A Comparison of Soil C, N, and P Stoichiometry Characteristics under Different Thinning Intensities in a Subtropical Moso bamboo (Phyllostachys edulis) Forest of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Soil Sampling
- No thinning (NT, 3 plots): no management activities were carried out after the abandonment of management.
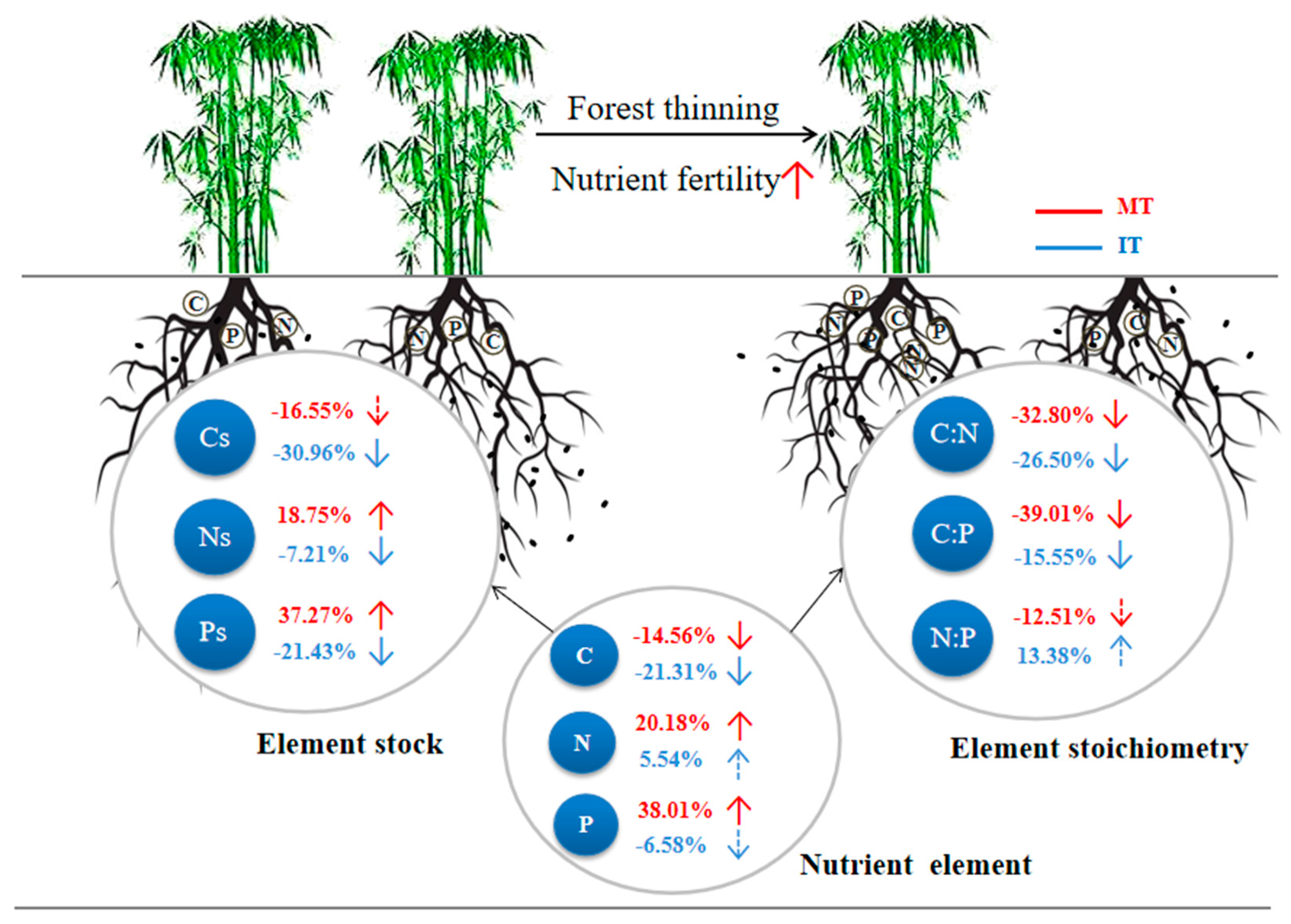
- Moderate thinning (MT, 3 plots): In 2017, 2018, and 2019, about 15% of stem numbers of mature Moso bamboo were removed by selective cutting each year.
- Intensive thinning (IT, 3 plots): In 2017, 2018, and 2019, about 33% of stem numbers of mature Moso bamboo were removed by selective cutting each year.
