Nutrient Resorption and C:N:P Stoichiometry Responses of a Pinus massoniana Plantation to Various Thinning Intensities in Southern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Thinning Experiment
2.2. Sample Collection and Analysis
2.3. Data Calculations and Analysis
3. Results
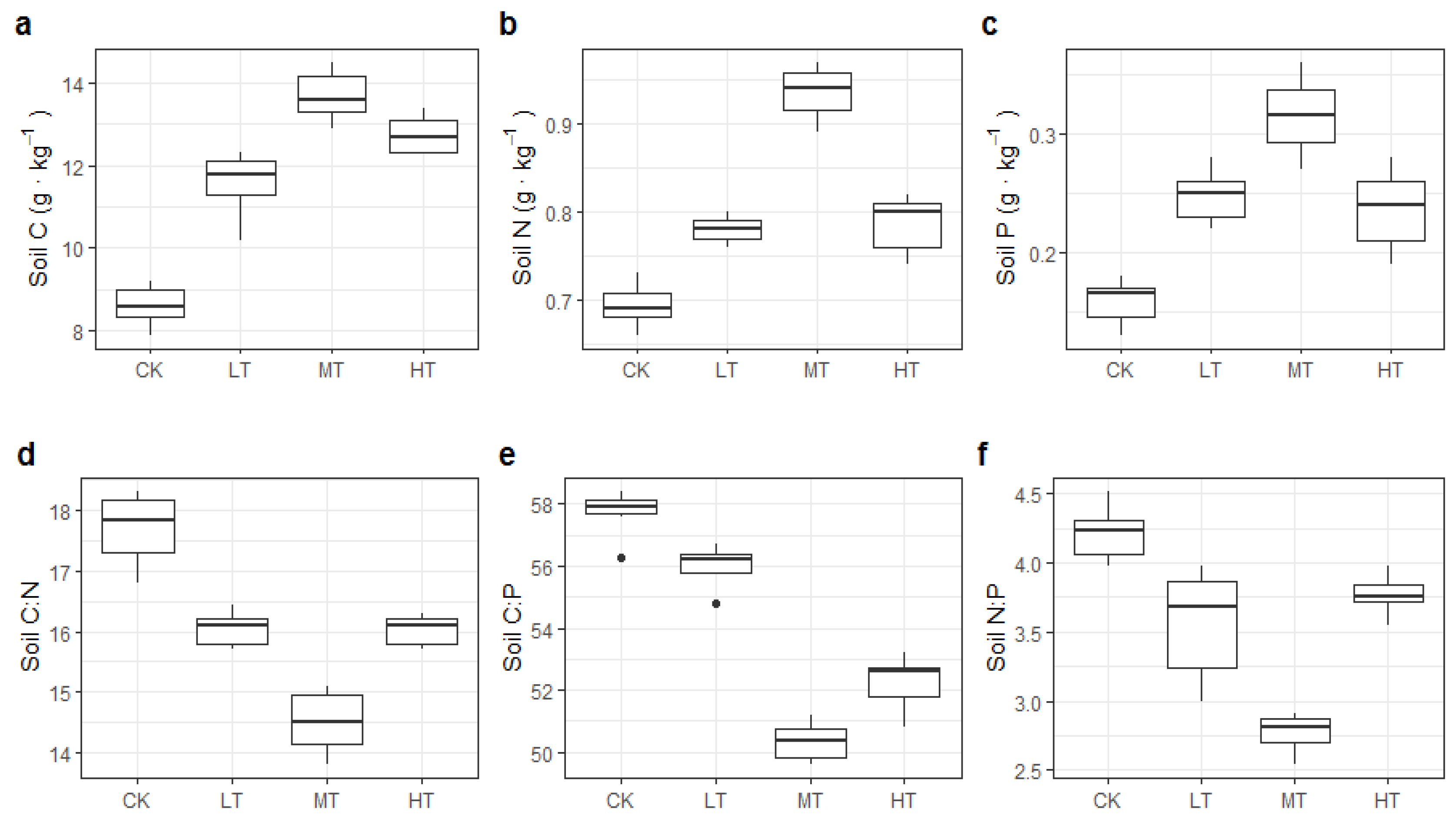
3.1. Soil Properties and C:N:P Stoichiometry
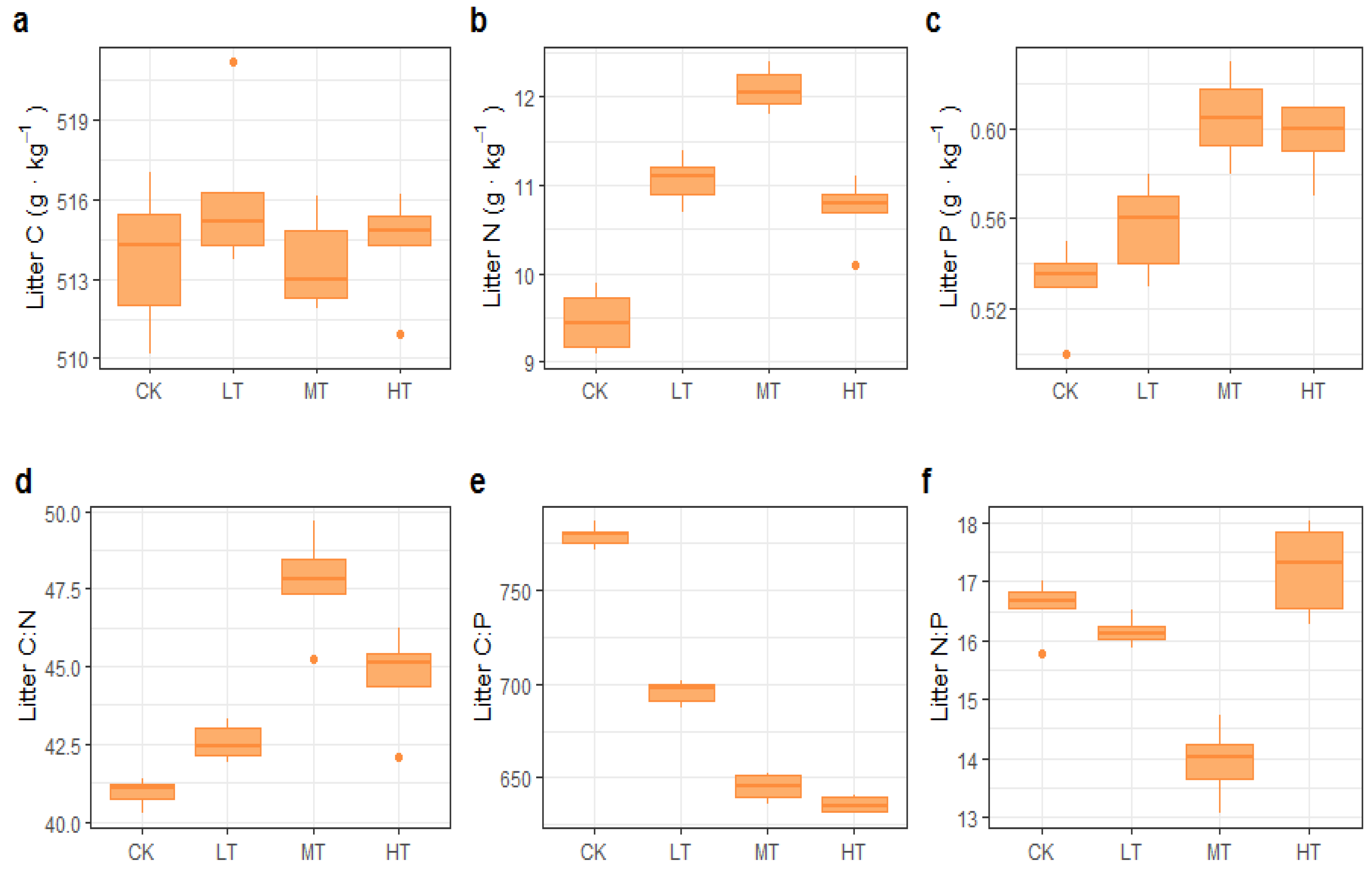
3.2. C:N:P Stoichiometry in Leaves and Litter
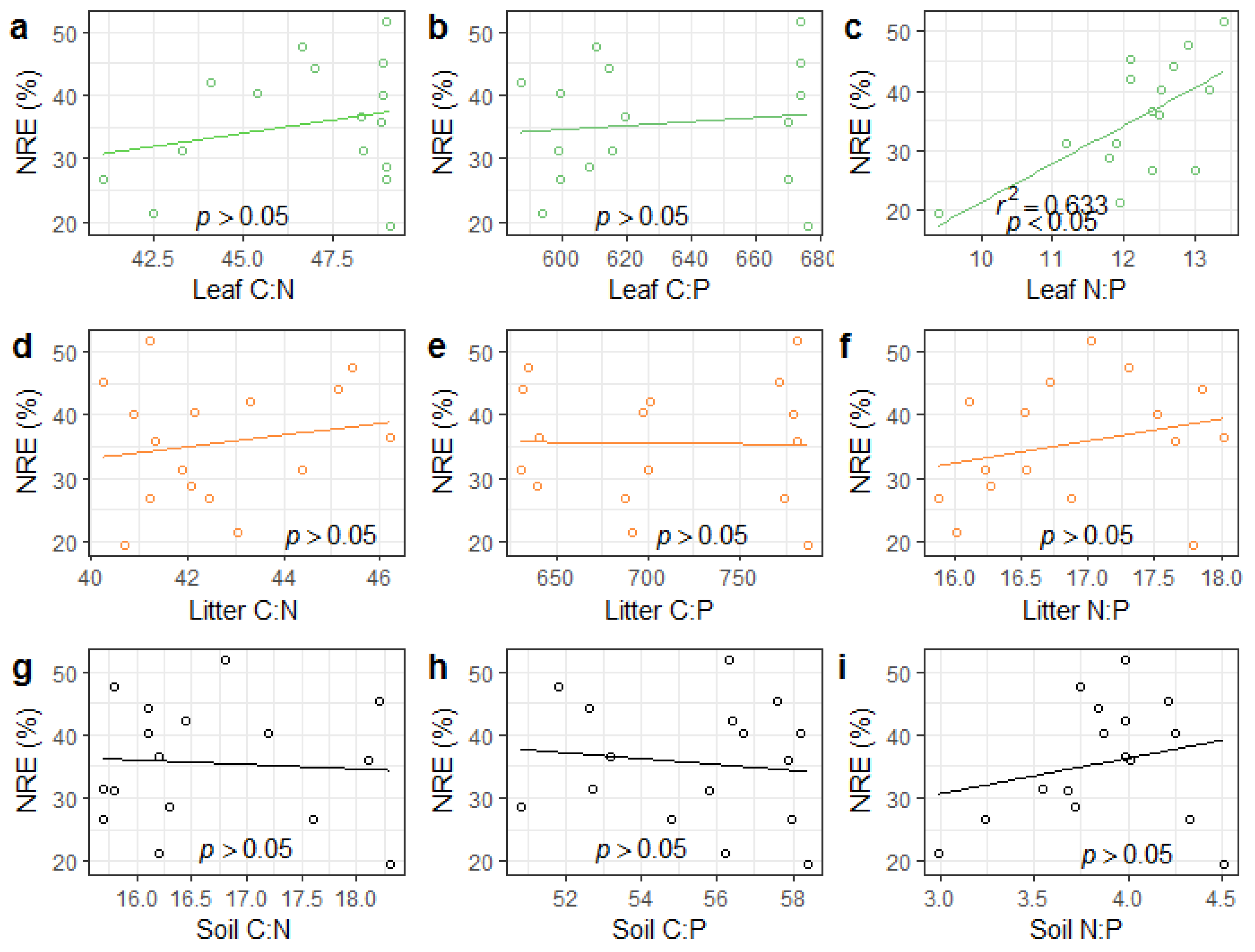
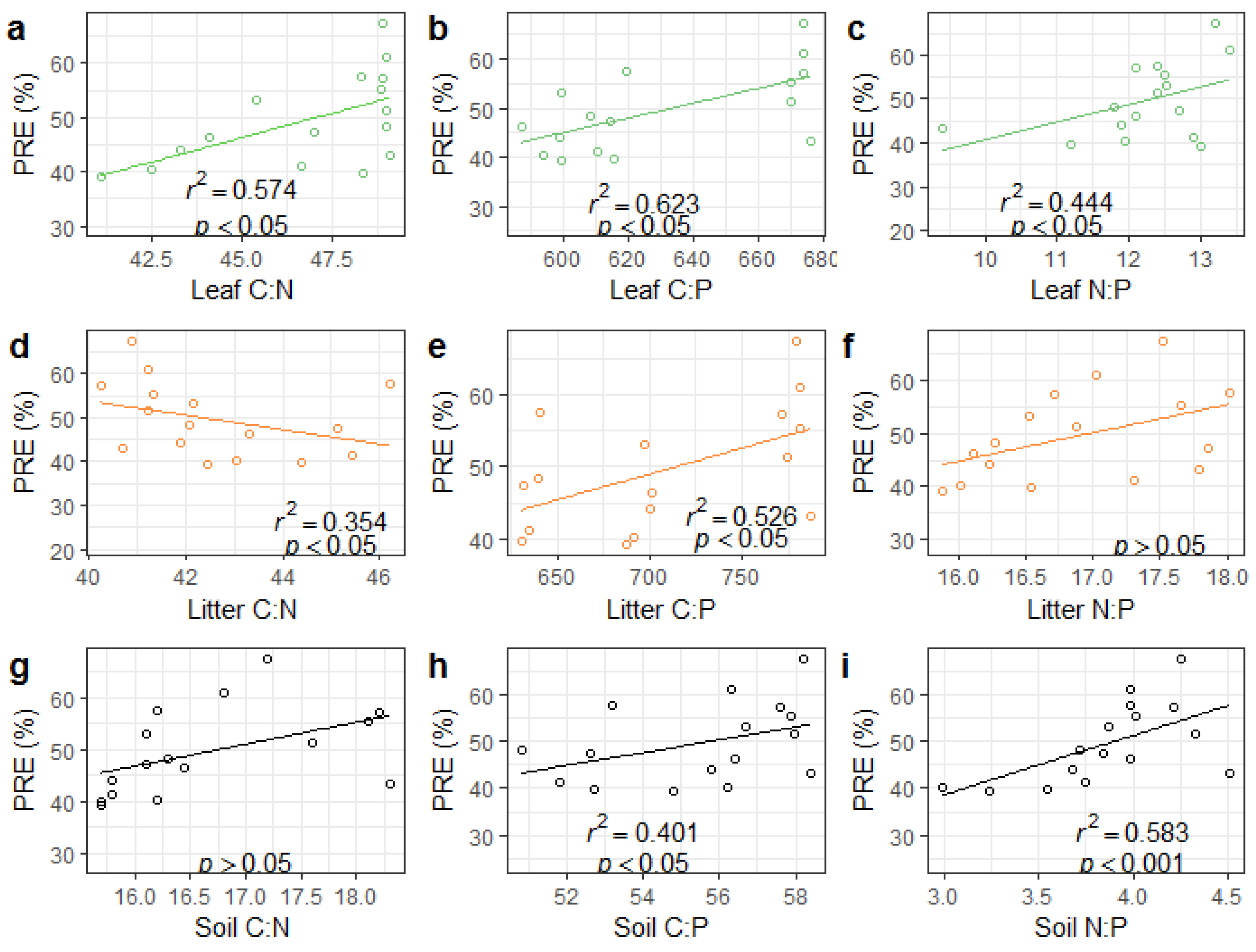
3.3. NRE, PRE, and Response to Leaf, Litter, and Soil Stoichiometry
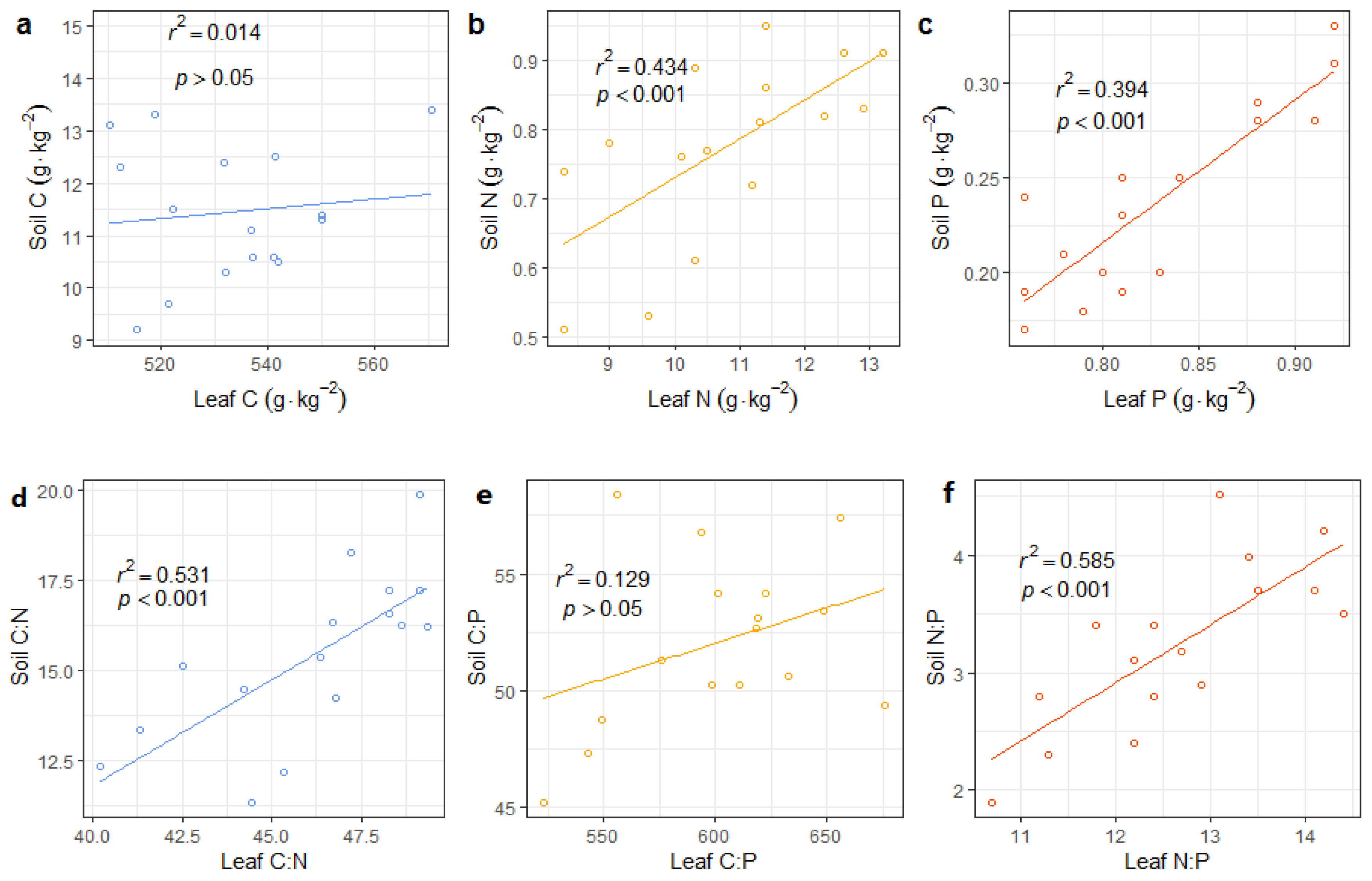
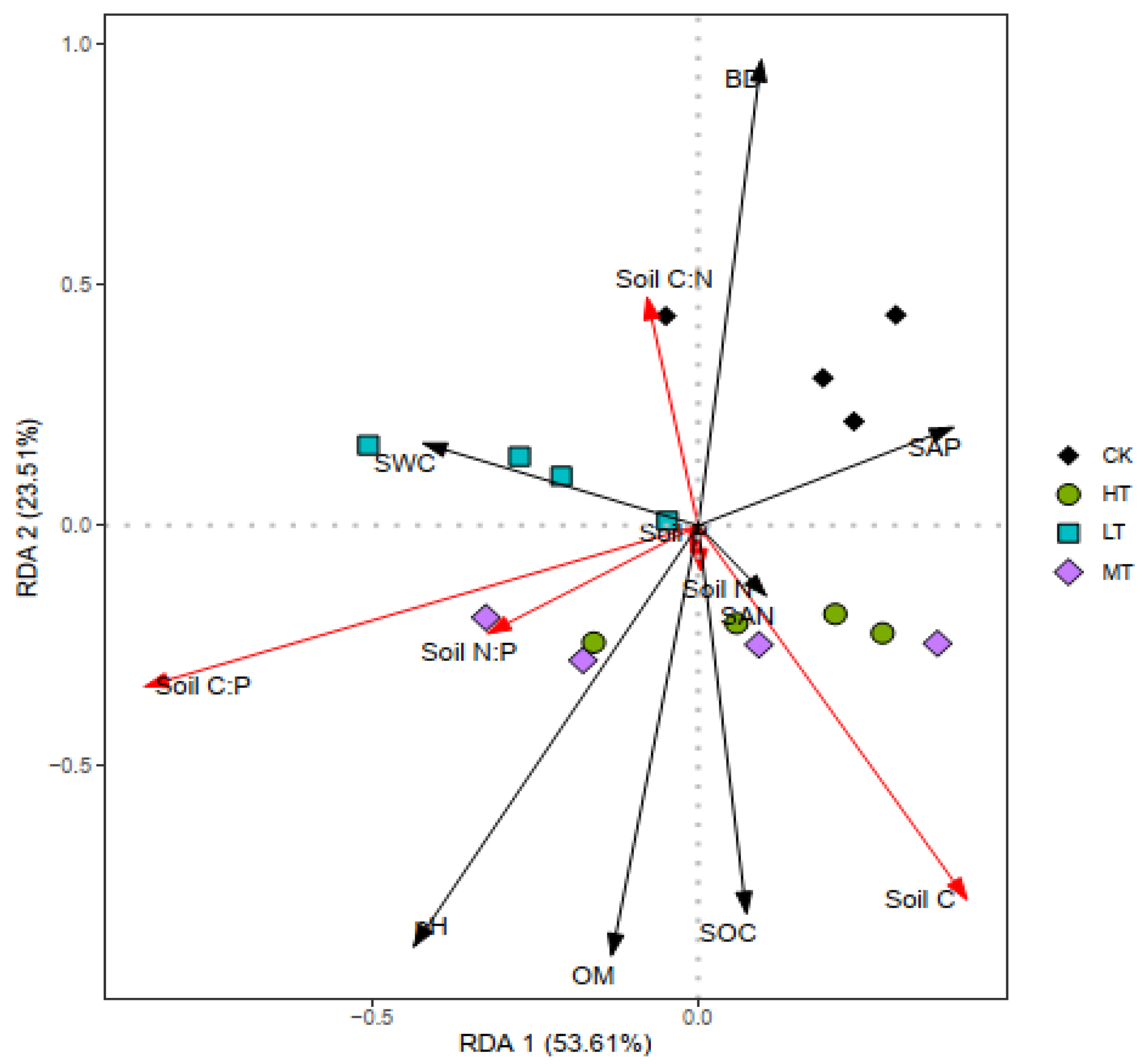
3.4. Relationships between Soil Properties, Stoichiometry, and NuRE
4. Discussion
4.1. Effects of Thinning Intensity on Soil C, N, and P Content and Stoichiometry
4.2. Effects of Thinning Intensity on Leaf and Litter C, N, and P Content and Stoichiometry
4.3. Effects of Thinning Intensity on NRE and PRE
4.4. Implications for Future Forest Management Practices
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Chapin, F.S. The mineral nutrition of wild plants. Annu. Rev. Ecol. Syst. 1980, 11, 233–260. [Google Scholar] [CrossRef]
- Ågren, G.I. Stoichiometry and nutrition of plant growth in natural communities. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 153–170. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Kerkhoff, A.J.; Swenson, N.G.; Enquist, B.J. Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. New Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef] [Green Version]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: London, UK, 2011. [Google Scholar]
