The Interconnected Relationship between Auxin Concentration Gradient Changes in Chinese Fir Radial Stems and Dynamic Cambial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Decapitation and Exogenous IAA Treatments
2.2. Measurement of Endogenous Hormones
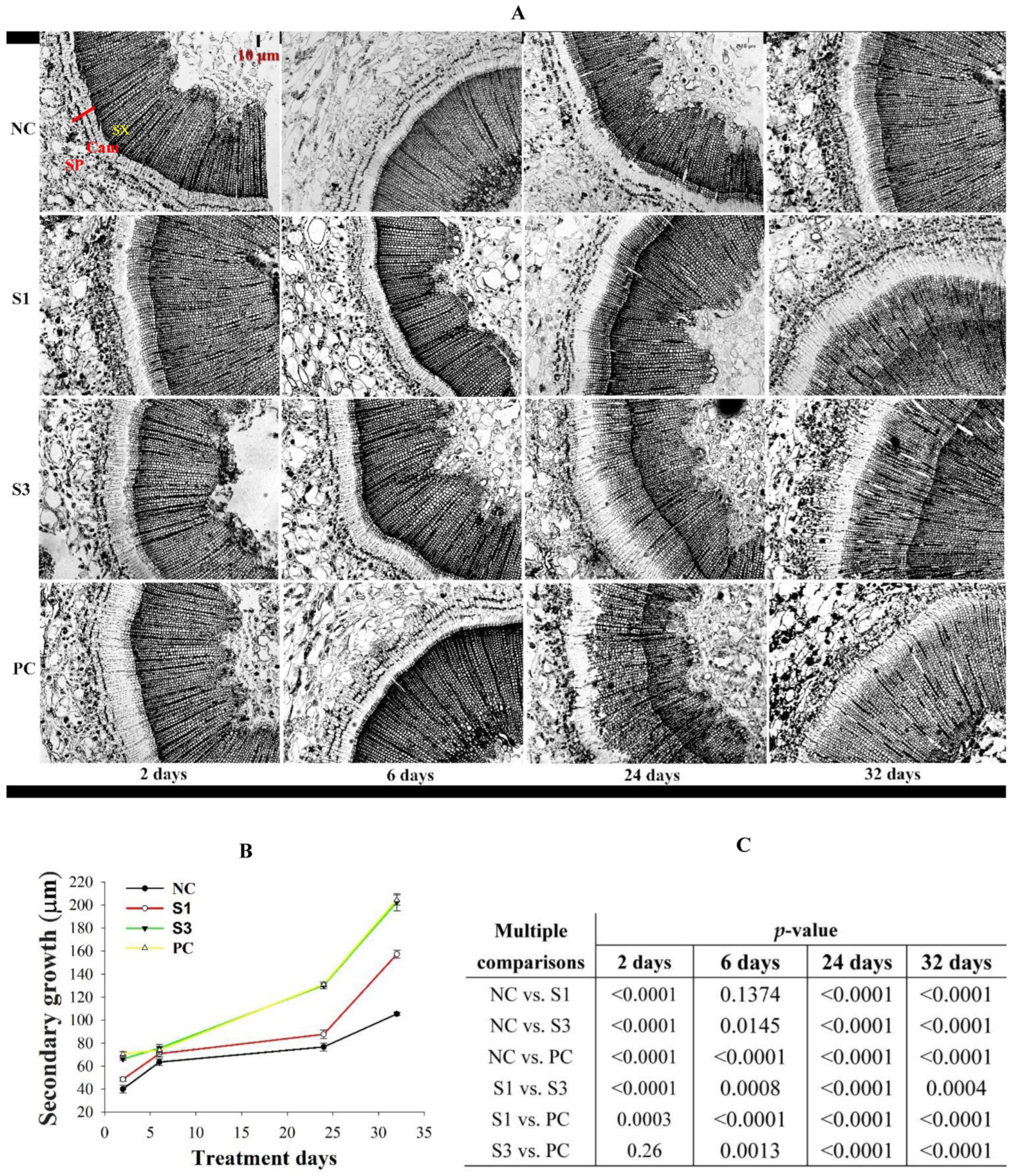
2.3. Anatomical Observation and Analysis of Secondary Growth Changes
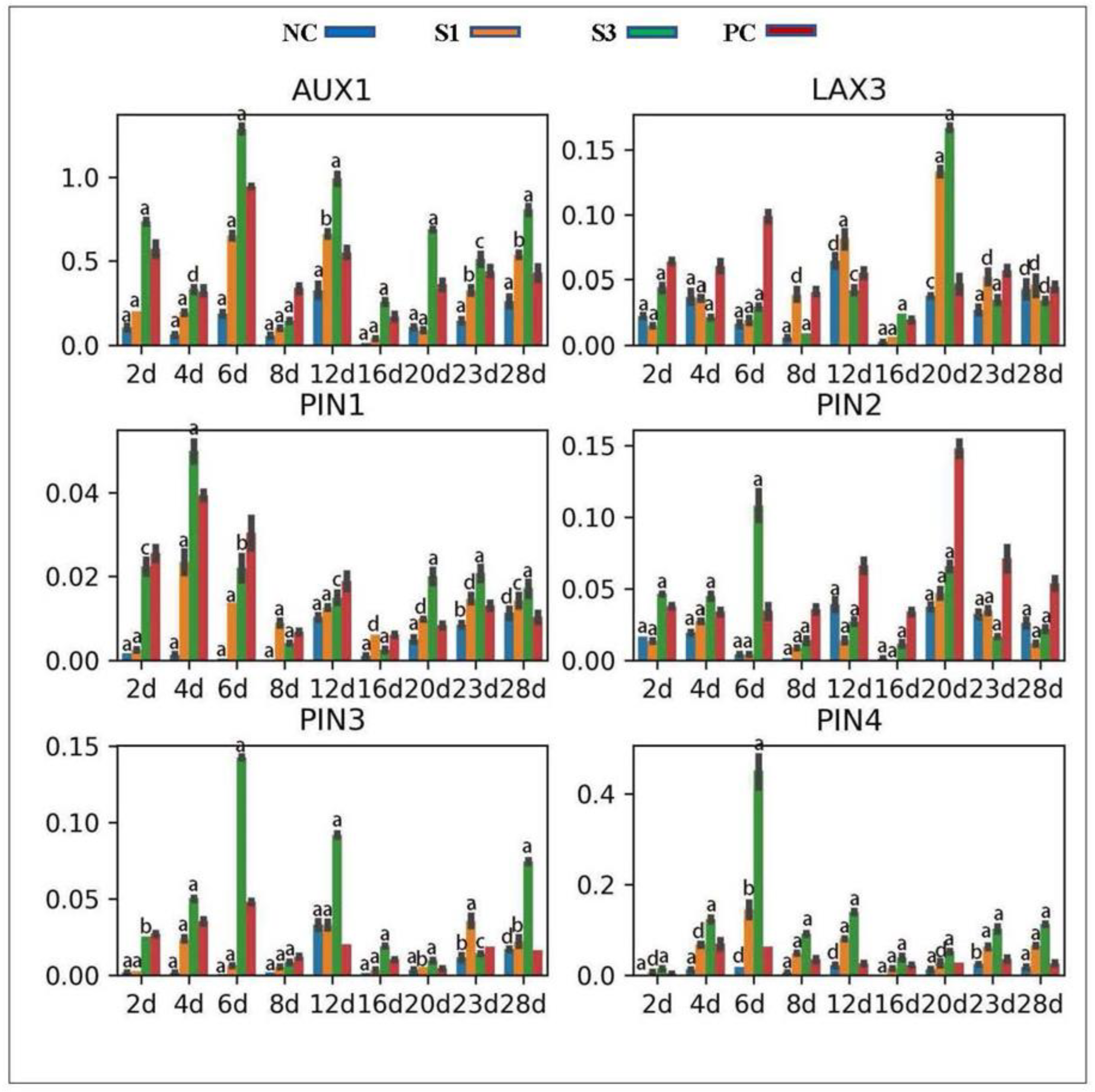
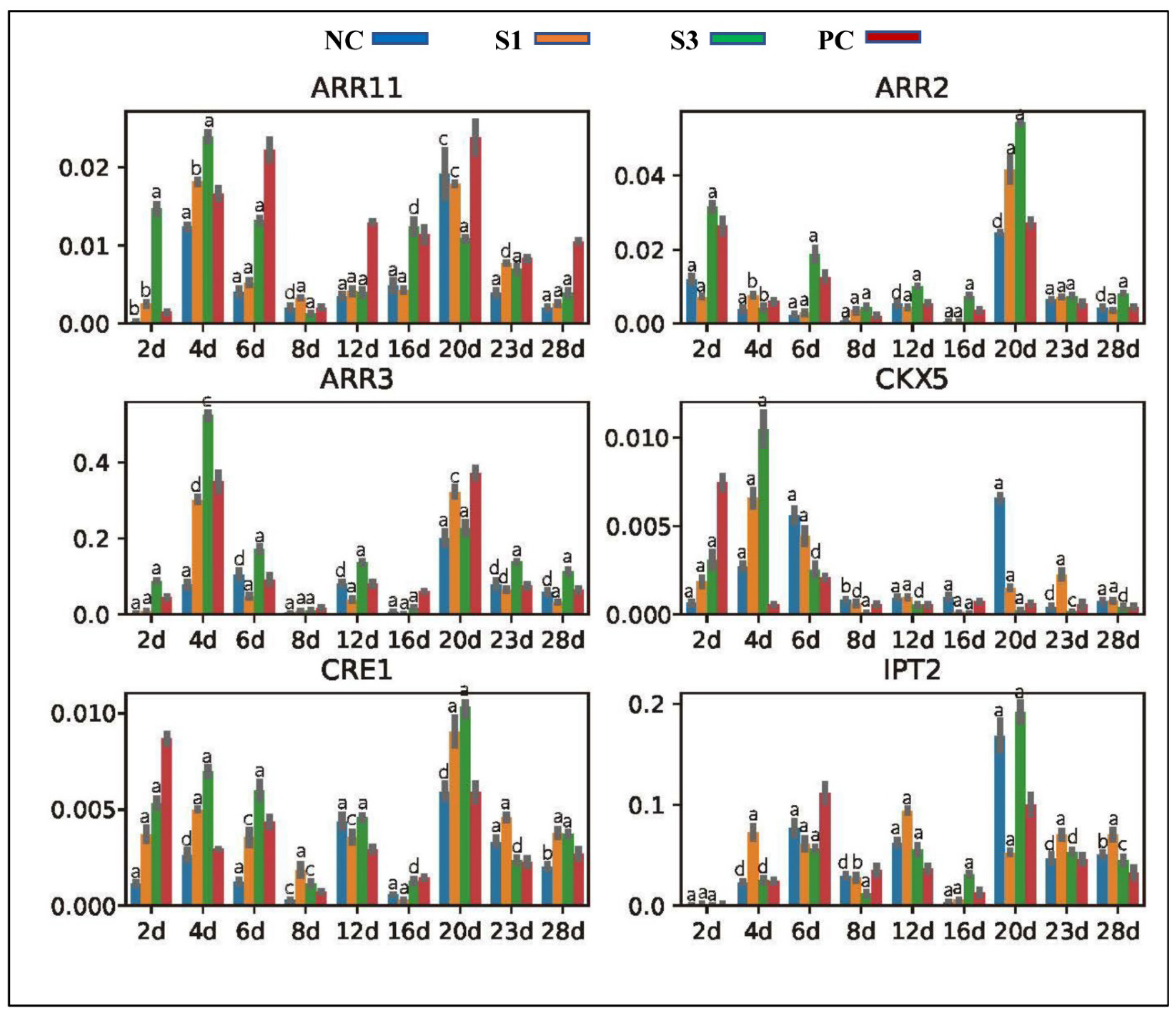
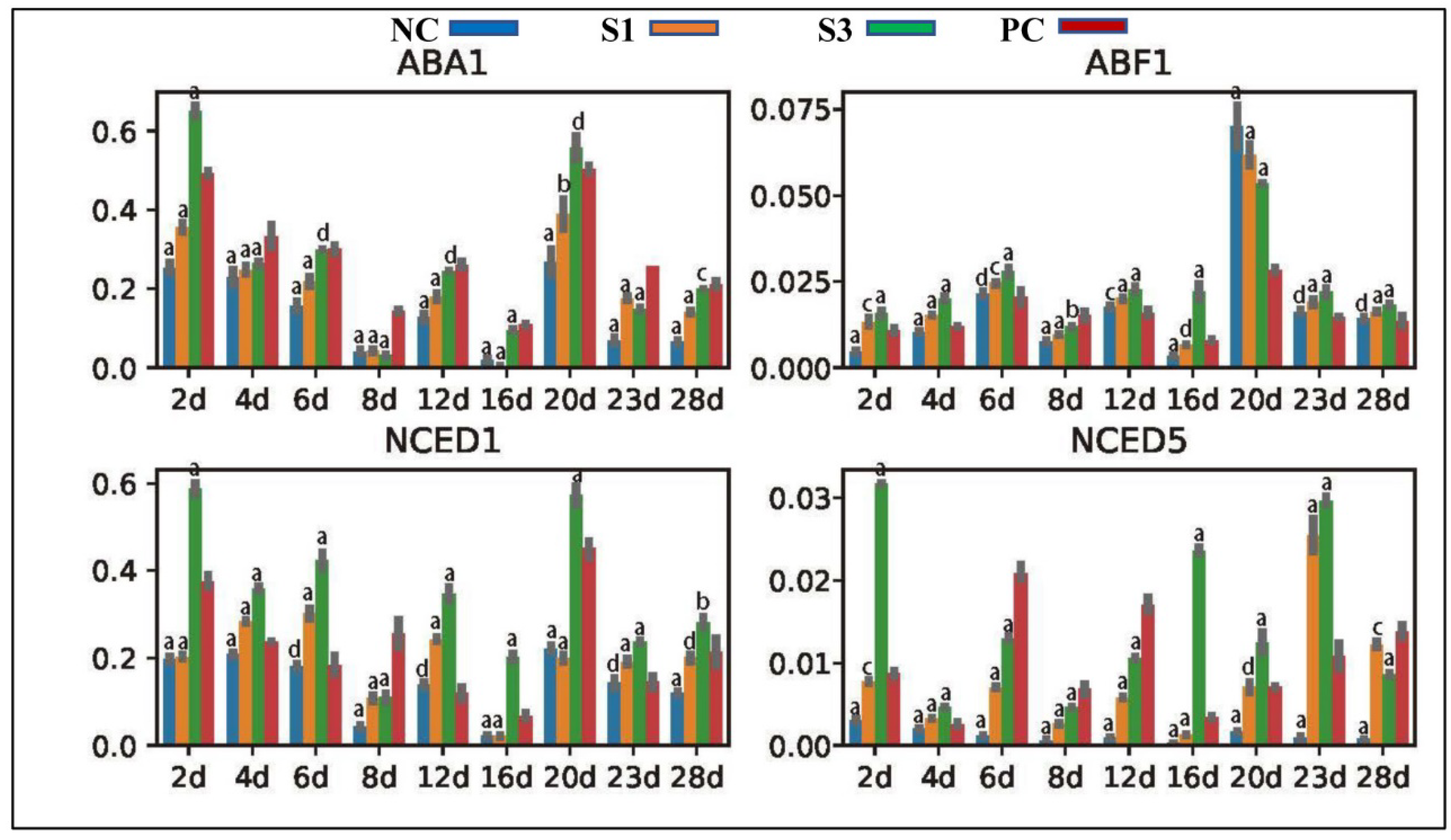
2.4. Gene Location and Expression Profile Analysis
3. Results
3.1. Changes in Endogenous Hormones
3.1.1. IAA
3.1.2. ZR
3.1.3. GA3
3.1.4. ABA
3.2. Changes in Cambial Activity
3.3. Changes in Gene Expression
3.3.1. PAT Carrier Genes
3.3.2. Auxin Signaling Genes
3.3.3. Genes Participating in CK Production and Signal Transduction
3.3.4. Genes Participating in GA Production and Signal Transduction
3.3.5. Genes Participating in ABA Production and Signal Transduction