2.3. Calculation of Soil Bulk Density and Porosity
2.4. Calculation of SOC, TN, and TP Stocks
2.5. Statistical Analyses
3. Results
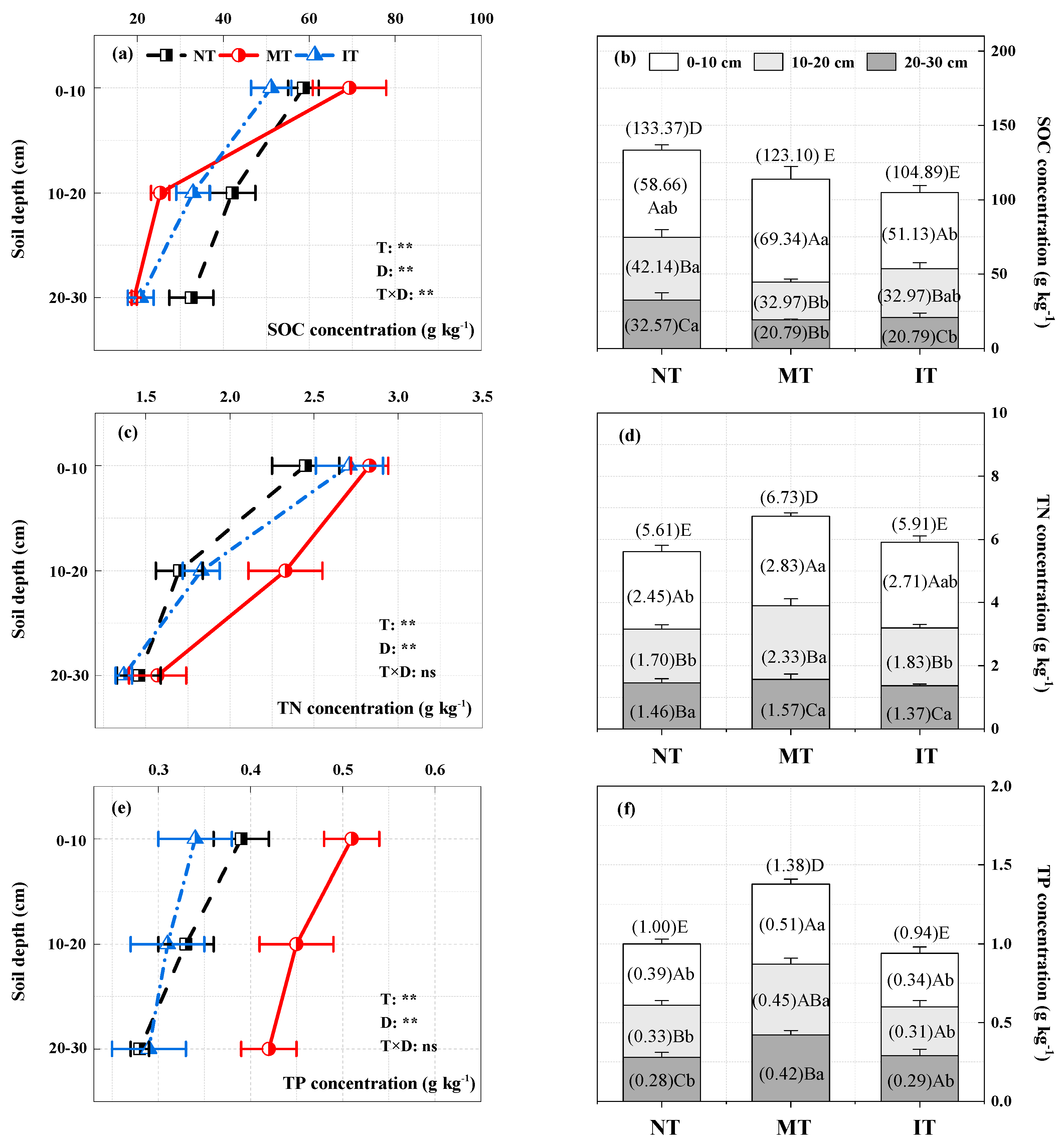
3.1. Impacts of Thinning on Soil Physical and Chemical Properties
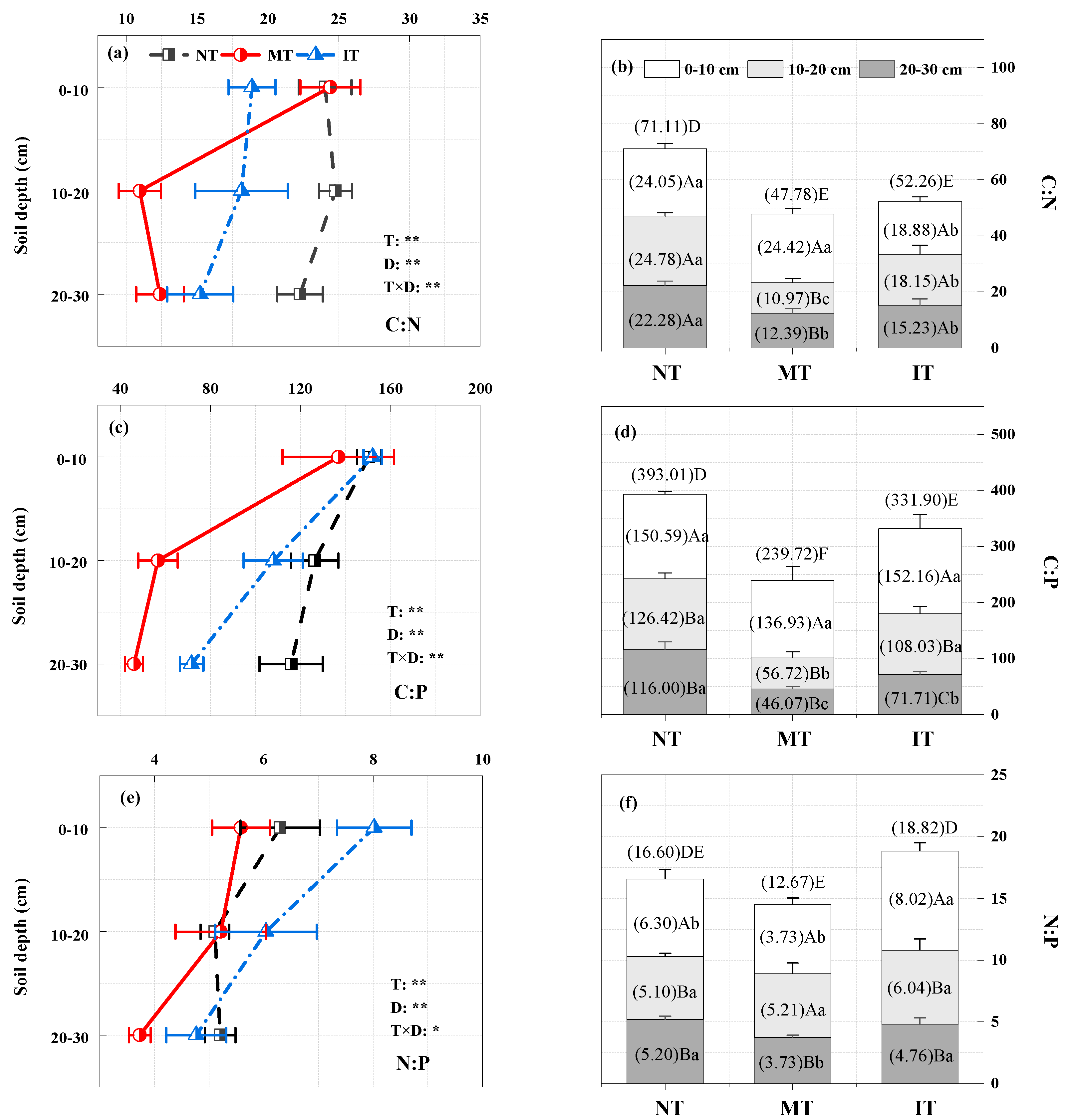
3.2. Soil C, N, and P Stocks and Stoichiometric Characteristics under Thinning
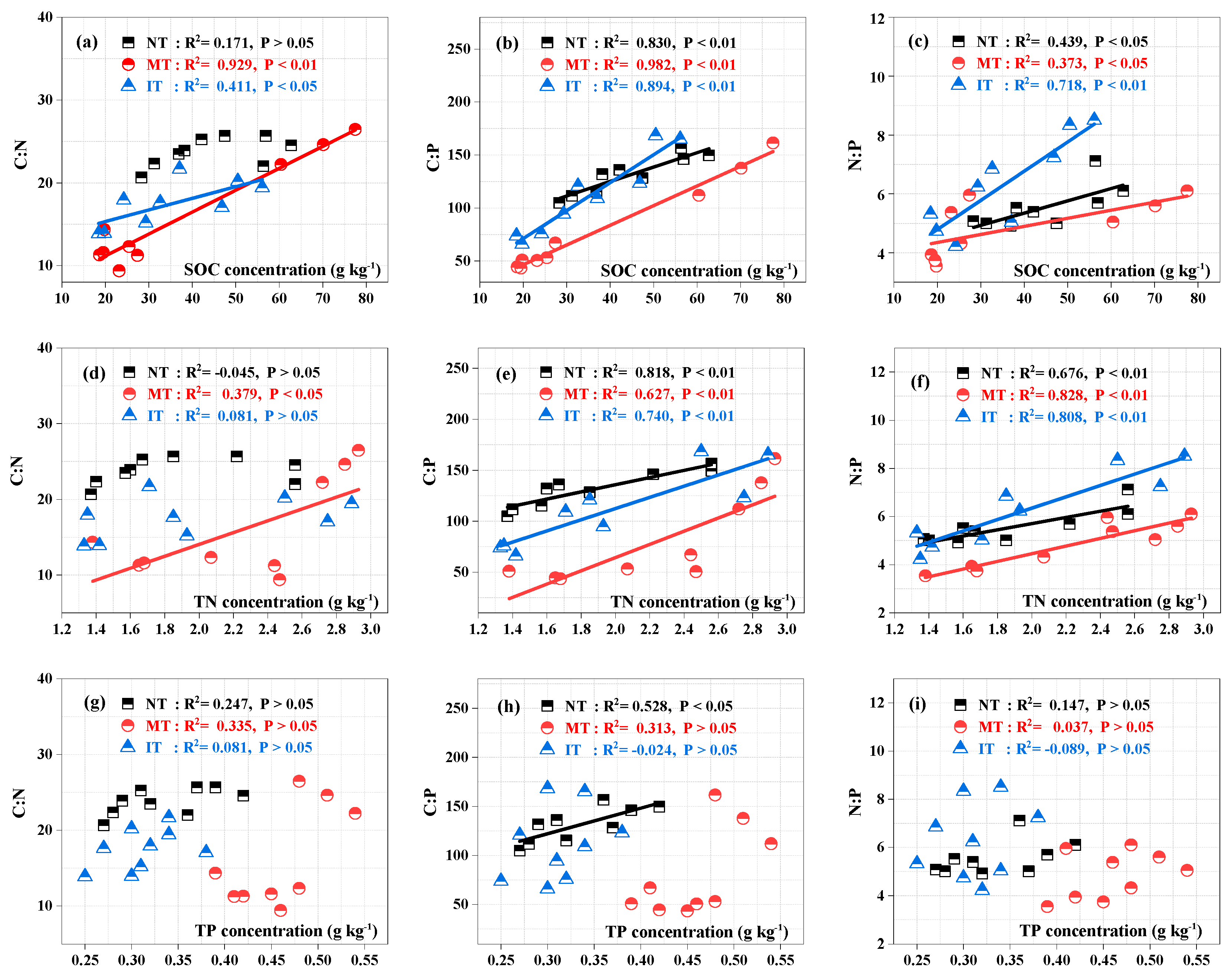
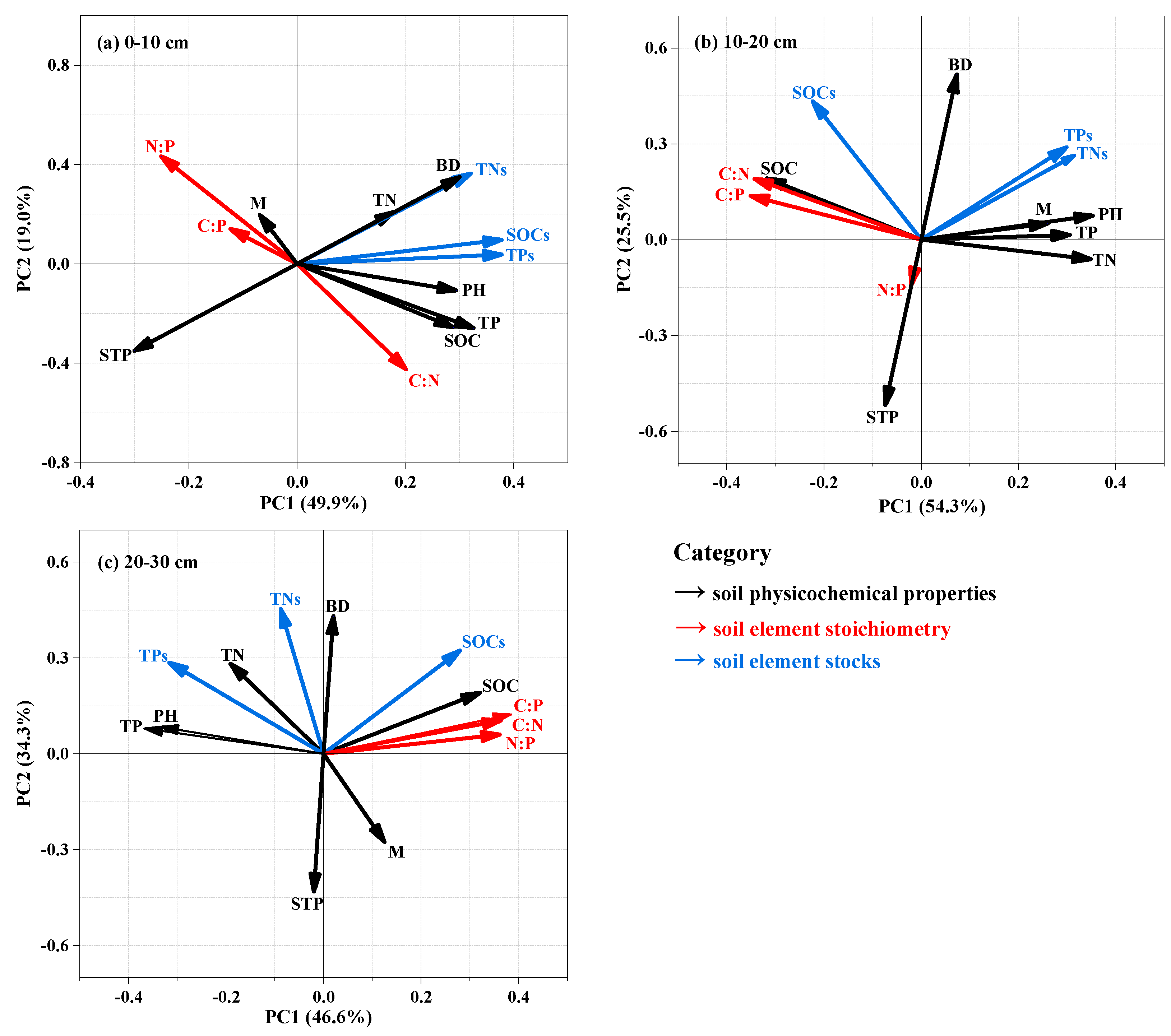
3.3. Relationships among Nutrient Stocks, Stoichiometric Ratios, and Soil Physicochemical Properties
4. Discussion
4.1. Effects of Thinning Intensity on Soil C, N, and P Concentrations and Stocks
4.2. Effects of Thinning on Soil C:N, C:P, and N:P Stoichiometry
4.3. Changes in Soil C, N, and P Stocks and Stoichiometric Characteristics with Soil Depth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elser, J.; Sterner, R.; Gorokhova, E.; Fagan, W.; Markow, T.; Cotner, J.; Harrison, J.; Hobbie, S.; Odell, G.; Weider, L. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 2000, 3, 540–550. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Chen, G.; Zhang, C.; Melillo, J.M.; Hall, C.A.S. Pattern and variation of C:N:P ratios in China’s soils: A synthesis of observational data. Biogeochemistry 2009, 98, 139–151. [Google Scholar] [CrossRef]
- Bai, Y.F.; Chen, S.Y.; Shi, S.R.; Qi, M.J.; Liu, X.H.; Wang, H.; Wang, Y.X.; Jiang, C.Q. Effects of different management approaches on the stoichiometric characteristics of soil C, N, and P in a mature Chinese fir plantation. Sci. Total Environ. 2020, 723, 137868. [Google Scholar] [CrossRef]
- Wang, Q.K.; Yu, Y.Z.; He, T.X.; Wang, Y.P. Aboveground and belowground litter have equal contributions to soil CO2 emission: An evidence from a 4-year measurement in a subtropical forest. Plant Soil 2017, 421, 7–17. [Google Scholar] [CrossRef]
- Wang, Y.L.; Song, C.S.; Peng, L.H.; Cui, C.W.; Zheng, M.M.; Lin, K.M.; Ren, Z.B.; Xie, R.G.; Hu, Y.Y. Effects of thinning on soil carbon and nitrogen factions in a Cunningham lanceolate plantation. J. Soil Water Conserv. 2021, 5, 9. [Google Scholar]