- Koerselman, W.; Meuleman, A.F.M. The Vegetation N:P Ratio: A New Tool to Detect the Nature of Nutrient Limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Güsewell, S.N. P ratios in terrestrial plants: Variation and functional signifcance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Li, N.; Wang, W.; Liu, X.; Du, F.; Yao, D. Nutrients resorption and stoichiometry characteristics of different-aged plantations of Larix kaempferi in the Qinling Mountains, central China. PLoS ONE 2017, 12, e0189424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amazonas, N.T.; Martinelli, L.A.; Piccolo, M.C.; Rodrigues, R.R. Nitrogen dynamics during ecosystem development in tropical forest restoration. For. Ecol. Manag. 2011, 262, 1551–1557. [Google Scholar] [CrossRef]
- Freschet, G.T.; Cornelissen, J.H.C.; van Logtestijn, R.S.P.; Aerts, R. Substantial nutrient resorption from leaves, stems and roots in a subarctic flora: What is the link with other resource economics traits? New Phytol. 2010, 186, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Gusewell, S. Nutrient resorption of wetland graminoids is related to the type of nutrient limitation. Funct. Ecol. 2005, 19, 344–354. [Google Scholar] [CrossRef]
- Gyenge, J.; Fernández, M.E. Patterns of resource use efficiency in relation to intraspecific competition, size of the trees and resource availability in ponderosa pine. For. Ecol. Manage. 2014, 312, 231–238. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Zhou, Z.; Zhang, Y. Inorganic Nitrogen Addition Affects Soil Respiration and Belowground Organic Carbon Fraction for a Pinus tabuliformis Forest. Forests 2019, 10, 369. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Geng, Q.; Li, Q.; Luo, Y.; Vogel, J.; Shi, Z.; Ruan, H.; Xu, X. Nitrogen and Phosphorus Resorption in Planted Forests Worldwide. Forests 2019, 10, 201. [Google Scholar] [CrossRef] [Green Version]