3.3.6. Genetic Regulators
4. Discussion
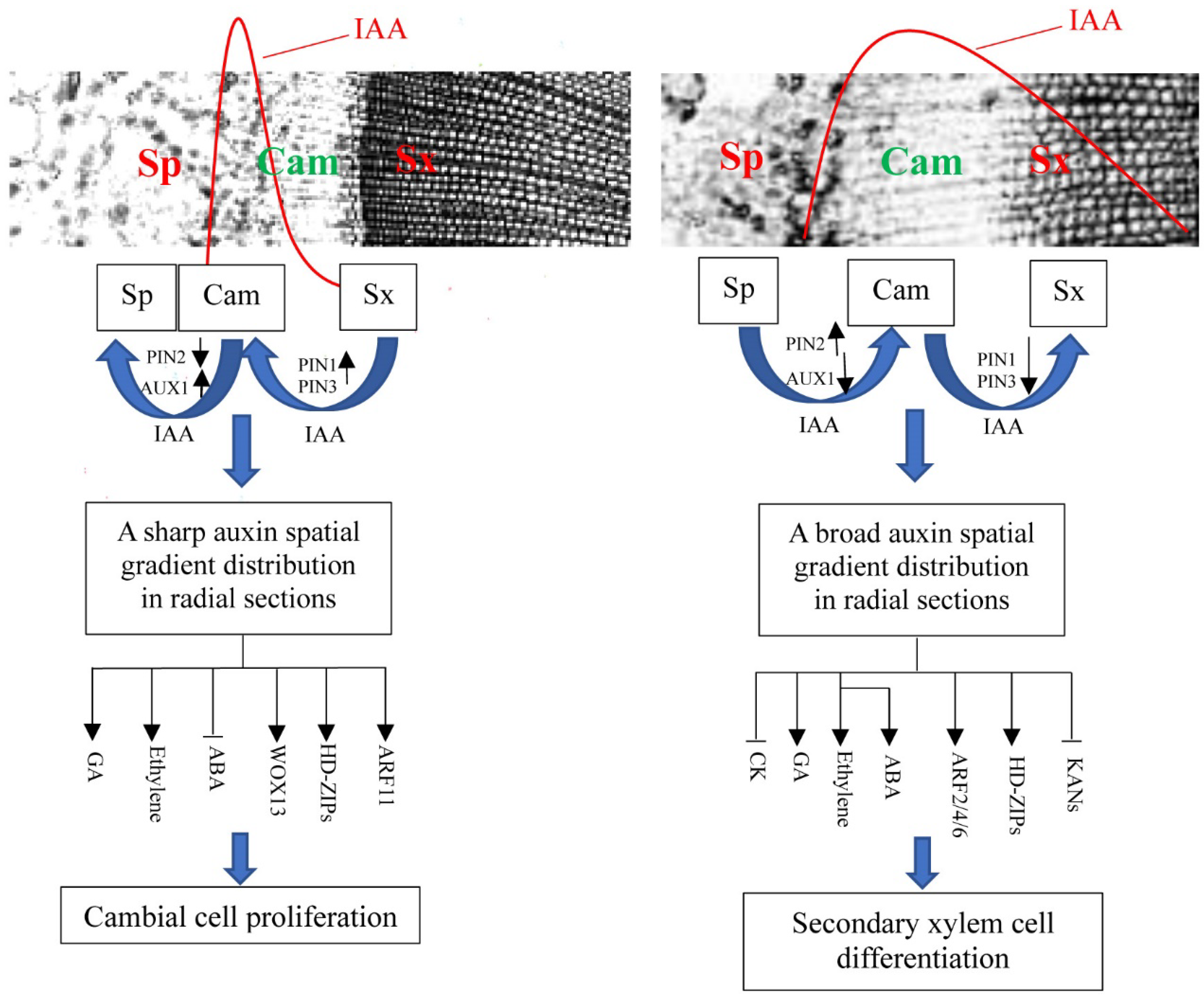
4.1. Auxin Concentration Gradient Changes Provide a Basic Cue for Cambial Activity
4.2. Pathways of Auxin Concentration Gradient Triggering Cambial Activity
4.2.1. Auxin Signaling Pathways
4.2.2. Differential Response of Other Plant Hormones to PAT Changes in Chinese Fir Shoots
4.2.3. Response of Key Genetic Regulators to PAT Changes in Chinese Fir Shoots
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Plomion, C.; Leprovost, G.; Stokes, A. Wood formation in trees. Plant Physiol. 2001, 127, 1513–1523. [Google Scholar] [CrossRef]
- Uggla, C.; Moritz, T.; Sandberg, G.; Sundberg, B. Auxin as a positional signal in pattern formation in plants. Proc. Natl. Acad. Sci. USA 1996, 93, 9282–9286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundberg, B.; Uggla, C.; Tuominen, H. Cambial Growth and Auxin Gradients. In Cell and Molecular Biology of Wood Formation; Savidge, R.A., Barnett, J.R., Napier, R., Eds.; BIOS Scientific: Oxford, UK, 2000; pp. 169–188. [Google Scholar]
