management of uneven-aged Norway spruce stands in southern Finland. Eur. J. For. Res. 2020, 139, 989–998. [Google Scholar] [CrossRef]
- Zwetsloot, M.J.; Bauerle, T.L. Repetitive seasonal drought causes substantial species-specific shifts in fine-root longevity and spatio-temporal production patterns in mature temperate forest trees. New Phytol. 2021, 231, 974–986. [Google Scholar] [CrossRef]
- Vlad, R.; Zhiyanski, M.; Dincă, L.; Sidor, C.G.; Constandache, C.; Pei, G.; Ispravnic, A.; Blaga, T. Assessment of the density of wood with stem decay of Norway spruce trees using drill resistance. Comptes R. Acad. Bulg. Sci. 2018, 71, 1502–1510. [Google Scholar] [CrossRef]
- Gömöry, D.; Longauer, R.; Hlásny, T.; Pacalaj, M.; Strmeň, S.; Krajmerová, D. Adaptation to common optimum in different populations of Norway spruce (Picea abies Karst.). Eur. J. For. Res. 2011, 131, 401–411. [Google Scholar] [CrossRef]
- Kapeller, S.; Lexer, M.J.; Geburek, T.; Hiebl, J.; Schueler, S. Intraspecific variation in climate response of Norway spruce in the eastern Alpine range: Selecting appropriate provenances for future climate. For. Ecol. Manag. 2012, 271, 46–57. [Google Scholar] [CrossRef]
- Anderegg, W.R.; Klein, T.; Bartlett, M.; Sack, L.; Pellegrini, A.F.; Choat, B.; Jansen, S. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc. Natl. Acad. Sci. USA 2016, 113, 5024–5029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jupa, R.; Plavcová, L.; Gloser, V.; Jansen, S. Linking xylem water storage with anatomical parameters in five temperate tree species. Tree Physiol. 2016, 36, 756–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffenrem, A.; Solheim, H.; Skrøppa, T. Genetic parameters for wood quality traits and resistance to the pathogens Heterobasidion parviporum and Endoconidiophora polonica in a Norway spruce breeding population. Eur. J. For. Res. 2016, 135, 815–825. [Google Scholar] [CrossRef]
- Zeltinš, P.; Katrevičs, J.; Gailis, A.; Maaten, T.; Bāders, E.; Jansons, Ā. Effect of stem diameter, genetics, and wood properties on stem cracking in Norway spruce. Forests 2018, 9, 546. [Google Scholar] [CrossRef] [Green Version]
- Budeanu, M.; Apostol, E.N.; Popescu, F.; Postolache, D.; Ioniță, L. Testing of the narrow crowned Norway spruce ideotype (Picea abies f. pendula) and the hybrids with normal crown form (pyramidalis) in multisite comparative trials. Sci. Total Environ. 2019, 689, 980–990. [Google Scholar] [CrossRef]
- Klisz, M.; Ukalska, J.; Koprowski, M.; Tereba, A.; Puchałka, R.; Przybylski, P.; Jastrzębowski, S.; Nabais, C. Effect of provenance and climate on intra-annual density fluctuations of Norway spruce Picea abies (L.) Karst. in Poland. Agric. For. Meteorol. 2019, 269, 145–156. [Google Scholar] [CrossRef]
- Hayatgheibi, H.; Haapanen, M.; Lundströmer, J.; Berlin, M.; Kärkkäinen, K.; Helmersson, A. Impact of Drought Stress on Height Growth of Young Norway Spruce Full-Sib and Half-Sib Clonal Trials in Sweden and Finland. Forests 2021, 12, 498. [Google Scholar] [CrossRef]
- Geburek, T.; Turok, J. Conservation and Management of Forest Genetic Resources in Europe; Arbora Publishers: Zvolen, Slovakia, 2005; 693p. [Google Scholar]
- Budeanu, M.; Apostol, E.N.; Besliu, E.; Crișan, V.E.; Petritan, A.M. Phenotypic variability and differences in the drought response of Norway spruce pendula and pyramidalis half-sib families. Forests 2021, 12, 947. [Google Scholar] [CrossRef]
- Kelblerová, R.; Dvořák, J.; Korecký, J. Genetic Diversity Maximization as a Strategy for Resilient Forest Ecosystems: A Case Study on Norway Spruce. Forests 2022, 13, 489. [Google Scholar] [CrossRef]
- Gyllenstrand, N.; Clapham, D.; Källman, T.; Lagercrantz, U.; Richardt, S.; Lang, D.; Reski, R.; Frank, W.; Rensing, S.A. A Norway spruce FLOWERING LOCUS T homolog is implicated in control of growth rhythm in conifers. Plant Physiol. 2007, 144, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Pretzsch, H.; Schütze, G.; Uhl, E. Resistance of European tree species to drought stress in mixed vs. pure forests: Evidence of stress release by inter-specific facilitation. Plant Biol. 2013, 15, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Kolář, T.; Čermák, P.; Trnka, M.; Žid, T.; Rybníček, M. Temporal changes in the climate sensitivity of Norway spruce and European beech along an elevation gradient in Central Europe. Agric. For. Meteorol. 2017, 239, 24–33. [Google Scholar] [CrossRef]
- Trujillo-Moya, C.; George, J.P.; Fluch, S.; Geburek, T.; Grabner, M.; Karanitsch-Ackerl, S.; Konrad, H.; Mayer, K.; Sehr, E.M.; Wischnitzki, E.; et al. Drought sensitivity of Norway spruce at the species’ warmest fringe: Quantitative and molecular analysis reveals high genetic variation among and within provenances. G3 Genes Genomes Genet. 2018, 8, 1225–1245. [Google Scholar] [CrossRef] [PubMed]