- Dang, P.; Gao, Y.; Liu, J.; Yu, S.; Zhao, Z. Effects of thinning intensity on understory vegetation and soil microbial communities of a mature Chinese pine plantation in the Loess Plateau. Sci. Total Environ. 2018, 630, 171. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, Y.C.; Wang, L.; Liu, X.Z.; Cai, D.X.; Jia, H.Y.; Ming, A.G.; Chen, B.B. Facilitation by tree species in variable retention harvesting for the restoration of monoculture plantations in southern china. Trop. Conv. Sci. 2019, 12, 1940082918824836. [Google Scholar] [CrossRef]
- Wic Baena, C.; Andrés-Abellán, M.; Lucas-Borja, M.E.; Martínez-García, E.; García-Morote, F.A.; Rubio, E.; López-Serrano, F.R. Thinning and recovery effects on soil properties in two sites of a Mediterranean forest, in Cuenca Mountain (South-eastern of Spain). For. Ecol. Manage. 2013, 308, 223–230. [Google Scholar] [CrossRef]
- Olajuyigbe, S.; Tobin, B.; Saunders, M.; Nieuwenhuis, M. Forest thinning and soil respiration in a Sitka spruce forest in Ireland. Agric. For. Meteorol. 2012, 157, 86–95. [Google Scholar] [CrossRef]
- Trentini, C.P.; Campanello, P.I.; Villagra, M.; Ritter, L.; Ares, A.; Goldstein, G. Thinning of loblolly pine plantations in sub-tropical Argentina: Impact on microclimate and understory vegetation. For. Ecol. Manage. 2017, 384, 236–247. [Google Scholar] [CrossRef]
- Zhao, B.; Cao, J.; Geng, Y.; Zhao, X.; von Gadow, K. Inconsistent responses of soil respiration and its components to thinning intensity in a Pinus tabuliformis plantation in northern China. Agric. For. Meteorology. 2019, 265, 370–380. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, H.; Peng, D.; Liu, X.; Cheng, S. Thinning drives C:N:P stoichiometry and nutrient resorption in Larix princi-pis-rupprechtii plantations in North China. For. Ecol. Manage. 2020, 462, 117984. [Google Scholar] [CrossRef]