- Sundberg, B.; Uggla, C. Origin and dynamics of indoleacetic acid under polar transport in Pinus sylvestris. Physiol. Plant. 1998, 104, 22–29. [Google Scholar] [CrossRef]
- Little, C.; Savidge, R. The role of plant growth regulators in forest tree cambial growth. In Hormonal Control of Tree growth; Springer: Berlin/Heidelberg, Germany, 1987; pp. 137–169. [Google Scholar]
- Tuominen, H.; Puech, L.; Fink, S.; Sundberg, B. A Radial Concentration Gradient of Indole-3-Acetic Acid Is Related to Secondary Xylem Development in Hybrid Aspen. Plant Physiol. 1997, 115, 577–585. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, J.; Karlberg, A.; Antti, H.; Lopez-Vernaza, M.; Mellerowicz, E. Dissecting the molecular basis of the regulation of wood formation by auxin in hybrid aspen. Plant Cell 2008, 20, 843–855. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Nieminen, K.; Serra, J.A.; Helariutta, Y. The formation of wood and its control. Curr. Opin. Plant Biol. 2014, 17, 56–63. [Google Scholar] [CrossRef]
- Tanaka, H.; Dhonukshe, P.; Brewer, P.B.; Friml, J. Spatiotemporal asymmetric auxin distribution: A means to coordinate plant development. Cell. Mol. Life Sci. 2006, 63, 2738–2754. [Google Scholar] [CrossRef]
- Michniewicz, M.; Brewer, P.B.; Friml, J. Polar Auxin Transport and Asymmetric Auxin Distribution. Arabidopsis Book 2007, 5, e0108. [Google Scholar]
- Friml, J.; Palme, K. Polar auxin transport—Old questions and new concepts? Plant Mol. Biol. 2002, 49, 273–284. [Google Scholar] [CrossRef]
- Kramer, E.M. PIN and AUX/LAX proteins: Their role in auxin accumulation. Trends Plant Sci. 2004, 9, 578–582. [Google Scholar] [CrossRef]
- Goldsmith, M. The Polar Transport of Auxin. Annu. Rev. Plant Physiol. 1977, 28, 439–478. [Google Scholar] [CrossRef]
