Influence of Water Limitation and Provenance on Reproductive Traits in a Common Garden of Frangula alnus Mill.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Application of Water Limitation
2.2. Measurements, Counts and Phenological Observations
2.3. Statistical Analysis
3. Results
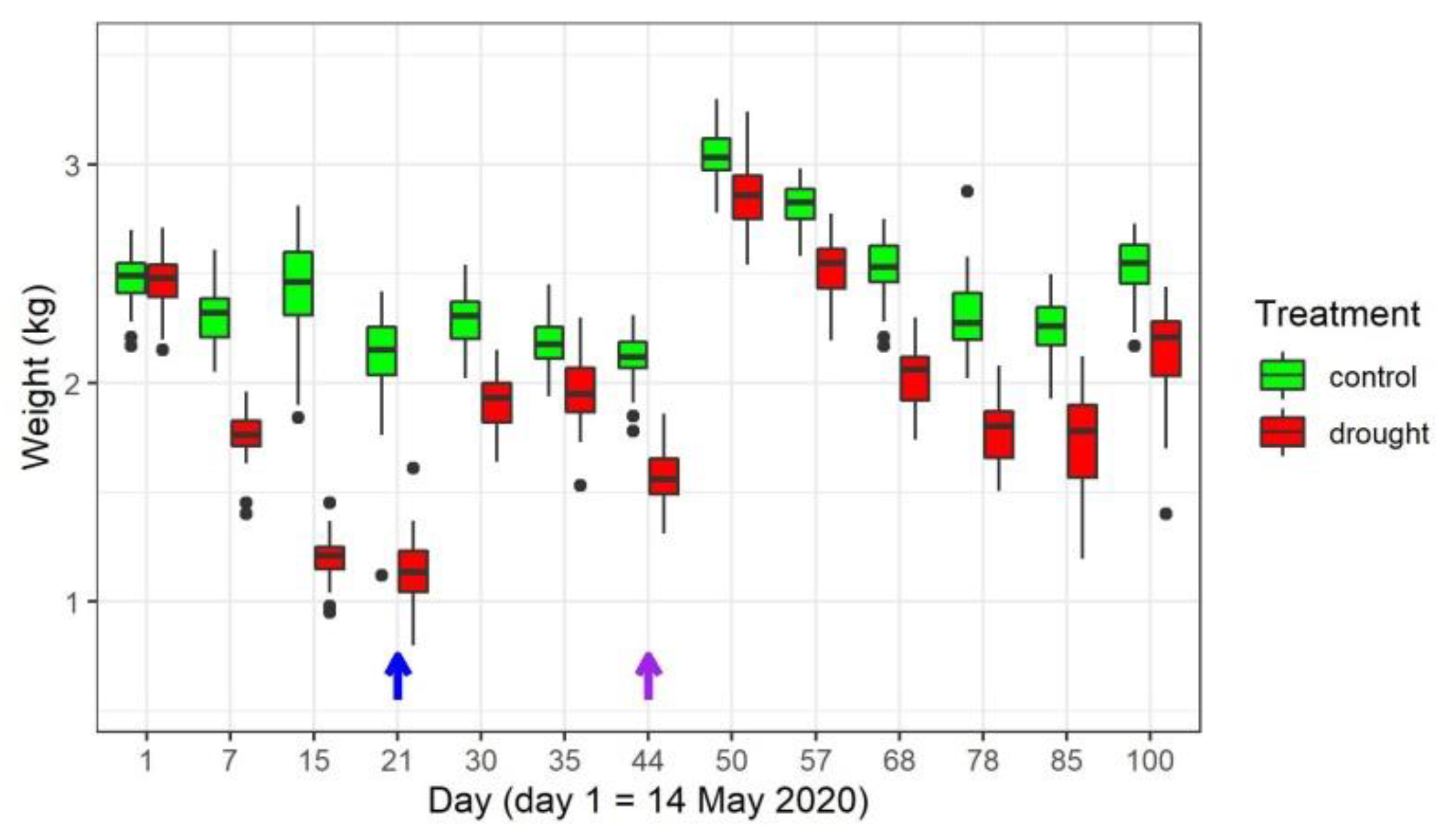
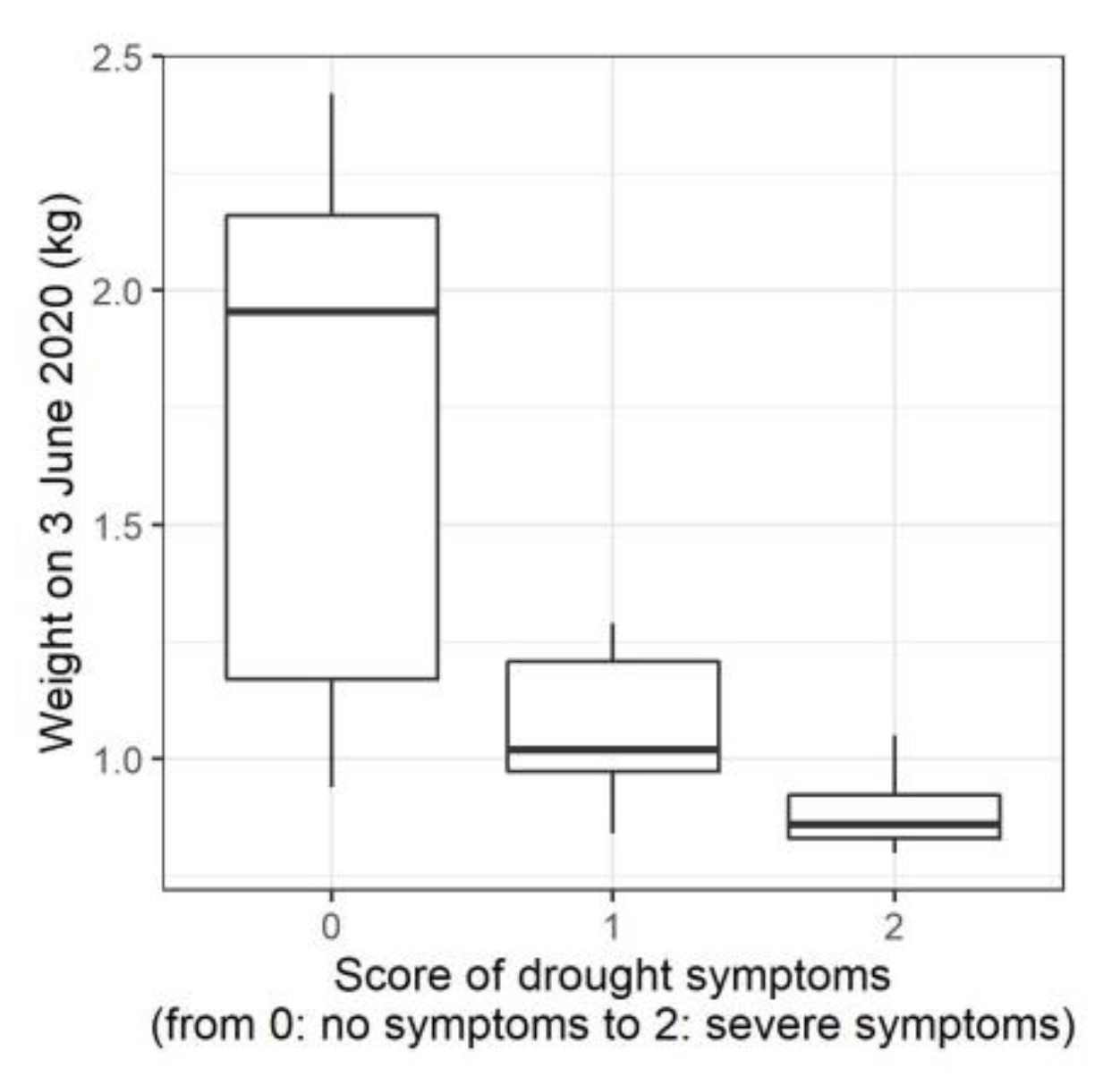
3.1. Water Limiting Treatment and Stress Symptoms
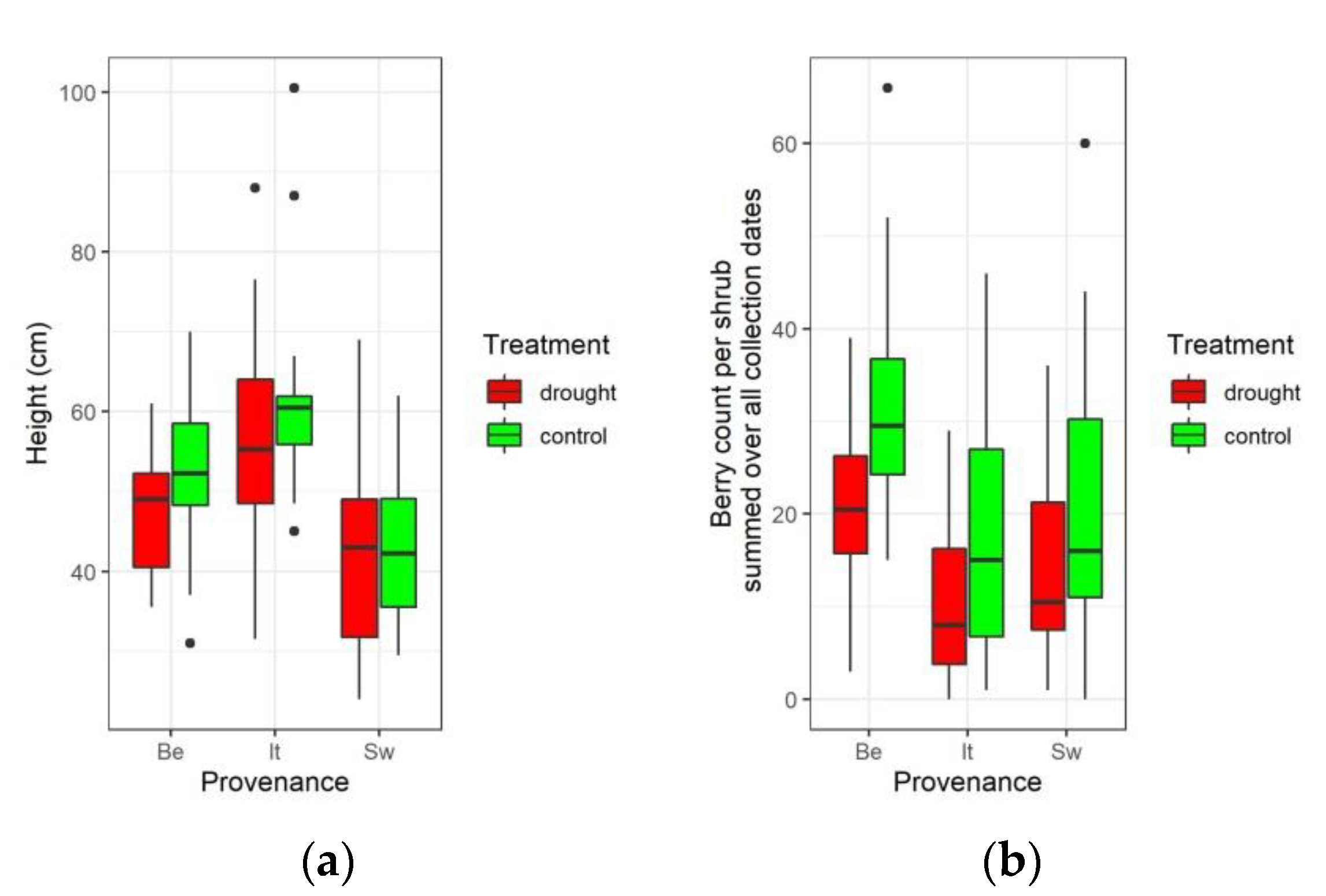
3.2. Influence of Water Limitation on Vegetative Traits
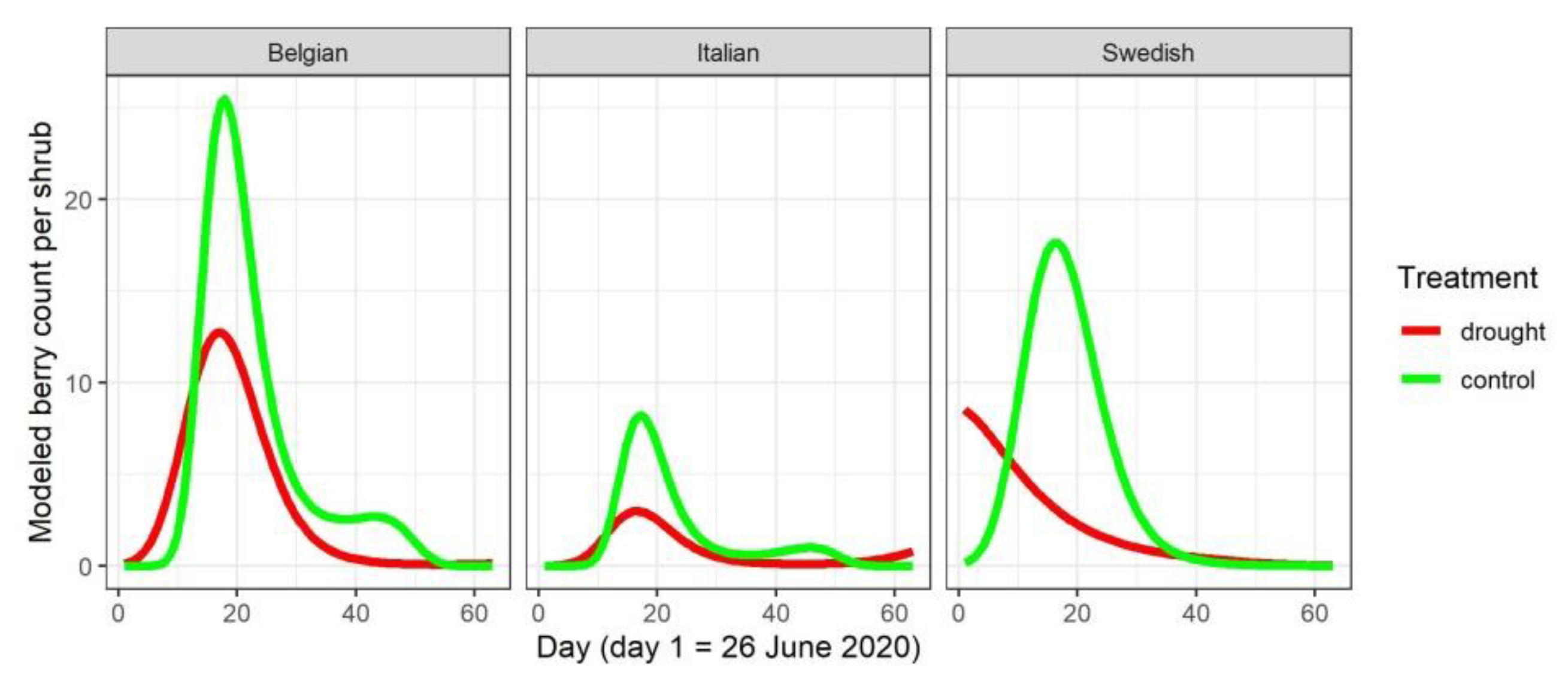
3.3. Influence of Water Limitation on Mature Berry Counts
3.4. Influence of Water Limitation on Stone Traits
4. Discussion
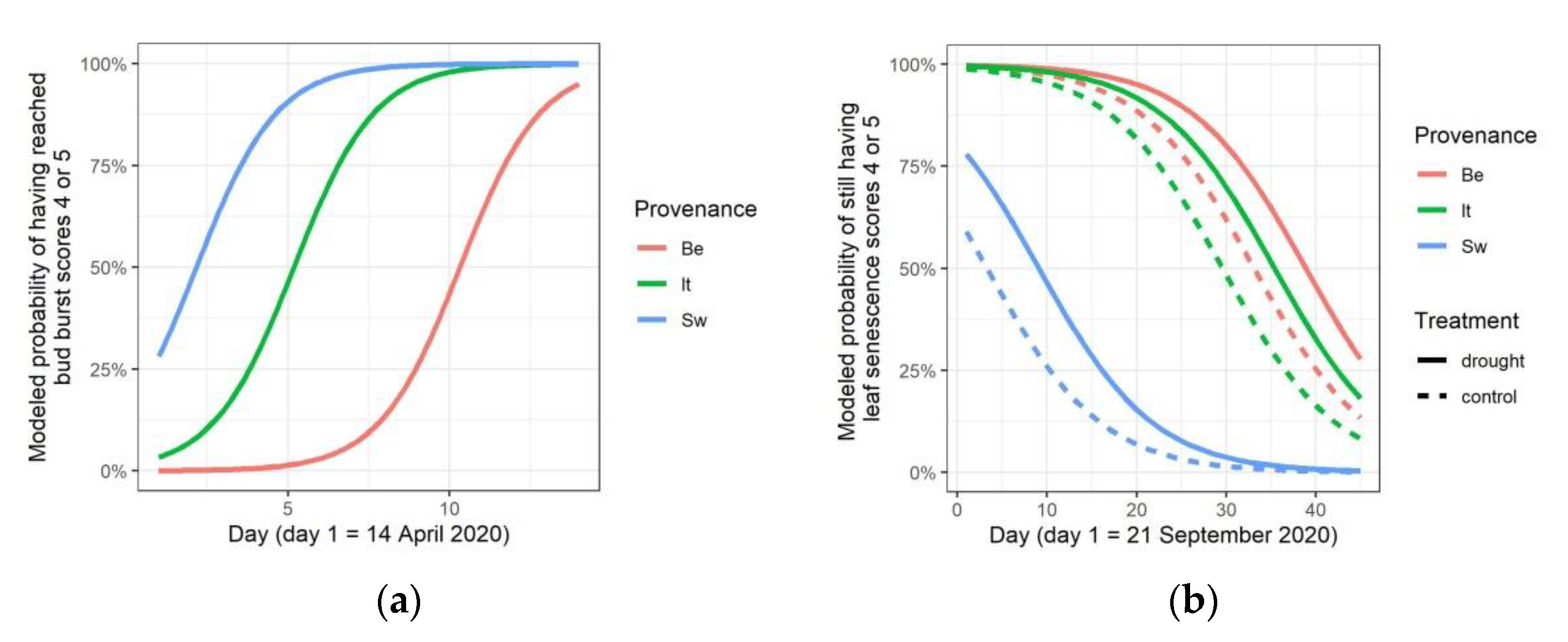
4.1. Drought Escape and Compensation Growth
4.2. Drought Avoidance and Trade-Off between Growth and Reproduction
4.3. Stone Traits Independent of Water Limitation and Size/Number Trade-Off
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trenberth, K.E. Changes in precipitation with climate change. Clim. Res. 2011, 47, 123–138. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.S.; Running, S.W. Drought-Induced Reduction in Global Terrestrial Net Primary Production from 2000 Through 2009. Science 2010, 329, 940–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, J.S.; Iverson, L.; Woodall, C.W.; Allen, C.D.; Bell, D.M.; Bragg, D.C.; D’Amato, A.W.; Davis, F.W.; Hersh, M.H.; Ibanez, I.; et al. The impacts of increasing drought on forest dynamics, structure, and biodiversity in the United States. Glob. Chang. Biol. 2016, 22, 2329–2352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hampe, A.; Petit, R.J. Conserving biodiversity under climate