- Liu, L.; Cai, M.K.; Liu, X.J.; Han, H.R.; Cheng, X.Q.; Tian, H.X.; Guo, Q.L. Effects of thinning on ecological stoichiometry C, N and P in leaves, roots and soil of Larix principis-rupprechtii plantation. Chin. J. Northeast. For. Univ. 2019, 47, 1–7. [Google Scholar]
- Qiu, X.C.; Wang, H.B.; Peng, D.L.; Xin, L.; Yang, F.; Li, Z.; Cheng, S. Thinning drives C: N: P stoichiometry and nutrient resorption in Larix principis-rupprechtii plantations in North China. For. Ecol. Manag. 2020, 462, 117984. [Google Scholar] [CrossRef]
- Johnson, D.W.; Curtis, P.S. Effects of forest management on soil C and N storage: Meta analysis. For. Ecol. Manag. 2001, 140, 227–238. [Google Scholar] [CrossRef]
- Hu, B.; Yang, B.; Pang, X.; Bao, W.; Tian, G. Responses of soil phosphorus fractions to gap size in a reforested spruce forest. Geoderma 2016, 279, 61–69. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, C.K.; Zhou, Z.H. Thinning promotes the nitrogen and phosphorous cycling in forest soils. Agric. For. Meteorol. 2021, 311, 108665. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.S.; Geng, Y.; Niu, S.L.; Tian, D.S.; Yan, T.; Liu, W.G.; Pan, J.X.; Zhao, X.H.; Zhang, C.Y. Heavy thinning reduces soil organic carbon: Evidence from a 9-year thinning experiment in a pine plantation. Catena 2022, 211, 106013. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Guan, D.X.; Li, W.B.; Sun, D.; Jin, C.J.; Yuan, F.H.; Wang, A.Z.; Wu, J.B. The effects of forest thinning on soil carbon stocks and dynamics: A meta-analysis. For. Ecol. Manag. 2018, 429, 36–43. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, J.; Liu, H.M.; Huang, K.; Yang, Q.S.; Lu, R.L.; Yan, L.M.; Wang, X.H.; Xia, J.Y. Depth-dependent soil C-N-P stoichiometry in a mature subtropical broadleaf forest. Geoderma 2020, 370, 114357. [Google Scholar] [CrossRef]
- Kou, D.; Ma, W.; Ding, J.; Zhang, B.; Fang, K.; Hu, H.; Yu, J.; Wang, T.; Qin, S.; Zhao, X.; et al. Dryland soils in northern China sequester carbon during the early 2000s warming hiatus period. Funct. Ecol. 2018, 32, 1620–1630. [Google Scholar] [CrossRef]
- Goulding, K.W.T.; Bailey, N.J.; Bradbury, N.J.; Hargreaves, P.; Howe, M.; Murphy, D.V.; Poulton, P.R.; Willison, T.W. Nitrogen deposition and its contribution to nitrogen cycling and associated soil processes. New Phytol. 1998, 139, 49–58. [Google Scholar] [CrossRef]