- Zaímalová, E.; Keek, P.; Skpa, P.; Hoyerová, K.; Petráek, J. Polar transport of the plant hormone auxin–the role of PIN-FORMED (PIN) proteins. Cell. Mol. Life Sci. Cmls 2007, 64, 1621–1637. [Google Scholar] [CrossRef]
- Mazur, E.; Gallei, M.; Adamowski, M.; Han, H.; Robert, H.S.; Friml, J. Clathrin-mediated trafficking and PIN trafficking are required for auxin canalization and vascular tissue formation in Arabidopsis. Plant Sci. 2020, 293, 110414. [Google Scholar] [CrossRef]
- Galweiler, L.; Guan, C.; Muller, A.; Wisman, E.; Mendgen, K.; Yephremov, A.; Palme, K. Regulation of Polar Auxin Transport by AtPIN1 in Arabidopsis Vascular Tissue. Science 1998, 282, 2226–2230. [Google Scholar] [CrossRef] [Green Version]
- Schrader, J.; Bhalerao, R.P.; May, S.T.; Palme, K.; Bennett, M.J.; Sandberg, G. “Polar Auxin Transport in Hybrid Aspen”. Aspen Bibliography. Paper 969. 1999. Available online: https://digitalcommons.usu.edu/aspen_bib/969 (accessed on 18 September 2022).