change: The rear edge matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Orangeville, L.; Maxwell, J.; Kneeshaw, D.; Pederson, N.; Duchesne, L.; Logan, T.; Houle, D.; Arseneault, D.; Beier, C.M.; Bishop, D.A.; et al. Drought timing and local climate determine the sensitivity of eastern temperate forests to drought. Glob. Chang. Biol. 2018, 24, 2339–2351. [Google Scholar] [CrossRef] [PubMed]
- Breda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef] [Green Version]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolstrom, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Loarie, S.R.; Duffy, P.B.; Hamilton, H.; Asner, G.P.; Field, C.B.; Ackerly, D.D. The velocity of climate change. Nature 2009, 462, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; Wang, T.L.; Curtis-McLane, S. Adaptation, migration or extirpation: Climate change outcomes for tree populations. Evol. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Moran, E.; Lauder, J.; Musser, C.; Stathos, A.; Shu, M. The genetics of drought tolerance in conifers. New Phytol. 2017, 216, 1034–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberto, F.; Bouffier, L.; Louvet, J.M.; Lamy, J.B.; Delzon, S.; Kremer, A. Adaptive responses for seed and leaf phenology in natural populations of sessile oak along an altitudinal gradient. J. Evol. Biol. 2011, 24, 1442–1454. [Google Scholar] [CrossRef] [PubMed]
- Sáenz-Romero, C.; Lamy, J.-B.; Ducousso, A.; Musch, B.; Ehrenmann, F.; Delzon, S.; Cavers, S.; Chałupka, W.; Dağdaş, S.; Hansen, J.K.; et al. Adaptive and plastic responses of Quercus petraea populations to climate across Europe. Glob. Chang. Biol. 2017, 23, 