- Manzoni, S.; Jackson, R.B.; Trofymow, J.A.; Porporato, A. The global stoichiometry of litter nitrogen mineralization. Science 2008, 321, 684. [Google Scholar] [CrossRef]
- Houlton, B.Z.; Morford, S.L.; Dahlgren, R.A. Convergent evidence for widespread rock nitrogen sources in Earth’s surface environment. Science 2018, 360, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Li, J.J.; Fan, M.C.; Shangguan, Z.P. Ecological stoichiometry characteristics of soil carbon, nitrogen, and phosphorus of the Robinia pseudoacacia forest on the north-south strip of the Loess Plateau. Chin. J. Acta Ecol. Sin. 2019, 39, 7996–8002. [Google Scholar]
- Wang, Y.X.; Bai, S.B.; Binkley, D.; Zhou, G.M.; Fang, F.Y. The independence of clonal shoot’s growth from light availability supports Moso bamboo invasion of closed-canopy forest. For. Ecol. Manag. 2016, 368, 105–110. [Google Scholar] [CrossRef]
- Zhou, G.M.; Jiang, P.K. Density, storage and spatial distribution of carbon in phyllostachy pubescens forest. Chin. J. Sci. Silvae Sin. 2004, 40, 20–24. [Google Scholar]
- Xu, L.; Deng, X.; Ying, J.Y.; Zhou, G.M.; Shi., Y.J. Silicate fertilizer application reduces soil greenhouse gas emissions in a Moso bamboo forest. Sci. Total Environ. 2020, 747, 141380. [Google Scholar] [CrossRef]
- Gu, H.H.; Weng, J.; Kong, J.J.; Ye, X.M.; Liu, Y.J.; Qi, L.H.; Song, X.Z. Ecological stoichiometry of Phullostachys edulis leaves with extensive and intensive management. Chin. J. Zhejiang AF Univ. 2015, 32, 661–667. [Google Scholar]
- State Forestry Administration, PR China (SFAPRC). Forest Soil Analysis Methods in China; State Forestry Administration, PR China (SFAPRC): Beijing, China, 2000; p. 334. [Google Scholar]
- Bao, S.D. Soil Analysis in Agricultural Chemistry, 3rd ed.; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta analysis. Glob. Change Biol. 2010, 8, 345–360. [Google Scholar] [CrossRef]
- Bauer, A.; Black, A.L. Quantification of the effect of soil organic matter content on soil productivity. Soil Sci. Soc. Am. J. 1994, 58, 185. [Google Scholar] [CrossRef]
- Doran, J.W.; Coleman, D.C.; Bezdicek, D.F. Defining Soil Quality for a Sustainable Environment; Special Publication No. 35; Soil Science Society of America: Madison, WI, USA, 1994; pp. 3–21. [Google Scholar]