- Hellgren, J.M.; Olofsson, K.; Sundberg, B. Patterns of auxin distribution during gravitational induction of reaction wood in poplar and pine. Plant Physiol. 2004, 135, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Quint, M.; Gray, W.M. Auxin signaling. Curr. Opin. Plant Biol. 2006, 9, 448–453. [Google Scholar] [CrossRef]
- Brackmann, K.; Qi, J.; Gebert, M.; Jouannet, V.; Schlamp, T.; Grünwald, K.; Wallner, E.S.; Novikova, D.D.; Levitsky, V.G.; Agustí, J.; et al. Spatial specificity of auxin responses coordinates wood formation. Nat. Commun. 2018, 9, 875. [Google Scholar] [CrossRef] [Green Version]
- Hirakawa, Y.; Kondo, Y.; Fukuda, H. TDIF peptide signaling regulates vascular stem cell proliferation via the WOX4 homeobox gene in Arabidopsis. Plant Cell 2010, 22, 2618–2629. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.; Strable, J.; Shimizu, R.; Koenig, D.; Sinha, N.; Scanlon, M.J. WOX4 promotes procambial development. Plant Physiol. 2010, 152, 1346–1356. [Google Scholar] [CrossRef] [Green Version]
- Suer, S.; Agusti, J.; Sanchez, P.; Schwarz, M.; Greb, T. WOX4 imparts auxin responsiveness to cambium cells in Arabidopsis. Plant Cell 2011, 23, 3247–3259. [Google Scholar] [CrossRef] [Green Version]
- Etchells, J.P.; Provost, C.M.; Mishra, L.; Turner, S.R. WOX4 and WOX14 act downstream of the PXY receptor kinase to regulate plant vascular proliferation independently of any role in vascular organisation. Development 2013, 140, 2224–2234. [Google Scholar] [CrossRef]
- Donner, T.J.; Sherr, I.; Scarpella, E. Regulation of preprocambial cell state acquisition by auxin signaling in Arabidopsis leaves. Development 2009, 136, 3235–3246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouannet, V.; Brackmann, K.; Greb, T. (Pro) cambium formation and proliferation: Two sides of the same coin? Curr. Opin. Plant Biol. 2015, 23, 54–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, C.J.; Valdés, A.E.; Wang, G.; Ramachandran, P.; Beste, L.; Uddenberg, D.; Carlsbecker, A. PHABULOSA mediates an auxin signaling loop to regulate vascular patterning in Arabidopsis. Plant Physiol. 2016, 170, 956–970. [Google Scholar] [CrossRef] [Green Version]
- Mishra, L.S. The role of the PXY-CLE signalling pathway in regulating cell division during wood formation in Poplar. Ph.D. Dissertation, The University of Manchester, Manchester, UK, 2016. [Google Scholar]
- Li, J.; Jia, H.; Zhang, J.; Liu, B.; Lu, M. Effect of Overexpression of Populus tomentosa WUSCHEL-related homeobox 4 (PtoWOX4a) on the Secondary Growth of Poplar. Sci. Silvae Sin. 2018, 54, 52–59. [Google Scholar]
- Johnson, L.A. An Investigation into the Molecular Basis of Secondary Vascular Tissue Formation in Poplar and Arabidopsis with an Emphasis on the Role of Auxin and the Auxin Response Factor MONOPTEROS. Ph.D. Dissertation, University of British Columbia, Vancouver, BC, Canada, 2006. [Google Scholar]
- Björklund, S. Plant Hormones in wood formation: A novel insight into the roles of ethylene and gibberellins. Ph.D. Thesis, Swedish University of Agricultural Sciences, Umea, Sweden, 2007; pp. 19–22. [Google Scholar]
- Buttò, V.; Deslauriers, A.; Rossi, S.; Rozenberg, P.; Morin, H. The role of plant hormones in tree-ring formation. Trees 2020, 34, 315–335. [Google Scholar] [CrossRef]
- Fu, X.; Su, H.; Liu, S.; Du, X.; Xu, C.; Luo, K. Cytokinin signaling localized in phloem noncell-autonomously regulates cambial activity during secondary growth of Populus stems. New Phytol. 2021, 230, 1476–1488. [Google Scholar] [CrossRef]
- Immanen, J.; Nieminen, K.; Smolander, O.P.; Kojima, M.; Serra, J.A.; Koskinen, P.; Zhang, J.; Elo, A.; Mähönen, A.P.; Street, N.; et al. Cytokinin and auxin display distinct but interconnected distribution and signaling profiles to stimulate cambial activity. Curr. Biol. 2016, 26, 1990–1997. [Google Scholar] [CrossRef] [Green Version]
- Nieminen, K.; Immanen, J.; Laxell, M.; Kauppinen, L.; Tarkowski, P.; Dolezal, K.; Tähtiharju, S.; Elo, A.; Decourteix, M.; Ljung, K.; et al. Cytokinin signaling regulates cambial development in poplar. Proc. Natl. Acad. Sci. USA 2008, 105, 20032–20037. [Google Scholar] [CrossRef] [Green Version]