2831–2847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberto, F.J.; Aitken, S.N.; Alia, R.; Gonzalez-Martinez, S.C.; Hanninen, H.; Kremer, A.; Lefevre, F.; Lenormand, T.; Yeaman, S.; Whetten, R.; et al. Potential for evolutionary responses to climate change—evidence from tree populations. Glob. Chang. Biol. 2013, 19, 1645–1661. [Google Scholar] [CrossRef] [Green Version]
- Zas, R.; Sampedro, L.; Solla, A.; Vivas, M.; Lombardero, M.J.; Alía, R.; Rozas, V. Dendroecology in common gardens: Population differentiation and plasticity in resistance, recovery and resilience to extreme drought events in Pinus pinaster. Agric. For. Meteorol. 2020, 291, 108060. [Google Scholar] [CrossRef]
- Bruschi, P. Geographical variation in morphology of Quercus petraea (Matt.) Liebl. as related to drought stress. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2010, 144, 298–307. [Google Scholar] [CrossRef]
- Rose, L.; Leuschner, C.; Köckemann, B.; Buschmann, H. Are marginal beech (Fagus sylvatica L.) provenances a source for drought tolerant ecotypes? Eur. J. For. Res. 2009, 128, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Arend, M.; Kuster, T.; Gunthardt-Goerg, M.S.; Dobbertin, M. Provenance-specific growth responses to drought and air warming in three European oak species (Quercus robur, Q. petraea and Q. pubescens). Tree Physiol. 2011, 31, 287–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depardieu, C.; Girardin, M.P.; Nadeau, S.; Lenz, P.; Bousquet, J.; Isabel, N. Adaptive genetic variation to drought in a widely distributed conifer suggests a potential for increasing forest resilience in a drying climate. New Phytol. 2020, 227, 427–439. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Camarero, J.J.; Rozas, V.; Génova, M.; Olano, J.M.; Arzac, A.; Gazol, A.; Caminero, L.; Tejedor, E.; de Luis, M.; et al. Resist, recover or both? Growth plasticity in response to drought is geographically structured and linked to intraspecific variability in Pinus pinaster. J. Biogeogr. 2018, 45, 1126–1139. [Google Scholar] [CrossRef]
- Vander Mijnsbrugge, K.; De Clerck, L.; Van der Schueren, N.; Moreels, S.; Lauwers, A.; Steppe, K.; De Ligne, L.; Campioli, M.; Van den Bulcke, J. Counter-Intuitive Response to Water Limitation in a Southern European Provenance of Frangula alnus Mill. in a Common Garden Experiment. Forests 2020, 11, 1186. [Google Scholar] [CrossRef]
- Büsgen, M.; Münch, E.; Thomson, T. The structure and life of forest trees; Chapman and Hall: London, UK, 1929. [Google Scholar]
- Hacket-Pain, A.; Bogdziewicz, M. Climate change and plant reproduction: Trends and drivers of mast seeding change. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20200379. [Google Scholar] [CrossRef] [PubMed]
- Pearse, I.S.; Koenig, W.D.; Kelly, D. Mechanisms of mast seeding: Resources, weather, cues, and selection. New Phytol. 2016, 212, 546–562. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramos, I.M.; Ourcival, J.M.; Limousin, J.M.; Rambal, S. Mast seeding under increasing drought: Results from a long-term data set and from a rainfall exclusion experiment. Ecology 2010, 91, 3057–3068. [Google Scholar] [CrossRef] [Green Version]
- Bogdziewicz, M.; Fernández-Martínez, M.; Bonal, R.; Belmonte, J.; Espelta, J.M. The Moran effect and environmental vetoes: Phenological synchrony and drought drive seed production in a Mediterranean oak. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauder, J.D.; Moran, E.V.; Hart, S.C. Fight or flight? Potential tradeoffs between drought defense and reproduction in conifers. Tree Physiol. 2019, 39, 1071–1085. [Google Scholar] [CrossRef]