- Campbell, J.; Alberti, G.; Martin, J.; Law, B.E. Carbon dynamics of a ponderosa pine plantation following a thinning treatment in the northern Sierra Nevada. For. Ecol. Manag. 2009, 257, 453–463. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.Y.H.; Chen, X.; Wang, J.; Chen, B.; Wang, D.; Guan, Q. Soil labile organic carbon and carbon-cycle enzyme activities under different thinning intensities in Chinese fir plantations. Appl. Soil Ecol. 2016, 107, 162–169. [Google Scholar] [CrossRef]
- Tian, H.; Cheng, X.; Han, H. Effects of post-thinning precipitation on soil acid phosphomonoesterase activity in Larix principis-rupprechtii Mayr. Plantations. Forests 2019, 10, 734. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Ade, L.; Wu, X.; Zi, H.; Luo, X.; Wang, C. Changes in Soil C:N:P Stoichiometry and Microbial Structure along Soil Depth in Two Forest Soils. Forests 2019, 10, 113. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Guo, W.; Yu, M.; Wang, G.G.; Wu, T. Latitudinal patterns of leaf N, P stoichiometry and nutrient resorption of Metasequoia glyptostroboides along the eastern coastline of China. Sci. Total Environ. 2018, 618, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Wilcox, K.; La Pierre, K.; Knapp, A.K.; Han, X.G.; Smith, M.D. Stoichiometric homeostasis predicts plant species dominance, temporal stability, and responses to global change. Ecology 2015, 96, 2328–2335. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.R.; Wardle, D.A.; Bardgett, R.D.; Clarkson, B.D. The use of chronosequences in studies of ecological succession and soil development. J. Ecol. 2010, 98, 725–736. [Google Scholar] [CrossRef]
- Aerts, R. Nutrient resorption from senescing leaves of perennials: Are there general patterns? J. Ecol. 1996, 84, 597–608. [Google Scholar] [CrossRef]
- Turner, B.L.; Condron, L.M.; Wells, A.; Andersen, K.M. Soil nutrient dynamics during podzol development under lowland temperate rain forest. Catena 2012, 97, 50–62. [Google Scholar] [CrossRef]
- Tian, D.L. Structure and Function of Masson Pine and Slash Pine Ecosystem; Science Press: Beijing, China, 2005. [Google Scholar]