- Ioio, R.D.; Nakamura, K.; Moubayidin, L.; Perilli, S.; Taniguchi, M.; Morita, M.T.; Aoyama, T.; Costantino, P.; Sabatini, S. A genetic framework for the control of cell division and differentiation in the root meristem. Science 2008, 322, 1380–1384. [Google Scholar] [CrossRef] [Green Version]
- Müller, B.; Sheen, J. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 2008, 453, 1094–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, M.E.; Israelsson, M.; Olsson, O.; Moritz, T. Increased gibberellin biosynthesis in transgenic trees promotes growth, biomass production and xylem fiber length. Nat. Biotechnol. 2000, 18, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Björklund, S.; Antti, H.; Uddestrand, I.; Moritz, T.; Sundberg, B. Cross-talk between gibberellin and auxin in development of Populus wood: Gibberellin stimulates polar auxin transport and has a common transcriptome with auxin. Plant J. 2007, 52, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Klintborg, A.; Eklund, L.; Little, C.A. Ethylene metabolism in Scots pine (Pinus sylvestris) shoots during the year. Tree Physiol. 2002, 22, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Muday, G.K.; Rahman, A.; Binder, B.M. Auxin and ethylene: Collaborators or competitors? Trends Plant Sci. 2012, 17, 181–195. [Google Scholar] [CrossRef]
- Hellgren, J.M. Ethylene and auxin in the control of wood formation. Ph.D. Thesis, Department of Forest, Genetics and Plant Physiology, Swedish University of Agricultural Sciences, Umea, Sweden, 2003. [Google Scholar]
- Junghans, U.; Langenfeld-Heyser, R.; Polle, A.; Teichmann, T. Effect of auxin transport inhibitors and ethylene on the wood anatomy of poplar. Plant Biol. 2004, 6, 22–29. [Google Scholar]
- Thomashow, M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N. Hormone signaling pathways under stress combinations. Plant Signal. Behav. 2016, 11, e1247139. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N.; Bassil, E.; Hamilton, J.S.; Inupakutika, M.A.; Zandalinas, S.I.; Tripathy, D.; Luo, Y.; Dion, E.; Fukui, G.; Kumazaki, A.; et al. ABA is required for plant acclimation to a combination of salt and heat stress. PLoS ONE 2016, 11, e0147625. [Google Scholar] [CrossRef] [Green Version]
- Baba, K.; Karlberg, A.; Schmidt, J.; Schrader, J.; Hvidsten, T.R.; Bako, L.; Bhalerao, R.P. Activity-dormancy transition in the cambial meristem involves stage-specific modulation of auxin response in hybrid aspen. Proc. Natl. Acad. Sci. USA 2011, 108, 3418–3423. [Google Scholar] [CrossRef] [Green Version]
- Hou, H.W.; Zhou, Y.T.; Mwange, K.N.; Li, W.F.; He, X.Q.; Cui, K.M. ABP1 expression regulated by IAA and ABA is associated with the cambium periodicity in Eucommia ulmoides Oliv. J. Exp. Bot. 2006, 57, 3857–3867. [Google Scholar] [CrossRef] [Green Version]
- Hansen, H.; Grossmann, K. Auxin-induced ethylene triggers abscisic acid biosynthesis and growth inhibition. Plant Physiol. 2000, 124, 1437–1448. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.H.; Xu, L.L.; Tong, Z.K.; Lin, E.P.; Liu, Q.P.; Cheng, L.J.; Zhu, M.Y. De novo characterization of the Chinese fir (Cunninghamia lanceolata) transcriptome and analysis of candidate genes involved in cellulose and lignin biosynthesis. BMC Genom. 2012, 13, 648. [Google Scholar] [CrossRef]
- Takei, K.; Yamaya, T.; Sakakibara, H. Arabidopsis CYP735A1 and CYP735A2 Encode Cytokinin Hydroxylases That Catalyze the Biosynthesis of trans-Zeatin. J. Biol. Chem. 2004, 279, 41866–41872. [Google Scholar] [CrossRef] [Green Version]
- Frebort, I.; Kowalska, M.; Hluska, T.; Frebortova, J.; Galuszka, P. Evolution of cytokinin biosynthesis and degradation. J. Exp. Bot. 2011, 62, 2431–2452. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Sheen, J.; Muller, B. Cytokinin signaling networks. Annu. Rev. Plant Biol. 2012, 63, 353–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanov, G.A.; Lomin, S.N.; Schmulling, T. Cytokinin signaling: From the ER or from the PM? That is the question! New Phytol. 2018, 218, 41–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helliwell, C.A.; Sheldon, C.C.; Olive, M.R.; Walker, A.R.; Zeevaart, J.A.; Peacock, W.J.; Dennis, E.S. Cloning of the Arabidopsis ent-kaurene oxidase gene GA3. Proc. Natl. Acad. Sci. USA 1998, 95, 9019–9024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef]