- Robert, J.; Marcus, L.; Lars, V.; Bram, B.; Rainer, B.; Frank, H.; Dale, W.; Kari, M.; Kenneth, A. How strongly can forest management influence soil carbon sequestration? Geoderma 2006, 137, 253–268. [Google Scholar]
- Gao, Y.; He, N.P.; Yu, G.R. Long-term effects of different land use types on C, N, and P stoichiometry and storage in subtropical ecosystems: A case study in China. Ecol. Eng. 2014, 67, 171–181. [Google Scholar] [CrossRef]
- Wang, X.L.; Zhao, J.; Wu, J.P.; Chen, H.; Lin, Y.B.; Zhou, L.X.; Fu, S.L. Impacts of understory species removal and/or addition on soil respiration in a mixed forest plantation with native species in southern China. For. Ecol. Manag. 2010, 261, 1053–1060. [Google Scholar] [CrossRef]
- Harald, P.; Keith, V.C. Weight loss of litter and cellulose bags in a thinned white spruce forest in interior Alaska. Can. J. For. Res. 1978, 8, 42–46. [Google Scholar]
- Yang, M.; Li, Y.C.; Xiao, Y.H.; Yue, T.; Jiang, P.K.; Zhou, G.M.; Liu, J. Effects of intensive management on soil C and N pools and soil enzyme activities in Moso bamboo plantations. Chin. J. Appl. Ecol. 2016, 27, 3455–3462. [Google Scholar]
- Gong, C.; Tan, Q.Y.; Liu, G.B.; Xu, M.X. Forest thinning increases soil carbon stocks in China. For. Ecol. Manag. 2021, 482, 118812. [Google Scholar] [CrossRef]
- Rodrigo, V.; Edith, B.A.; Michael, F.A. Effects of Vegetation Thinning on Above- and Belowground Carbon in a Seasonally Dry Tropical Forest in Mexico. Biotrophic 2009, 41, 302–311. [Google Scholar]
- Chen, X.; Ma, L.Y.; Jia, Z.K. Research Progress on Effect of Intermediate Cutting to Forest Soil. Soil Water Conserv. China 2012, 9, 27. [Google Scholar]
- Deng, X.; Yin, J.Y.; Xu, L.; Shi, Y.J.; Zhou, G.M.; Li, Y.F.; Chen, G.S.; Ye, Y.Z.; Zhang, F.G.; Zhou, Y.F.; et al. Effects of abandonment management on soil C and N pools in Moso bamboo forests. Sci. Total Environ. 2020, 729, 138949. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.J.; Li, W.Z.; Wei, W.; Yang, Y.L.; Wu, Q.G. Effects of alnus cremastogyne-cupressus funebris strip reform on soil C: N: P stoichiometry of cupressus funebris forests in a hilly area of central Sichuan Basin. Chin. J. Appl. Environ. Biol. 2020, 26, 425–432. [Google Scholar]