- IUSS Working Group WRB. World reference base for soil resources. In World Soil Resources Reports No. 103, 2nd ed.; Food and Agriculture Organization (FAO): Rome, Italy, 2006. [Google Scholar]
- Vergutz, L.; Manzoni, S.; Porporato, A.; Novais, R.F.; Jackson, R.B. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol. Monogr. 2012, 82, 205–220. [Google Scholar] [CrossRef] [Green Version]
- Bao, S. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Killingbeck, K.T. Nutrients in senesced leaves: Keys to the search for potential resorption and resorption proficiency. Ecology 1996, 77, 1716–1727. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Shao, M.A. Spatial variability of soil physical properties in a region of the Loess Plateau of Pr China subject to wind and water erosion. Land Degrad. Dev. 2013, 24, 296–304. [Google Scholar] [CrossRef]
- Mora, J.L.; Lázaro, R. Seasonal changes in bulk density under semiarid patchy vegetation: The soil beats. Geoderma 2014, 235, 30–38. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Shah, J.J.F. Ecoenzymatic Stoichiometry and Ecological Theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 313–343. [Google Scholar] [CrossRef] [Green Version]
- Matzek, V.; Vitousek, P.M. N:P stoichiometry and protein: RNA ratios in vascular plants: An evaluation of the growth-rate hypothesis. Ecol. Lett. 2009, 12, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wang, S.; Ren, C.; Zhang, W.; Zhao, F.; Li, X.; Zhang, D.; Han, X.; Yang, G. Nitrogen and Phosphorus Resorption in Relation to Nutrition Limitation along the Chronosequence of Black Locust (Robinia pseudoacacia L.) Plantation. Forests 2019, 10, 261. [Google Scholar] [CrossRef] [Green Version]
- Gergócs, V.; Hufnagel, L. The effect of microarthropods on litter decomposition depends on litter quality. Eur. J. Soil Biol. 2016, 75, 24–30. [Google Scholar] [CrossRef]