- Hacket-Pain, A.J.; Lageard, J.G.A.; Thomas, P.A. Drought and reproductive effort interact to control growth of a temperate broadleaved tree species (Fagus sylvatica). Tree Physiol. 2017, 37, 744–754. [Google Scholar] [CrossRef]
- Bogdziewicz, M.; Fernández-Martínez, M.; Espelta, J.M.; Ogaya, R.; Penuelas, J. Is forest fecundity resistant to drought? Results from an 18-yr rainfall-reduction experiment. New Phytol. 2020, 227, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Zecchin, B.; Caudullo, G.; de Rigo, D. Frangula alnus in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; Publication Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Vander Mijnsbrugge, K.; Turcsán, A.; Michiels, B. Population differentiation and phenotypic plasticity in temperature response of bud burst in Frangula alnus provenances of different latitude. Plant Syst. Evol. 2016, 302, 257–264. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Caudullo, G.; Welk, E.; San-Miguel-Ayanz, J. Chorological data for the main European woody species. Mendeley Data V14 2021. [Google Scholar] [CrossRef]
- R_Core_Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Bates, D.; Machler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. nlme: Linear and Nonlinear Mixed Effects Models, R package version 3.1-113; R Development Core Team: Vienna, Austria, 2013. [Google Scholar]
- Christensen, R.H.B. Ordinal: Regression Models for Ordinal Data. R package version 2015.6-28. 2015. Available online: http://www.cran.r-project.org/package=ordinal/ (accessed on 25 September 2019).