- Wang, R.Z.; Li, J.Q.; Yang, G.L.; Zuo, M.; Yang, Y.L. Soil Nutrient storage and its value assessment of typical forest ecosystems in the Mopan Mountains. Chin. J. West China For. Sci. 2019, 48, 93–99. [Google Scholar]
- Guo, Z.W.; Cheng, S.L.; Yang, Q.P.; Li, Y.C. Responses of N and P stoichiometry on mulching management in the stand of Phyllostachys praecox. Chin. J. Acta Ecol. Sin. 2012, 32, 6361–6368. [Google Scholar]
- Lv, J.L.; Yan, M.J.; Song, B.L.; Guan, J.H.; Shi, W.Y.; Du, S. Ecological stoichiometry characteristics of soil carbon, nitrogen, and phosphorus in an oak forest and a black locust plantation in the Loess hilly region. Chin. J. Acta Ecol. Sin. 2017, 37, 3385–3393. [Google Scholar]
- Kunhamu, T.K.; Kumar, B.M.; Viswanath, S. Does thinning affect litterfall, litter decomposition, and associated nutrient release in Acacia mangium stands of Kerala in peninsular India? Can. J. For. Res. 2009, 39, 792–801. [Google Scholar] [CrossRef]
- Marty, C.; Houle, D.; Gagnon, C.; Courchesne, F. The relationships of soil total nitrogen concentrations, pools and C: N ratios with climate, vegetation types and nitrate deposition in temperate and boreal forests of eastern Canada. Catena 2017, 152, 163–172. [Google Scholar] [CrossRef]
- Bui, E.N.; Henderson, B.L. C:N:P stoichiometry in Australian soils with respect to vegetation and environmental factors. Plant Soil 2013, 373, 553–568. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Sun, J.; Ren, C.J.; Kang, D.; Deng, J.; Han, X.H.; Yang, G.H.; Feng., Y.Z.; Ren, G.X. Land use change influences soil C, N, and P stoichiometry under ‘Grain-to-Green Program’ in China. Sci. Rep. 2015, 5, 10195. [Google Scholar]
- Bing, H.; Wu, Y.; Zhou, J.; Sun, H.; Luo, J.; Wang, J.; Yu, D. Stoichiometric variation of carbon, nitrogen, and phosphorus in soils and its implication for nutrient limitation in alpine ecosystem of Eastern Tibetan Plateau. J. Soils Sediments 2015, 16, 405–416. [Google Scholar] [CrossRef]
- Peng, P.Q.; Zhang, W.J.; Tong, C.L.; Qiu, S.J.; Zhang, W.C. Soil C, N and P contents and their relationships with soil physical properties in wetlands of Dongting Lake floodplain. Chin. J. Appl. Ecol. 2015, 16, 1872–1878. [Google Scholar]
