Chemical Composition and Optimization of Liquefaction Parameters of Cytisus scoparius (Broom)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Composition
2.3. Liquefaction
3. Results and Discussion
3.1. Chemical Composition
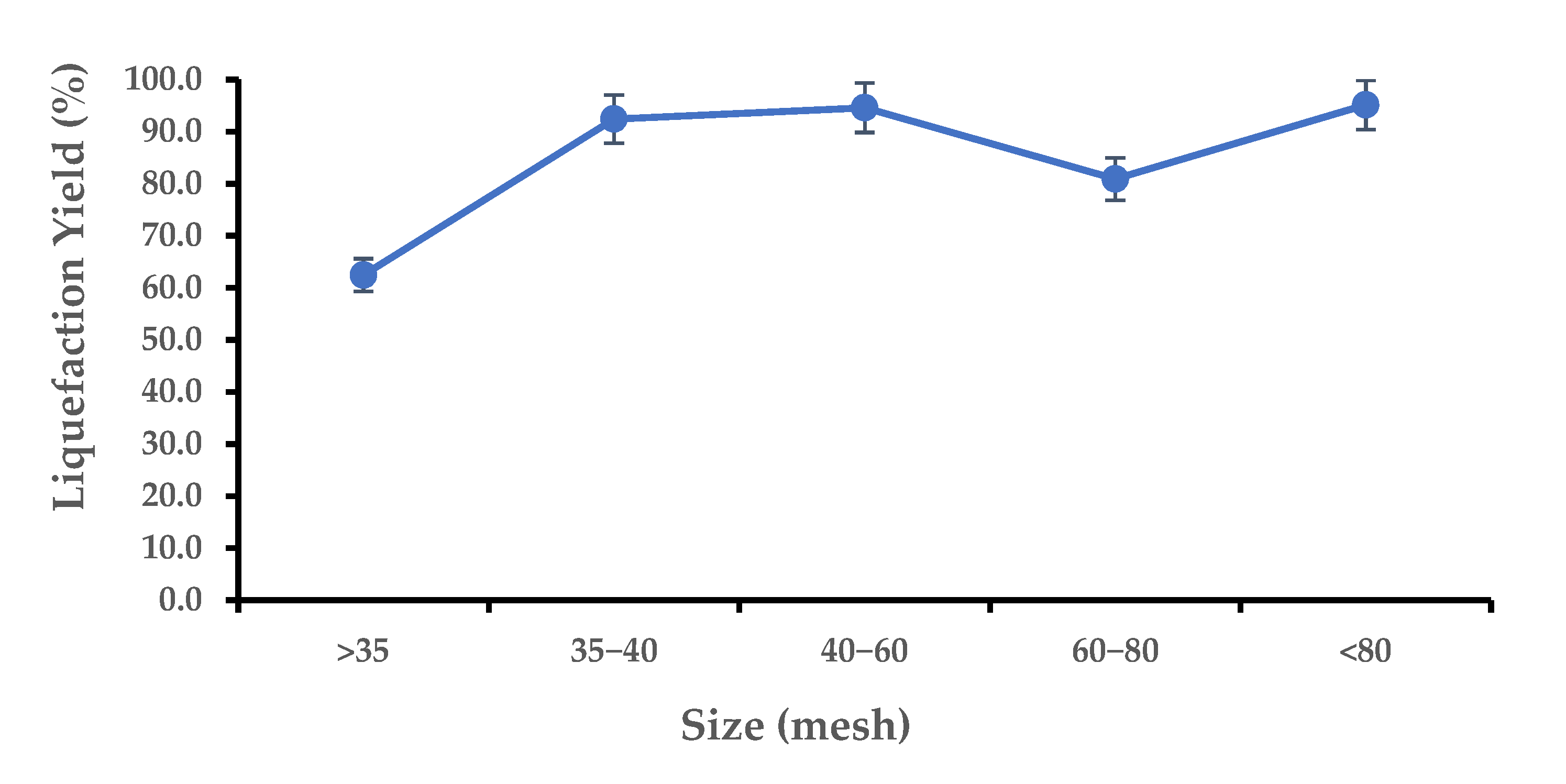
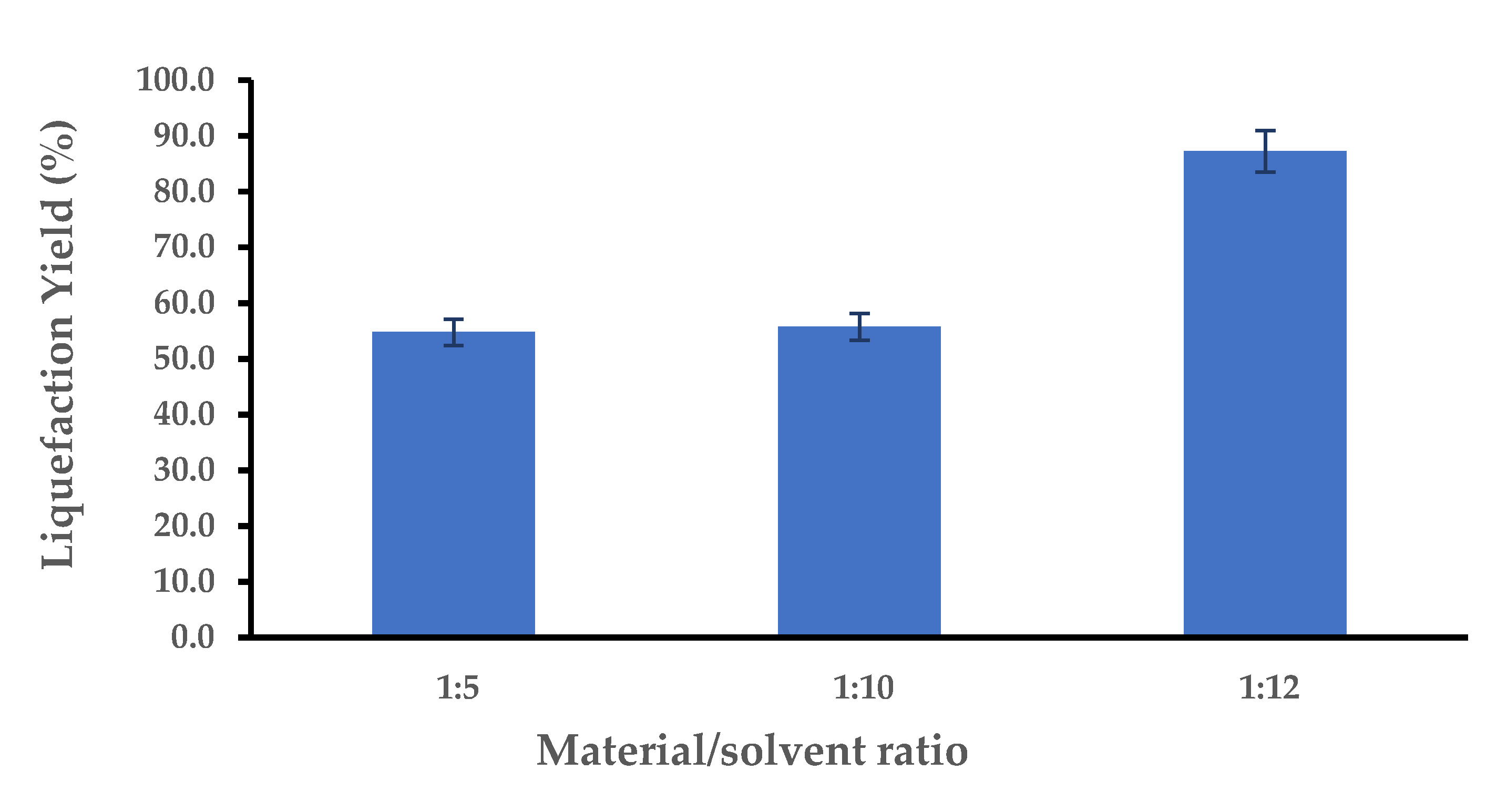
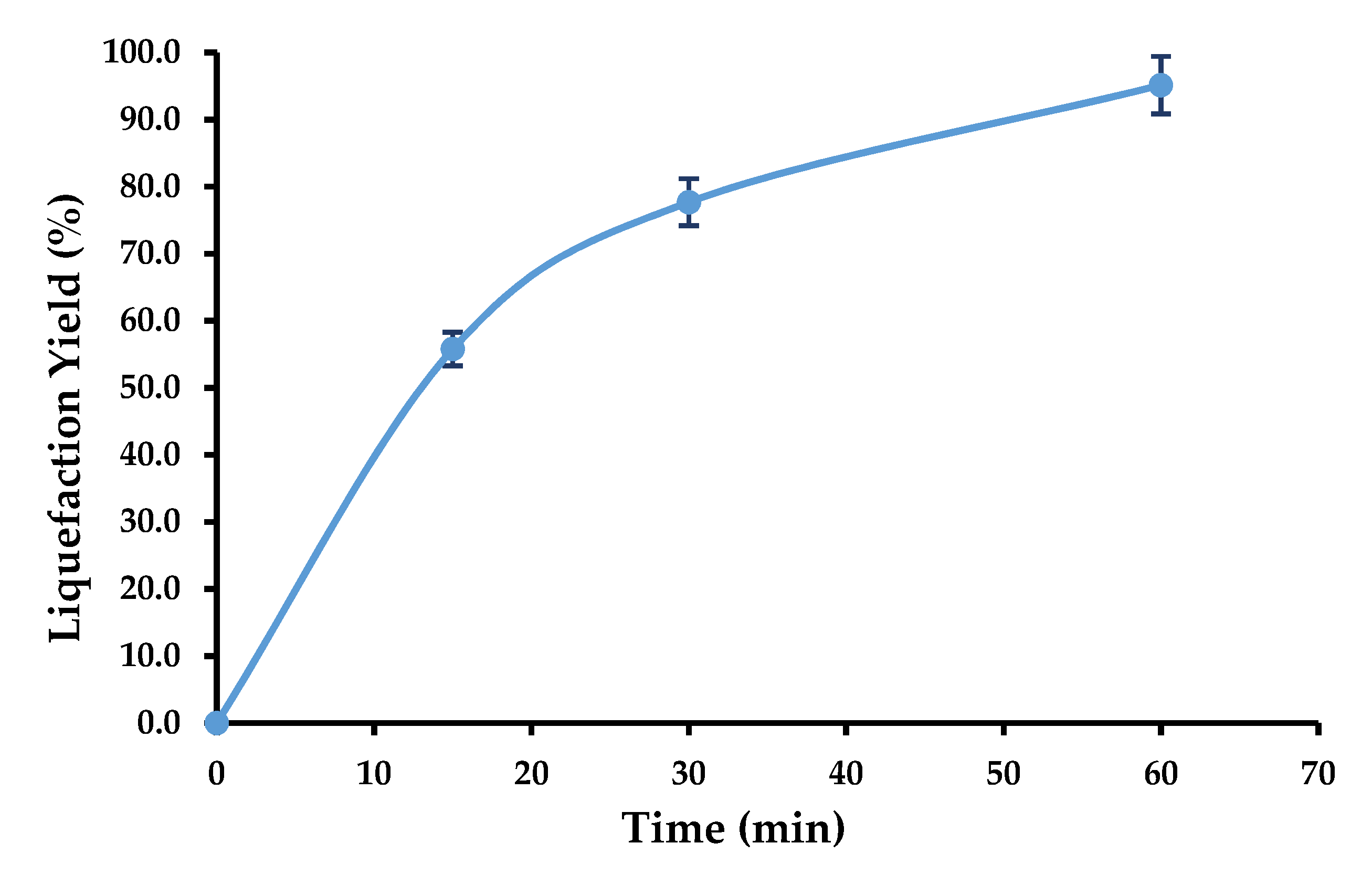
3.2. Liquefaction Optimization
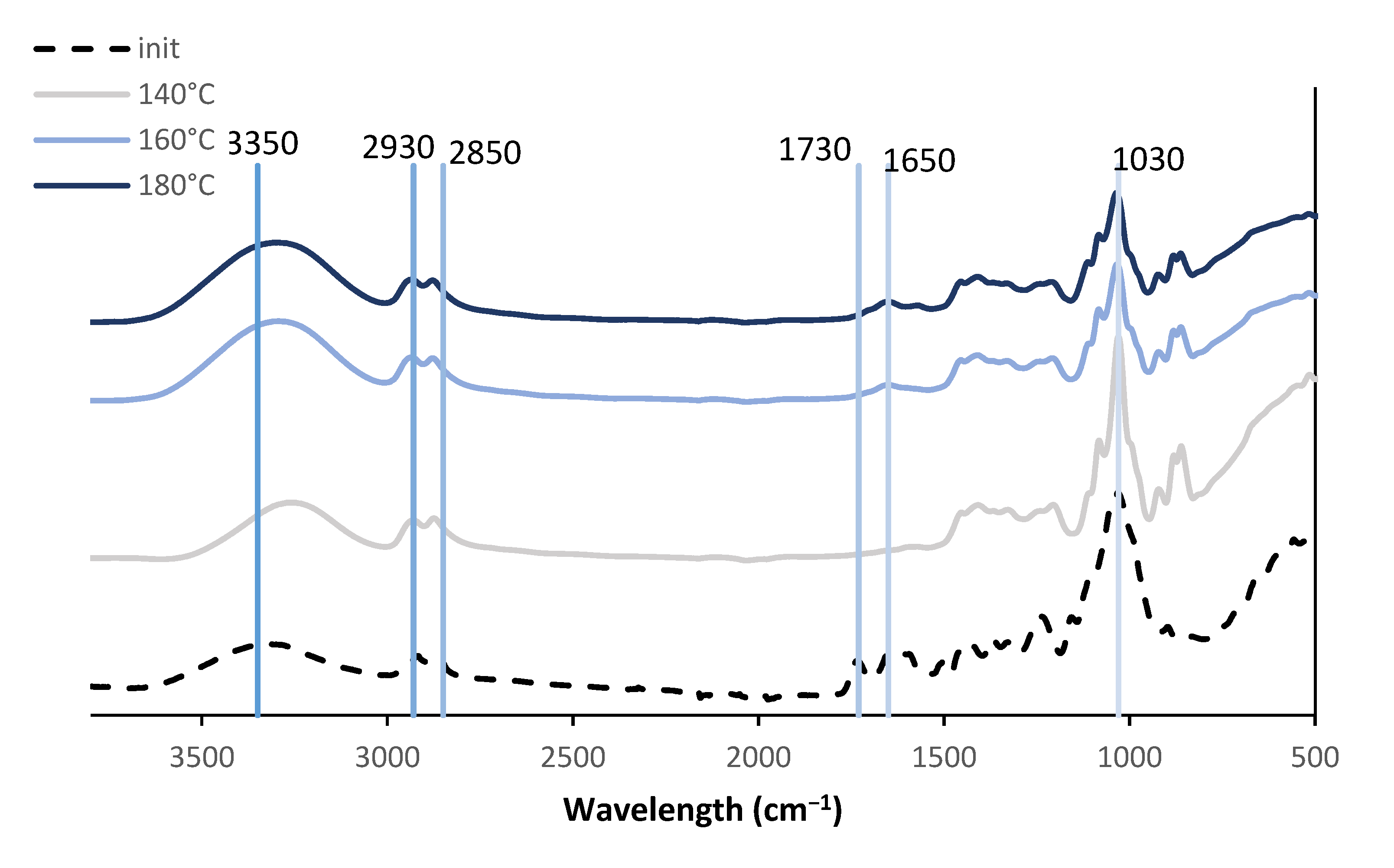
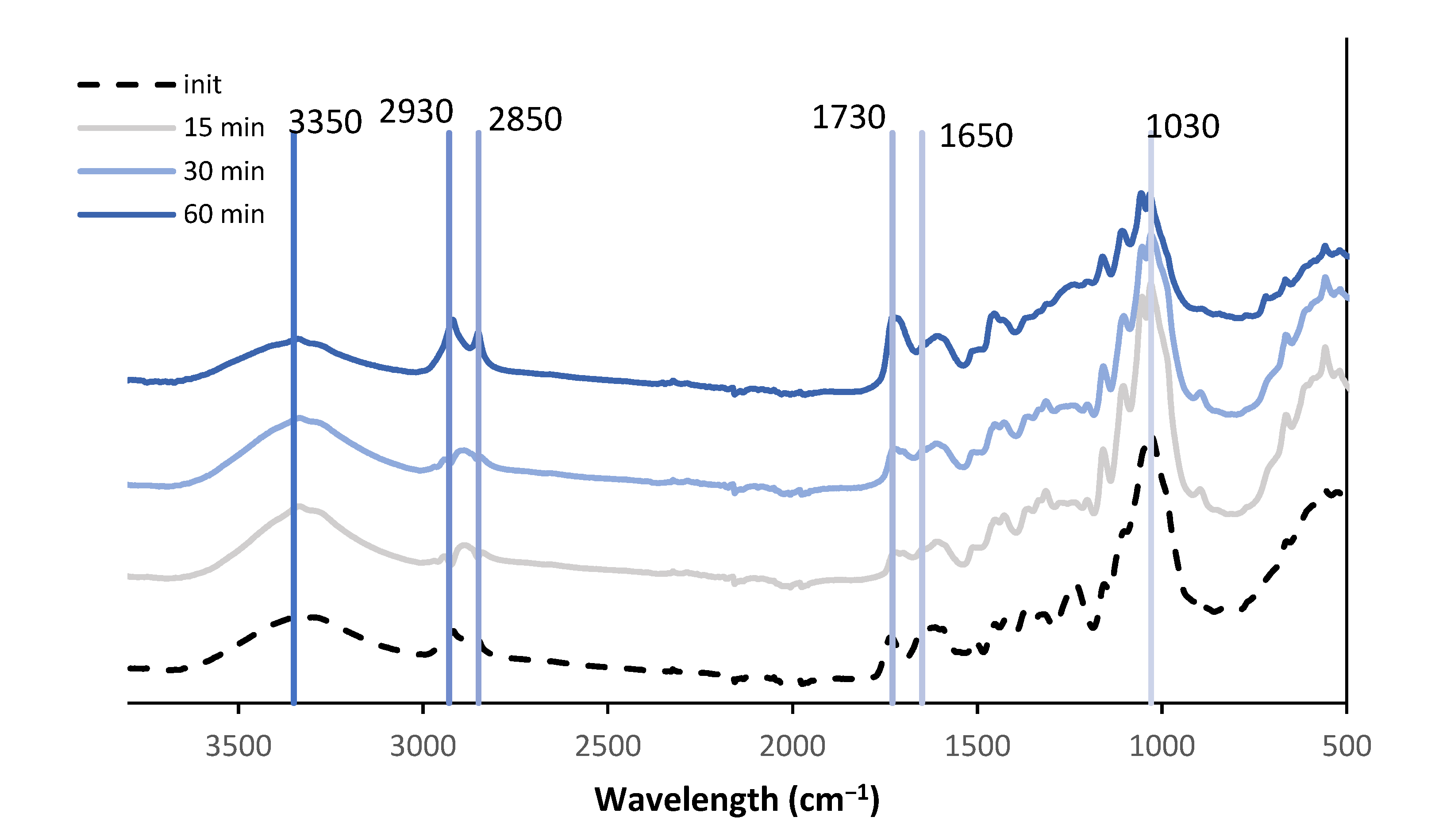
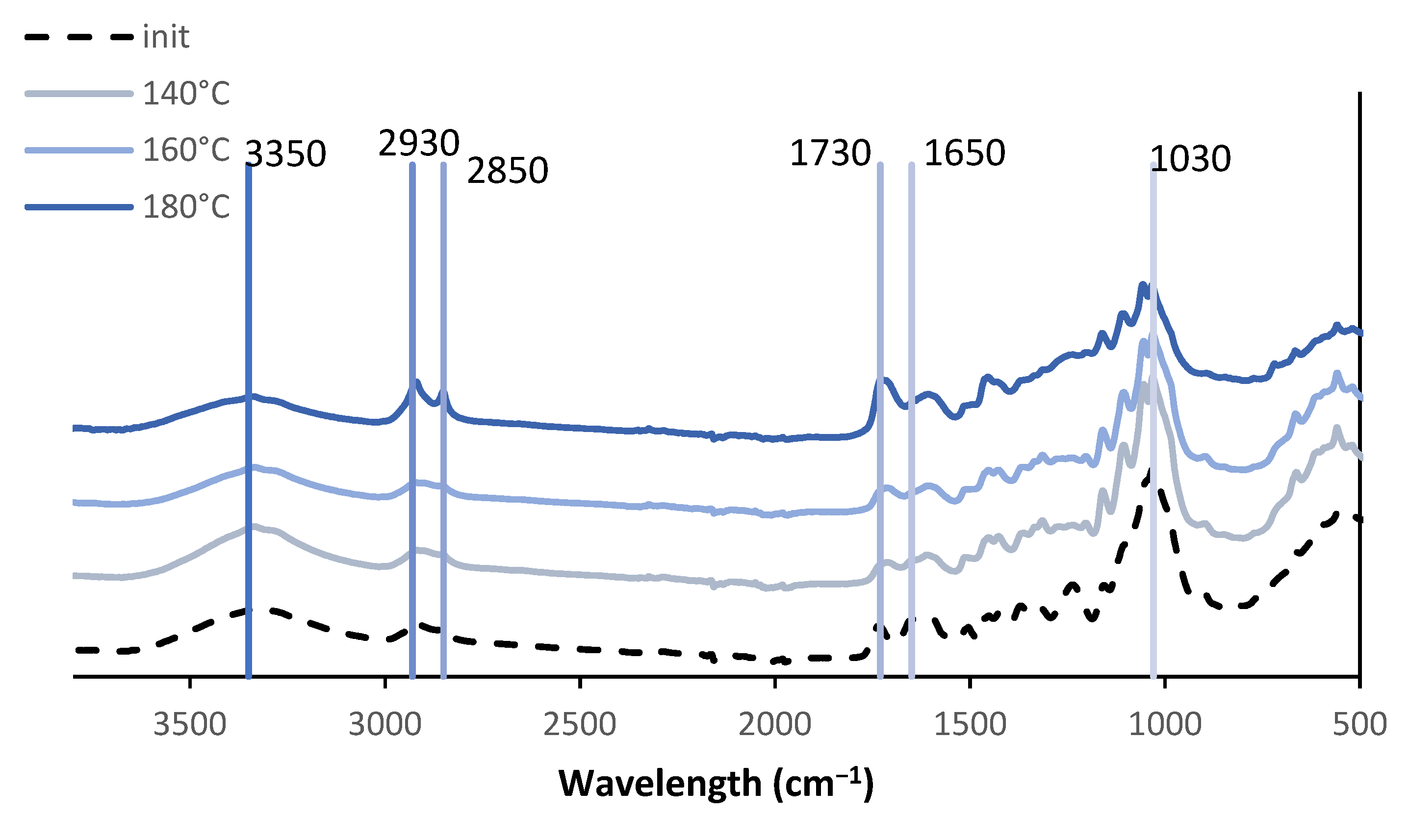
3.3. FTIR of Liquefaction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marchante, H.; Morais, M.; Freitas, H.; Marchante, E. Guia Prático para a Identificação de Plantas Invasoras em Portugal; Imprensa da Universidade de Coimbra/Coimbra University Press: Lisboa, Portugal, 2014; ISBN 978-989-26-0785-6. [Google Scholar]
- Costa, J.C.; Aguiar, C.; Capelo, J.; Lousã, M.; Antunes, J.H.S.; Honrado, J.J.; Izco, J.; Ladero, M. A Classe Cytisetea Scopario-Striati Em Portugal Continental. Quercetea 2003, 4, 45–70. [Google Scholar]
- o Inventário Florestal Nacional—PDF Free Download. Available online: https://docplayer.com.br/146300038-6-o-inventario-florestal-nacional.html (accessed on 17 July 2021).