- Daviere, J.M.; Achard, P. Gibberellin signaling in plants. Development 2013, 140, 1147–1151. [Google Scholar] [CrossRef] [Green Version]
- Dong, T.; Park, Y.; Hwang, I. Abscisic acid: Biosynthesis, inactivation, homoeostasis and signalling. Essays Biochem. 2015, 58, 29–48. [Google Scholar] [PubMed]
- Raghavendra, A.S.; Gonugunta, V.K.; Christmann, A.; Grill, E. ABA perception and signalling. Trends Plant Sci. 2010, 15, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Etchells, J.P.; Turner, S.R. The PXY-CLE41 receptor ligand pair defines a multifunctional pathway that controls the rate and orientation of vascular cell division. Development 2010, 137, 767–774. [Google Scholar] [CrossRef]
- Qiang, Y.; Wu, J.; Han, H.; Wang, G. CLE peptides in vascular development. J Integr Plant Biol. 2013, 55, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Dolzblasz, A.; Nardmann, J.; Clerici, E.; Causier, B.; van der Graaff, E.; Chen, J.; Davies, B.; Werr, W.; Laux, T. Stem Cell Regulation by Arabidopsis WOX Genes. Mol. Plant 2016, 9, 1028–1039. [Google Scholar] [CrossRef]
- Sieburth, L.E.; Deyholos, M.K. Vascular development: The long and winding road. Curr. Opin. Plant Biol. 2006, 9, 48–54. [Google Scholar] [CrossRef]
- Baima, S.; Possenti, M.; Matteucci, A.; Wisman, E.; Altamura, M.M.; Ruberti, I.; Morelli, G. The arabidopsis ATHB-8 HD-zip protein acts as a differentiation-promoting transcription factor of the vascular meristems. Plant Physiol. 2001, 126, 643–655. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Craig, J.C.; Petzold, H.E.; Dickerman, A.W.; Beers, E.P. The xylem and phloem transcriptomes from secondary tissues of the Arabidopsis root-hypocotyl. Plant Physiol. 2005, 138, 803–818. [Google Scholar] [CrossRef] [Green Version]
- Ilegems, M.; Douet, V.; Meylan-Bettex, M.; Uyttewaal, M.; Brand, L.; Bowman, J.L.; Stieger, P.A. Interplay of auxin, KANADI and Class III HD-ZIP transcription factors in vascular tissue formation. Development 2010, 137, 975–984. [Google Scholar] [CrossRef]




| Hormone | Variance Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|---|
| IAA | Treatments | 7425.00 | 3 | 2475.00 | 1934.00 | <0.001 |
| Treatment stages | 22,568.00 | 6 | 3761.00 | 2940.00 | <0.001 | |
| Interaction | 25,613.00 | 18 | 1423.00 | 1112.00 | <0.001 | |
| Error | 72 | 56 | 1.29 | |||
| Total | ||||||
| ZR | Treatments | 659.00 | 3 | 219.80 | 5261.00 | <0.001 |
| Treatment stages | 2375.00 | 6 | 395.90 | 9474.00 | <0.001 | |
| Interaction | 4638.00 | 18 | 257.70 | 6167.00 | <0.001 | |
| Error | 2.00 | 56 | 0.04 | |||
| Total | ||||||
| GA3 | Treatments | 23.50 | 3 | 7.84 | 319.60 | <0.001 |
| Treatment stages | 548.10 | 6 | 91.35 | 3722.00 | <0.001 | |
| Interaction | 836.50 | 18 | 46.47 | 1893.60 | <0.001 | |
| Error | 1.40 | 56 | 0.02 | |||
| Total | ||||||
| ABA | Treatments | 53,660.00 | 3 | 17,887.00 | 2192.20 | <0.001 |
| Treatment stages | 67,108.00 | 6 | 11,185.00 | 1370.80 | <0.001 | |
| Interaction | 53,403.00 | 18 | 2967.00 | 363.60 | <0.001 | |
| Error | 457.00 | 56 | 8.00 | |||
| Total |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Zhu, S. The Interconnected Relationship between Auxin Concentration Gradient Changes in Chinese Fir Radial Stems and Dynamic Cambial Activity. Forests 2022, 13, 1698. https://doi.org/10.3390/f13101698
Yang L, Zhu S. The Interconnected Relationship between Auxin Concentration Gradient Changes in Chinese Fir Radial Stems and Dynamic Cambial Activity. Forests. 2022; 13(10):1698. https://doi.org/10.3390/f13101698
Chicago/Turabian StyleYang, Liwei, and Sheng Zhu. 2022. "The Interconnected Relationship between Auxin Concentration Gradient Changes in Chinese Fir Radial Stems and Dynamic Cambial Activity" Forests 13, no. 10: 1698. https://doi.org/10.3390/f13101698
APA StyleYang, L., & Zhu, S. (2022). The Interconnected Relationship between Auxin Concentration Gradient Changes in Chinese Fir Radial Stems and Dynamic Cambial Activity. Forests, 13(10), 1698. https://doi.org/10.3390/f13101698