- Tian, H.; Cheng, X.; Han, H.; Jing, H.; Liu, X.; Li, Z. Seasonal variations and thinning effects on soil phosphorus fractions in Larix principis-rupprechtii Mayr. Plantations. Forests 2019, 10, 172. [Google Scholar] [CrossRef] [Green Version]
- Elser, J.J.; Acharyal, K.; Kylel, M.; Cotner, J.; Makino, W.; Markow, T.; Watts, T.; Hobbie, S.; Fagan, W.; Schade, J.; et al. Growth rate-stoichiometry couplings in diverse biota. Ecol. Lett. 2003, 6, 936–943. [Google Scholar] [CrossRef] [Green Version]
- Feng, D.F.; Bao, W.K. Review of the temporal and spatial patterns of soil C:N:P stoichiometry and its driving factors. Chin. J. Appl. Environ. Biol. 2017, 23, 400–408. [Google Scholar]
- Jack, T.T.; Dudley, J.R. Use of nitrogen to phosphorus ratios in plant tissue as an indicator of nutrient limitation and nitrogen saturation. J. Appl. Ecol. 2003, 40, 523–534. [Google Scholar]
- Qiu, Y.B.; Ling, G.C.; Zheng, W.H.; Xing, W.L.; Cheng, X.R. Effects of Thinning on Contents and Stoichiometry of C, N, and P in Different Components of Chinese Fir Plantation. Chin. J. For. Res. 2019, 32, 64–69. [Google Scholar]
- Zhao, R.Y.; Li, Z.C.; Wang, B.; Ge, X.G.; Dai, Y.X.; Zhao, Z.X.; Zhang, Y.J. Duration of mulching caused variable pools of labile organic carbon in a Phyllostachys edulis plantation. Chin. J. Plant Ecol. 2017, 41, 418–429. [Google Scholar]
- Shuai, O.Y.; Xiang, W.H.; Gou, M.M.; Lei, P.F.; Chen, L.; Deng, X.W.; Zhao, Z.H. Variations in soil carbon, nitrogen, phosphorus and stoichiometry along forest succession in southern China. Biogeosciences Discuss. 2017, 408, 1–27. [Google Scholar]
- Lv, D.; Yang, Y.H.; Zhao, W.H.; Lei, S.Y.; Zhang, X.P. Fine root biomass distribution and coupling to soil physicochemical properties under different restored vegetation types. Chin. J. Acta Ecol. Sin. 2018, 38, 3979–3987. [Google Scholar]
- Zhang, Y.J.; Wang, K.Q.; Song, Y.L.; Li, J.W. Ecological stoichiometry of soil carbon, nitrogen and phosphorus in five forest types in Subalpine of Middle Yunnan Province. Chin. J. Ecol. Environ. Sci. 2019, 28, 73–82. [Google Scholar]