- Shavrukov, Y.; Kurishbayev, A.; Jatayev, S.; Shvidchenko, V.; Zotova, L.; Koekemoer, F.; de Groot, S.; Soole, K.; Langridge, P. Early Flowering as a Drought Escape Mechanism in Plants: How Can It Aid Wheat Production? Front. Plant Sci. 2017, 8, 1950. [Google Scholar] [CrossRef] [PubMed]
- Kooyers, N.J. The evolution of drought escape and avoidance in natural herbaceous populations. Plant Sci. 2015, 234, 155–162. [Google Scholar] [CrossRef]
- Marchin, R.; Zeng, H.; Hoffmann, W. Drought-deciduous behavior reduces nutrient losses from temperate deciduous trees under severe drought. Oecologia 2010, 163, 845–854. [Google Scholar] [CrossRef]
- Vander Mijnsbrugge, K.; Turcsan, A.; Maes, J.; Duchene, N.; Meeus, S.; Steppe, K.; Steenackers, M. Repeated Summer Drought and Re-watering during the First Growing Year of Oak (Quercus petraea) Delay Autumn Senescence and Bud Burst in the Following Spring. Front. Plant Sci. 2016, 7, 419. [Google Scholar] [CrossRef] [PubMed]
- Pflug, E.E.; Buchmann, N.; Siegwolf, R.T.W.; Schaub, M.; Rigling, A.; Arend, M. Resilient Leaf Physiological Response of European Beech (Fagus sylvatica L.) to Summer Drought and Drought Release. Front. Plant Sci. 2018, 9, 187. [Google Scholar] [CrossRef] [Green Version]
- Rice, K.J.; Knapp, E.E. Effects of Competition and Life History Stage on the Expression of Local Adaptation in Two Native Bunchgrasses. Restor. Ecol. 2008, 16, 12–23. [Google Scholar] [CrossRef]
- Stöcklin, J.; Kuss, P.; Pluess, A.R. Genetic diversity, phenotypic variation and local adaptation in the alpine landscape: Case studies with alpine plant species. Bot. Helv. 2009, 119, 125–133. [Google Scholar] [CrossRef]