- Caetano, M.; Igreja, C.; Marcelino, F.; Costa, H. Estatísticas e Dinâmicas Territoriais Multiescala de Portugal Continental 1995–2007–2010 Com Base Na Carta de Uso e Ocupação Do Solo (COS); Direção-Geral do Território: Lisbon, Portugal, 2017. [Google Scholar]
- Pinela, J.; Barros, L.; Carvalho, A.M.; Ferreira, I.C. Influence of the Drying Method in the Antioxidant Potential and Chemical Composition of Four Shrubby Flowering Plants from the Tribe Genisteae (Fabaceae). Food Chem. Toxicol. 2011, 49, 2983–2989. [Google Scholar] [CrossRef] [PubMed]
- González, N.; Otero, A.; Conde, E.; Falqué, E.; Moure, A.; Domínguez, H. Extraction of Phenolics from Broom Branches Using Green Technologies. J. Chem. Technol. Biotechnol. 2017, 92, 1345–1352. [Google Scholar] [CrossRef]
- Nirmal, J.; Babu, C.S.; Harisudhan, T.; Ramanathan, M. Evaluation of Behavioural and Antioxidant Activity of Cytisus Scoparius Link in Rats Exposed to Chronic Unpredictable Mild Stress. BMC Complement. Altern. Med. 2008, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Raja, S.; Ahamed, K.N.; Kumar, V.; Mukherjee, K.; Bandyopadhyay, A.; Mukherjee, P.K. Antioxidant Effect of Cytisus Scoparius against Carbon Tetrachloride Treated Liver Injury in Rats. J. Ethnopharmacol. 2007, 109, 41–47. [Google Scholar] [CrossRef]
- Gil, N.R.S. Pré-Tratamento de Materiais Lenhocelulósicos para Produção de Bioetanol. Ph.D. Thesis, Universidade da Beira Interior, Covilha, Portugal, 2008. [Google Scholar]
- Lin, R.; Sun, J.; Yue, C.; Wang, X.; Tu, D.; Gao, Z. Study on Preparation and Properties of Phenol-Formaldehyde-Chinese Fir Liquefaction Copolymer Resin. Maderas. Cienc. Tecnol. 2014, 16, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Mun, S.P.; Gilmour, I.A.; Jordan, P.J. Effect of Organic Sulfonic Acids as Catalysts during Phenol Liquefaction of Pinus Radiata Bark. J. Ind. Eng. Chem. 2006, 12, 720–726. [Google Scholar]
- Zhang, Q.; Zhao, G.; Jie, S. Liquefaction and Product Identification of Main Chemical Compositions of Wood in Phenol. For. Stud. China 2005, 7, 31–37. [Google Scholar] [CrossRef]
- Li, G.; Hse, C.; Qin, T. Wood Liquefaction with Phenol by Microwave Heating and FTIR Evaluation. J. For. Res. 2015, 26, 1043–1048. [Google Scholar] [CrossRef]
- Cruz-Lopes, L.P.; Domingos, I.; Ferreira, J.; Esteves, B. Chemical Composition and Study on Liquefaction Optimization of Chestnut Shells. Open Agric. 2020, 5, 905–911. [Google Scholar] [CrossRef]
- Kurimoto, Y.; Tamura, Y. Species Effects on Wood-Liquefaction in Polyhydric Alcohols. Holzforschung 1999, 53, 617–622. [Google Scholar] [CrossRef]
- D’Souza, J.; Wong, S.Z.; Camargo, R.; Yan, N. Solvolytic Liquefaction of Bark: Understanding the Role of Polyhydric Alcohols and Organic Solvents on Polyol Characteristics. ACS Sustain. Chem. Eng. 2015, 4, 851–861. [Google Scholar] [CrossRef]
- Esteves, B.; Dulyanska, Y.; Costa, C.; Ferreira, J.V.; Domingos, I.; Pereira, H.; de Lemos, L.T.; Cruz-Lopes, L.V. Cork Liquefaction for Polyurethane Foam Production. BioResources 2017, 12, 2339–2353. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Ruan, X.; Cheng, X.; Lü, Q. Liquefaction of Lignin by Polyethyleneglycol and Glycerol. Bioresour. Technol. 2011, 102, 3581–3583. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Tukamoto, K.; Tomita, B. Application of Liquefied Wood to a New Resin System-Synthesis and Properties of Liquefied Wood/Epoxy Resins. Holzforschung 2000, 54, 93–100. [Google Scholar] [CrossRef]
- Chen, F.; Lu, Z. Liquefaction of Wheat Straw and Preparation of Rigid Polyurethane Foam from the Liquefaction Products. J. Appl. Polym. Sci. 2009, 111, 508–516. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, Y.; Zheng, Z.; Zhou, Q.; Feng, H. Study on Liquefaction Technology of Cornstarch in Polyhydric Alcohols. J. Southwest For. Univ. 2011, 31, 72–74. [Google Scholar]