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.A.; Lindahl, B.D. Roots and associated fungi drive long-term carbon sequestration in Boreal forest. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
- Margit, V.L.; Ingrid, K.K. Temperature sensitivity of soil organic matter decomposition-what do we know? Biol. Fertil. Soils 2009, 46, 1–15. [Google Scholar]


| Treatment | Age (a) | Aspect | Slope (°) | Altitude(a.s.l. m) | Density (Stem ha−1) | DBH (cm) | Average Height (m) | Canopy Density | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Before Thinning (2017) | After Thinning * (2020) | Before Thinning (2017) | After Thinning * (2020) | |||||||
| NT | 10 | northeast | 15 | 205 | 3700 | 4092 | 11 | 11.1 | 14 | 0.8 |
| MT | 10 | northeast | 17 | 205 | 3725 | 1950 | 10.6 | 10.7 | 13.2 | 0.6 |
| IT | 10 | northeast | 15 | 205 | 3638 | 1738 | 11 | 10.3 | 15.5 | 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Jiang, C.; Wang, H.; Jiang, C.; Liu, J.; Zang, Y.; Li, S.; Wang, Y.; Bai, Y. A Comparison of Soil C, N, and P Stoichiometry Characteristics under Different Thinning Intensities in a Subtropical Moso bamboo (Phyllostachys edulis) Forest of China. Forests 2022, 13, 1770. https://doi.org/10.3390/f13111770
Hu X, Jiang C, Wang H, Jiang C, Liu J, Zang Y, Li S, Wang Y, Bai Y. A Comparison of Soil C, N, and P Stoichiometry Characteristics under Different Thinning Intensities in a Subtropical Moso bamboo (Phyllostachys edulis) Forest of China. Forests. 2022; 13(11):1770. https://doi.org/10.3390/f13111770
Chicago/Turabian StyleHu, Xingbao, Chunqian Jiang, Hui Wang, Chunwu Jiang, Jianzhong Liu, Yiming Zang, Shigui Li, Yixiang Wang, and Yanfeng Bai. 2022. "A Comparison of Soil C, N, and P Stoichiometry Characteristics under Different Thinning Intensities in a Subtropical Moso bamboo (Phyllostachys edulis) Forest of China" Forests 13, no. 11: 1770. https://doi.org/10.3390/f13111770
APA StyleHu, X., Jiang, C., Wang, H., Jiang, C., Liu, J., Zang, Y., Li, S., Wang, Y., & Bai, Y. (2022). A Comparison of Soil C, N, and P Stoichiometry Characteristics under Different Thinning Intensities in a Subtropical Moso bamboo (Phyllostachys edulis) Forest of China. Forests, 13(11), 1770. https://doi.org/10.3390/f13111770