- Lázaro, A.; Larrinaga, A.R. A multi-level test of the seed number/size trade-off in two Scandinavian communities. PLoS ONE 2018, 13, e0201175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shipley, B.; Dion, J. The Allometry of Seed Production in Herbaceous Angiosperms. Am. Nat. 1992, 139, 467–483. [Google Scholar] [CrossRef]
- Siemens, D.H.; Johnson, C.D. Number of Seeds per Pod in Three Species of Perennial Legumes: A Compromise between Ecological and Physiological Constraints? Am. Midl. Nat. 1995, 133, 197–205. [Google Scholar] [CrossRef]


| Bud Burst | Leaf Senescence | |||
|---|---|---|---|---|
| Variable | Estimate | p-Value | Estimate | p-Value |
| It | −4.12 | 0.017 * | 0.55 | 0.308 |
| Sw | −6.54 | <0.001 *** | 4.66 | <0.001 *** |
| D | −0.80 | <0.001 *** | 0.16 | <0.001 *** |
| T | 0.91 | <0.001 *** | ||
| Height Growth | Berry Count | |||
|---|---|---|---|---|
| Estimate | p-Value | Estimate | p-Value | |
| (Intercept) | 48.34 | <0.001 *** | 3.42 | <0.001 *** |
| It | 9.16 | 0.030 * | −0.97 | 0.006 ** |
| Sw | −7.30 | 0.055 | −0.62 | 0.049 * |
| T | 3.57 | 0.039.* | −0.51 | 0.001 ** |
| It:T | −0.08 | 0.733 | ||
| Sw:T | 0.15 | 0.482 | ||
| Belgian Provenance | Italian Provenance | Swedish Provenance | ||||
|---|---|---|---|---|---|---|
| Variable | Estimate | p-Value | Estimate | p-Value | Estimate | p-Value |
| (Intercept) | −0.11 | 0.473 | −0.32 | 0.139 | −1.00 | 0.016 * |
| D1 | −19.38 | <0.001 *** | −23.76 | <0.001 *** | −2.83 | 0.237 |
| D2 | −10.62 | <0.001 *** | −3.33 | 0.177 | −5.54 | 0.007 ** |
| D3 | 13.96 | <0.001 *** | −1.85 | 0.361 | 17.68 | <0.001 *** |