- Luyssaert, S.; Staelens, J.; De Schrijver, A. Does the commonly used estimator of nutrient resorption in tree foliage actually measure what it claims to? Oecologia 2005, 144, 177–186. [Google Scholar] [CrossRef]
- Ma, S.; Concilio, A.; Oakley, B.; North, M.; Chen, J. Spatial variability in microclimate in a mixed-conifer forest before and after thinning and burning treatments. For. Ecol. Manag. 2010, 259, 904–915. [Google Scholar] [CrossRef]
- Yan, T.; Lü, X.-T.; Zhu, J.-J.; Yang, K.; Yu, L.-Z.; Gao, T. Changes in nitrogen and phosphorus cycling suggest a transition to phosphorus limitation with the stand development of larch plantations. Plant Soil. 2018, 422, 385–396. [Google Scholar] [CrossRef]
- You, C.; Wu, F.; Yang, W.; Xu, Z.; Tan, B.; Zhang, L.; Yue, K.; Ni, X.; Li, H.; Chang, C.; et al. Does foliar nutrient resorption regulate the coupled relationship between nitrogen and phosphorus in plant leaves in response to nitrogen deposition? Sci. Total Environ. 2018, 645, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Drenovsky, R.E.; Pietrasiak, N.; Short, T.H.; Silva, T. Global temporal patterns in plant nutrient resorption plasticity. Glob. Ecol. Biogeogr. 2019, 28, 728–743. [Google Scholar] [CrossRef] [Green Version]
- Forrester, D.I.; Bauhus, J.; Cowie, A.L.; Vanclay, J.K. Mixed-species plantations of Eucalyptus with nitrogen-fixing trees: A review. For. Ecol. Manag. 2006, 233, 211–230. [Google Scholar] [CrossRef] [Green Version]
- Kou, L.; Chen, W.; Jiang, L.; Dai, X.; Fu, X.; Wang, H.; Li, S. Simulated nitrogen deposition affects stoichiometry of multiple elements in resource-acquiring plant organs in a seasonally dry subtropical forest. Sci. Total Environ. 2018, 624, 611–620. [Google Scholar] [CrossRef]


| Thinning Treatment | Thinning Intensity (%) | Prethinning Density (tree·ha−1) | Postthinning Density (tree·ha−1) | Slope (°) | Altitude (m) | Average DBH (cm) | Average Height (m) | Stand Age |