- Bosela, M.; Kulla, L.; Roessiger, J.; Seben, V.; Dobor, L.; Buntgen, U.; Lukac, M. Long-term effects of environmental change and species diversity on tree radial growth in a mixed European forest. For. Ecol. Manag. 2019, 446, 293–303. [Google Scholar] [CrossRef]
- Obladen, N.; Dechering, P.; Skiadaresis, G.; Tegel, W.; Keßler, J.; Höllerl, S.; Kaps, S.; Hertel, M.; Dulamsuren, C.; Seifert, T.; et al. Tree mortality of European beech and Norway spruce induced by 2018-2019 hot droughts in central Germany. Agric. For. Meteorol. 2021, 307, 108482. [Google Scholar] [CrossRef]
- Karki, L. Genetically narrow-crowned trees combine high timber quality and high stem wood production at low cost. In Crop Physiology of Forest Trees; Landsberg, J.J., Ed.; Tigerstedt Publishing House: Helsinki, Finland, 1985; pp. 245–256. [Google Scholar]
- Zubizarreta Gerendiain, A.; Peltola, H.; Pulkkinen, P.; Ikonen, V.P.; Jaatinen, R. Differences in growth and wood properties between narrow and normal crowned types of Norway spruce grown at narrow spacing in Southern Finland. Silva Fenn. 2008, 42, 423–437. [Google Scholar] [CrossRef] [Green Version]
- Zubizarreta Gerendiain, A.; Peltola, H.; Pulkkinen, P. Growth and wood property traits in narrow crowned Norway spruce (Picea abies f. pendula) clones grown in southern Finland. Silva Fenn. 2009, 43, 369–382. [Google Scholar] [CrossRef] [Green Version]
- Apostol, E.N.; Budeanu, M. Adaptability of narrow-crowned Norway spruce ideotype (Picea abies (L.) Karst. pendula form) in 25 years half-sib comparative trials in the eastern Carpathians. Forests 2019, 10, 395. [Google Scholar] [CrossRef] [Green Version]
- Marcu, M.; Budeanu, M.; Apostol, E.N.; Radu, G.R. Valuation of the economic benefits from using genetically improved forest reproductive materials in afforestation. Forests 2020, 11, 382. [Google Scholar] [CrossRef] [Green Version]
- Anonymous. Management Planning of Production Unit IV Suzana, Forest District Măneciu; Silvică: Voluntari, Romania, 2018. [Google Scholar]
- Caudullo, G.; Welk, E.; San-Miguel-Ayanz, J. Chorological maps for the main European woody species. Data Brief 2017, 12, 662–666. [Google Scholar] [CrossRef]
- QGIS.org. QGIS Geographic Information System; QGIS Association. 2022. Available online: http://www.qgis.org (accessed on 5 June 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.r-project.org/ (accessed on 10 June 2022).
- Charru, M.; Seynave, I.; Hervé, J.C.; Bontemps, J.D. Spatial patterns of historical growth changes in Norway spruce across western European mountains and the key effect of climate warming. Trees 2014, 28, 205–221. [Google Scholar] [CrossRef]
- Sterck, F.; Martinéz-Ramos, M.; Dyer-Leal, G.; Rodríguez-Velazquez, J.; Poorter, L. The consequences of crown traits for the growth and survival of tree saplings in a Mexican lowland rainforest. Funct. Ecol. 2003, 17, 194–200. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Benito, P.; Lines, E.R.; Gómez-Aparicio, L.; Zavala, M.A.; Coomes, D.A. Patterns and Drivers of Tree Mortality in Iberian Forests: Climatic Effects Are Modified by Competition. PLoS ONE 2013, 8, e56843. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.L.; MacLean, D.A. Spatiotemporal patterns of mortality in declining balsam fir and spruce stands. For. Ecol. Manag. 2007, 253, 188–201. [Google Scholar] [CrossRef]
- Bałazy, R.; Kamińska, A.; Ciesielski, M.; Socha, J.; Pierzchalski, M. Modeling the effect of environmental and topographic variables affecting the height increment of Norway spruce stands in mountainous conditions with the use of LiDAR data. Remote Sens. 2019, 11, 2407. [Google Scholar] [CrossRef]










| Survival Rate (%) | ||||
|---|---|---|---|---|
| Factors | DF | SS | MS | p |
| Replication | 3 | 1824 | 608.1 | 0.005 ** |
| Crown form | 1 | 1069 | 1068.8 | 0.006 ** |
| Provenance | 7 | 8792 | 1256.0 | 0.000 *** |
| Marginal effect | 1 | 932 | 931.6 | 0.010 * |
| Slope position | 2 | 3990 | 1995.2 | 0.000 *** |
| Error | 177 | 24,633 | 139.2 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besliu, E.; Budeanu, M.; Apostol, E.N.; Radu, R.G. Microenvironment Impact on Survival Rate, Growth and Stability Traits, in a Half-Sib Test of Pendula and Pyramidalis Varieties of Norway Spruce. Forests 2022, 13, 1691. https://doi.org/10.3390/f13101691
Besliu E, Budeanu M, Apostol EN, Radu RG. Microenvironment Impact on Survival Rate, Growth and Stability Traits, in a Half-Sib Test of Pendula and Pyramidalis Varieties of Norway Spruce. Forests. 2022; 13(10):1691. https://doi.org/10.3390/f13101691
Chicago/Turabian StyleBesliu, Emanuel, Marius Budeanu, Ecaterina Nicoleta Apostol, and Raul Gheorghe Radu. 2022. "Microenvironment Impact on Survival Rate, Growth and Stability Traits, in a Half-Sib Test of Pendula and Pyramidalis Varieties of Norway Spruce" Forests 13, no. 10: 1691. https://doi.org/10.3390/f13101691