- Zhang, J.; Hori, N.; Takemura, A. Optimization of Agricultural Wastes Liquefaction Process and Preparing Bio-Based Polyurethane Foams by the Obtained Polyols. Ind. Crops Prod. 2019, 138, 111455. [Google Scholar] [CrossRef]
- Pan, H.; Zheng, Z.; Hse, C.Y. Microwave-Assisted Liquefaction of Wood with Polyhydric Alcohols and Its Application in Preparation of Polyurethane (PU) Foams. Eur. J. Wood Wood Prod. 2012, 70, 461–470. [Google Scholar] [CrossRef]
- Zheng, Z.; Pan, H.; Huang, Y.; Chung, Y.; Zhang, X.; Feng, H. Rapid Liquefaction of Wood in Polyhydric Alcohols under Microwave Heating and Its Liquefied Products for Preparation of Rigid Polyurethane Foam. Open Mater. Sci. J. 2011, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Esteves, B.; Cruz-Lopes, L.; Ferreira, J.; Domingos, I.; Nunes, L.; Pereira, H. Optimizing Douglas-Fir Bark Liquefaction in Mixtures of Glycerol and Polyethylene Glycol and KOH. Holzforschung 2018, 72, 25–30. [Google Scholar] [CrossRef]
- Fernandes, A.; Cruz-Lopes, L.; Dulyanska, Y.; Domingos, I.; Ferreira, J.; Evtuguin, D.; Esteves, B. Eco Valorization of Eucalyptus globulus Bark and Branches through Liquefaction. Appl. Sci. 2022, 12, 3775. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, J.; Zhao, J.; Tu, S. Liquefaction of Bamboo Shoot Shell for the Production of Polyols. Bioresour. Technol. 2014, 153, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Du, M.; Hu, L. Factors Influencing Polyol Liquefaction of Nut Shells of Different Camellia Species. BioResources 2016, 11, 9956–9969. [Google Scholar] [CrossRef] [Green Version]
- Duarte, J.; Cruz-Lopes, L.; Dulyanska, Y.; Domingos, I.; Ferreira, J.; de Lemos, L.T.; Esteves, B. Orange Peel Liquefaction Monitored by FTIR. J. Int. Sci. Publ. 2017, 5, 309–313. [Google Scholar]
- 264 Om-97; Preparation of Wood in Chemical Analysis. Tappi Press: Atlanta, GA, USA, 1997.
- 211 Om–93; Ash in Wood, Pulp, Paper and Paperboard: Combustion at 525. Tappi Press: Atlanta, GA, USA, 1993.
- Prozil, S.O.; Evtuguin, D.V.; Lopes, L.P.C. Chemical Composition of Grape Stalks of Vitis vinifera L. from Red Grape Pomaces. Ind. Crops Prod. 2012, 35, 178–184. [Google Scholar] [CrossRef]
- Neto, C.P.; Seca, A.; Fradinho, D.; Coimbra, M.A.; Domingues, F.; Evtuguin, D.; Silvestre, A.; Cavaleiro, J.A.S. Chemical Composition and Structural Features of the Macromolecular Components of Hibiscus Cannabinus Grown in Portugal. Ind. Crops Prod. 1996, 5, 189–196. [Google Scholar] [CrossRef]
- González, D.; Campos, A.R.; Cunha, A.M.; Santos, V.; Parajó, J.C. Manufacture of Fibrous Reinforcements for Biodegradable Biocomposites from Citysus Scoparius. J. Chem. Technol. Biotechnol. 2011, 86, 575–583. [Google Scholar] [CrossRef]
- González, N.; Ribeiro, D.; Fernandes, E.; Nogueira, D.R.; Conde, E.; Moure, A.; Vinardell, M.P.; Mitjans, M.; Domínguez, H. Potential Use of Cytisus Scoparius Extracts in Topical Applications for Skin Protection against Oxidative Damage. J. Photochem. Photobiol. B Biol. 2013, 125, 83–89. [Google Scholar] [CrossRef]
- Esteves, B.; Ayata, U.; Cruz-Lopes, L.; Brás, I.; Ferreira, J.; Domingos, I. Changes in the Content and Composition of the Extractives in Thermally Modified Tropical Hardwoods. Maderas. Cienc. Tecnol. 2022, 24, 1–14. [Google Scholar] [CrossRef]
- Chupin, L.; Motillon, C.; Charrier-El Bouhtoury, F.; Pizzi, A.; Charrier, B. Characterisation of Maritime Pine (Pinus pinaster) Bark Tannins Extracted under Different Conditions by Spectroscopic Methods, FTIR and HPLC. Ind. Crops Prod. 2013, 49, 897–903. [Google Scholar] [CrossRef]
- Kofujita, H.; Ettyu, K.; Ota, M. Characterization of the Major Components in Bark from Five Japanese Tree Species for Chemical Utilization. Wood Sci. Technol. 1999, 33, 223–228. [Google Scholar] [CrossRef]