| D4 | −1.80 | 0.333 | −1.23 | 0.477 | −5.97 | 0.004 ** |
| T | −0.74 | 0.003 ** | −0.06 | 0.795 | −1.14 | <0.001 *** |
| D1:T | 9.19 | 0.060 | −6.24 | 0.245 | −5.24 | 0.331 |
| D2:T | −43.99 | <0.001 *** | −13.02 | 0.003 ** | −50.63 | <0.001 *** |
| D3:T | −6.72 | 0.013 * | 12.90 | <0.001 *** | −10.55 | 0.001 ** |
| D4:T | −26.38 | <0.001 *** | −1.77 | 0.493 | −27.19 | <0.001 *** |
| Stone Count Per Berry | Stone Weight | |||
|---|---|---|---|---|
| Variable | Estimate | p-Value | Estimate | p-Value |
| (Intercept) | 2.10 | <0.001 *** | 0.0175 | <0.001 *** |
| It | 0.06 | 0.2607 | 0.0033 | 0.003 ** |
| Sw | 0.15 | 0.003 ** | 0.0022 | 0.020 * |
| T | −0.04 | 0.2129 | 0.0002 | 0.628 |
| D1 | 1.04 | <0.001 *** | −0.0078 | 0.038 * |
| D2 | −0.97 | 0.001 ** | −0.0243 | <0.001 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vander Mijnsbrugge, K.; Schouppe, M.; Moreels, S.; Aguas Guerreiro, Y.; Decorte, L.; Stessens, M. Influence of Water Limitation and Provenance on Reproductive Traits in a Common Garden of Frangula alnus Mill. Forests 2022, 13, 1744. https://doi.org/10.3390/f13111744
Vander Mijnsbrugge K, Schouppe M, Moreels S, Aguas Guerreiro Y, Decorte L, Stessens M. Influence of Water Limitation and Provenance on Reproductive Traits in a Common Garden of Frangula alnus Mill. Forests. 2022; 13(11):1744. https://doi.org/10.3390/f13111744
Chicago/Turabian StyleVander Mijnsbrugge, Kristine, Marc Schouppe, Stefaan Moreels, Yorrick Aguas Guerreiro, Laura Decorte, and Marie Stessens. 2022. "Influence of Water Limitation and Provenance on Reproductive Traits in a Common Garden of Frangula alnus Mill." Forests 13, no. 11: 1744. https://doi.org/10.3390/f13111744