|---|---|---|---|---|---|---|---|---|
| CK | 0 | 1245 | 1231 | 23 | 354 | 14.38 ± 0.36 | 13.55 ± 0.22 | 25 |
| LT | 50 | 1203 | 590 | 25 | 362 | 14.43 ± 1.03 | 12.79 ± 0.31 | 25 |
| MT | 63 | 1116 | 450 | 22 | 273 | 14.55 ± 1.14 | 13.71 ± 0.28 | 25 |
| HT | 80 | 1224 | 225 | 18 | 286 | 14.29 ± 0.34 | 13.11 ± 0.19 | 25 |
| Thinning Treatment | BD | SWC | pH | OM | SOC | SAN | SAP |
|---|---|---|---|---|---|---|---|
| CK | 1.33 ± 0.10 a | 31.19 ± b | 4.29 ± 0.13 a | 10.12 ± 1.33 b | 14.52 ± 1.3 a | 120.44 ± 8.23 b | 0.51 ± 0.57 b |
| LT | 1.38 ± 0.09 a | 32.11 ± b | 4.93 ± 0.21 b | 12.22 ± 1.31 ab | 14.88 ± 1.1 a | 121.15 ± 13.21 b | 0.54 ± 0.33 b |
| MT | 1.42 ± 0.12 a | 35.26 ± a | 4.51 ± 0.28 b | 15.40 ± 1.04 a | 15.12 ± 1.22 a | 135.28 ± 11.21 a | 0.79 ± 0.12 a |
| HT | 1.51 ± 0.13 a | 36.48 ± a | 4.7 ± 0.14 b | 13.01 ± 1.59 ab | 15.01 ± 1.01 a | 128.14 ± 14.23 ab | 0.68 ± 0.26 ab |
| Thinning | Soil Stoichiometry | Thinning × Soil Stoichiometry | ||||
|---|---|---|---|---|---|---|
| F | p-Value | F | p-Value | F | p-Value | |
| Leaf C | 3.5 | 0.45 | 22.4 | 0.42 | 36.66 | 0.79 |
| Leaf N | 45.2 | <0.05 | 51.3 | <0.01 | 8.64 | <0.001 |
| Leaf P | 36.7 | <0.001 | 44.3 | <0.01 | 15.58 | 0.63 |
| Litter C | 0.54 | <0.05 | 21.6 | 0.34 | 10.32 | 0.44 |
| Litter N | 31.2 | <0.01 | 8.7 | 0.67 | 25.55 | 0.16 |
| Litter P | 26.5 | <0.01 | 31.4 | <0.05 | 48.26 | 0.11 |
| Leaf C:N | 10.2 | 0.73 | 61.3 | <0.001 | 27.46 | <0.001 |
| Leaf C:P | 15.1 | 0.56 | 37.5 | <0.05 | 13.22 | 0.22 |
| Leaf N:P | 12.6 | 0.36 | 49.4 | <0.05 | 2.73 | <0.05 |
| Litter C:N | 26.4 | 0.19 | 12.2 | 0.44 | 14.63 | 0.34 |
| Litter C:P | 41.2 | <0.01 | 36.1 | 0.18 | 9.63 | 0.17 |
| Litter N:P | 25.7 | <0.01 | 55.3 | <0.05 | 28.77 | 0.41 |
| BD | pH | SWC | OM | SOC | SAN | SAP | |
|---|---|---|---|---|---|---|---|
| Leaf C | −0.673 ** | −0.534 | 0.532 *** | 0.376 | 0.425 * | 0.216 | 0.102 |