- Smuga-Kogut, M.; Szymanowska, D.; Markiewicz, R.; Piskier, T.; Kobus-Cisowska, J.; Cielecka-Piontek, J.; Schöne, H. Evaluation of the Potential of Fireweed (Epilobium angustifolium L.), European Goldenrod (Solidago virgaurea L.), and Common Broom (Cytisus scoparius L.) Stems in Bioethanol Production. Energy Sci. Eng. 2020, 8, 3244–3254. [Google Scholar] [CrossRef]
- Gil, N.; Domingues, F.C.; Amaral, M.E.; Duarte, A.P. Optimization of diluted acid pretreatment of Cytisus striatus and Cistus ladanifer for bioethanol production. J. Biobased Mater. Bioenergy 2012, 6, 292–298. [Google Scholar] [CrossRef]
- Sen, A.; Miranda, I.; Esteves, B.; Pereira, H. Chemical Characterization, Bioactive and Fuel Properties of Waste Cork and Phloem Fractions from Quercus cerris L. Bark. Ind. Crops Prod. 2020, 157, 112909. [Google Scholar] [CrossRef]
- Domingos, I.; Ferreira, J.; Esteves, B.; Cruz-Lopes, L. Liquefaction and Chemical Composition of Walnut Shells. Open Agric. 2022, 7, 249–256. [Google Scholar] [CrossRef]
- Domingos, I.; Ferreira, J.; Cruz-Lopes, L.; Esteves, B. Polyurethane Foams from Liquefied Orange Peel Wastes. Food Bioprod. Process. 2019, 115, 223–229. [Google Scholar] [CrossRef]
- Gama, N.V.; Soares, B.; Freire, C.S.; Silva, R.; Brandão, I.; Neto, C.P.; Barros-Timmons, A.; Ferreira, A. Rigid Polyurethane Foams Derived from Cork Liquefied at Atmospheric Pressure: Rigid Polyurethane Foams Derived from Cork Liquefied. Polym. Int. 2015, 64, 250–257. [Google Scholar] [CrossRef]
- Cinelli, P.; Anguillesi, I.; Lazzeri, A. Green Synthesis of Flexible Polyurethane Foams from Liquefied Lignin. Eur. Polym. J. 2013, 49, 1174–1184. [Google Scholar] [CrossRef]
- Kahlerras, Z.; Irinislimane, R.; Bruzaud, S.; Belhaneche-Bensemra, N. Elaboration and Characterization of Polyurethane Foams Based on Renewably Sourced Polyols. J. Polym. Environ. 2020, 28, 3003–3018. [Google Scholar] [CrossRef]
- Esteves, B.; Velez Marques, A.; Domingos, I.; Pereira, H. Chemical Changes of Heat Treated Pine and Eucalypt Wood Monitored by FTIR. Maderas. Cienc. Tecnol. 2013, 15, 245–258. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Lopes, L.; Dulyanska, Y.; Domingos, I.; Ferreira, J.; Fragata, A.; Guiné, R.; Esteves, B. Influence of Pre-Hydrolysis on the Chemical Composition of Prunus Avium Cherry Seeds. Agronomy 2022, 12, 280. [Google Scholar] [CrossRef]
- Michell, A.; Higgins, H. Infrared Spectroscopy in Australian Forest Products Research; CSIRO Forestry: Victoria, Australia, 2002. [Google Scholar]


| Parameters | Content (% Dry Matter, w/w) | |
|---|---|---|
| Ashes | 0.694 ± 0.012 | |
| K | 0.194 ± 0.001 | |
| Ca | 0.046 ± 0.001 | |
| Na | 0.042 ± 0.001 | |
| Mg | 0.030 ± 0.001 | |
| P | 0.028 ± 0.001 | |
| Fe | <0.01 ± 0.000 | |
| Zn | <0.01 ± 0.000 | |
| Extractives | Dichloromethane | 0.689 ± 0.274 |
| Ethanol | 6.638 ± 1.466 | |
| Hot water | 1.977 ± 0.794 | |
| Total | 9.304 ± 1.466 | |
| 1% NaOH extract a | 20.77 ± 0.15 | |
| Klason Lignin b | 14.57 ± 0.06 | |
| Cellulose Kürscher and Höffer | 36.05 ± 1.15 | |
| Hemicelluloses | 18.61 ± 1.47 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Lopes, L.; Almeida, D.; Dulyanska, Y.; Domingos, I.; Ferreira, J.; Fragata, A.; Esteves, B. Chemical Composition and Optimization of Liquefaction Parameters of Cytisus scoparius (Broom). Forests 2022, 13, 1772. https://doi.org/10.3390/f13111772
Cruz-Lopes L, Almeida D, Dulyanska Y, Domingos I, Ferreira J, Fragata A, Esteves B. Chemical Composition and Optimization of Liquefaction Parameters of Cytisus scoparius (Broom). Forests. 2022; 13(11):1772. https://doi.org/10.3390/f13111772
Chicago/Turabian StyleCruz-Lopes, Luísa, Daniela Almeida, Yuliya Dulyanska, Idalina Domingos, José Ferreira, Anabela Fragata, and Bruno Esteves. 2022. "Chemical Composition and Optimization of Liquefaction Parameters of Cytisus scoparius (Broom)" Forests 13, no. 11: 1772. https://doi.org/10.3390/f13111772