| Leaf N | −0.397 * | −0.541 * | 0.613 ** | 0.446 ** | 0.254 | 0.365 | 0.053 |
| Leaf P | −0.558 ** | −0.818 *** | 0.684 *** | 0.546 ** | 0.013 | 0.485 | 0.542 |
| Litter C | −0.344 * | −0.256 | 0.634 * | 0.367 | 0.413 ** | 0.264 | 0.102 |
| Litter N | −0.611 ** | −0.489 * | 0.715 ** | 0.706 ** | 0.395 | 0.517 * | 0.097 * |
| Litter P | −0.734 *** | −0.738 *** | 0.687 ** | −0.213 | 0.275 | 0.657 * | 0.324 *** |
| Leaf C:N | 0.056 | 0.113 | −0.036 | 0.156 | 0.116 | −0.089 | −0.067 |
| Leaf C:P | 0.186 | 0.624 ** | −0.162 | 0.084 | 0.204 | −0.495 | −0.617 ** |
| Leaf N:P | 0.326 | 0.401 ** | −0.203 | 0.526 ** | 0.068 | −0.107 | −0.232 |
| Litter C:N | 0.184 | 0.058 | −0.205 | 0.534 * | 0.335 * | −0.016 | −0.048 |
| Litter C:P | 0.626 *** | 0.724 ** | −0.748 ** | 0.220 | 0.243 | −0.611 ** | −0.107 |
| Litter N:P | 0.703 ** | 0.806 ** | −0.824 *** | 0.617 ** | 0.513 | −0.724 ** | −0.265 |
| NRE | 0.212 | 0.156 | 0.035 | 0.104 | 0.097 | 0.048 | 0.205 |
| PRE | 0.638 ** | −0.679 ** | 0.322 | 0.103 | 0.054 | −0.059 | −0.334 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Lu, Y.; Chen, B.; Ming, A.; Pang, L. Nutrient Resorption and C:N:P Stoichiometry Responses of a Pinus massoniana Plantation to Various Thinning Intensities in Southern China. Forests 2022, 13, 1699. https://doi.org/10.3390/f13101699
Jiang J, Lu Y, Chen B, Ming A, Pang L. Nutrient Resorption and C:N:P Stoichiometry Responses of a Pinus massoniana Plantation to Various Thinning Intensities in Southern China. Forests. 2022; 13(10):1699. https://doi.org/10.3390/f13101699
Chicago/Turabian StyleJiang, Jun, Yuanchang Lu, Beibei Chen, Angang Ming, and Lifeng Pang. 2022. "Nutrient Resorption and C:N:P Stoichiometry Responses of a Pinus massoniana Plantation to Various Thinning Intensities in Southern China" Forests 13, no. 10: 1699. https://doi.org/10.3390/f13101699





